Manipulation, Sampling and Inactivation of the SARS-CoV-2 Virus Using Nonuniform Electric Fields on Micro-Fabricated Platforms: A Review
Abstract
1. Introduction
2. Virus Manipulation Using Electric Fields
2.1. Concentration of Moderately Large Viruses (>200 nm)
2.2. Concentration of Smaller Viruses
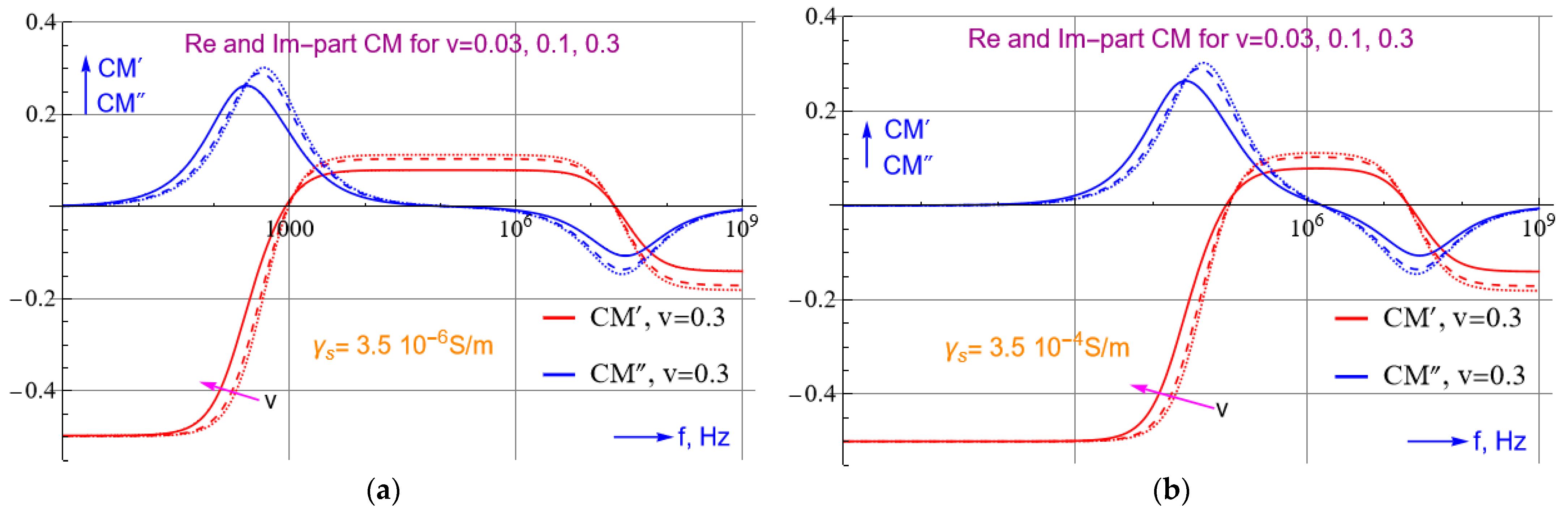
2.3. Trapping of SARS-CoV-2: Theoretical Approach
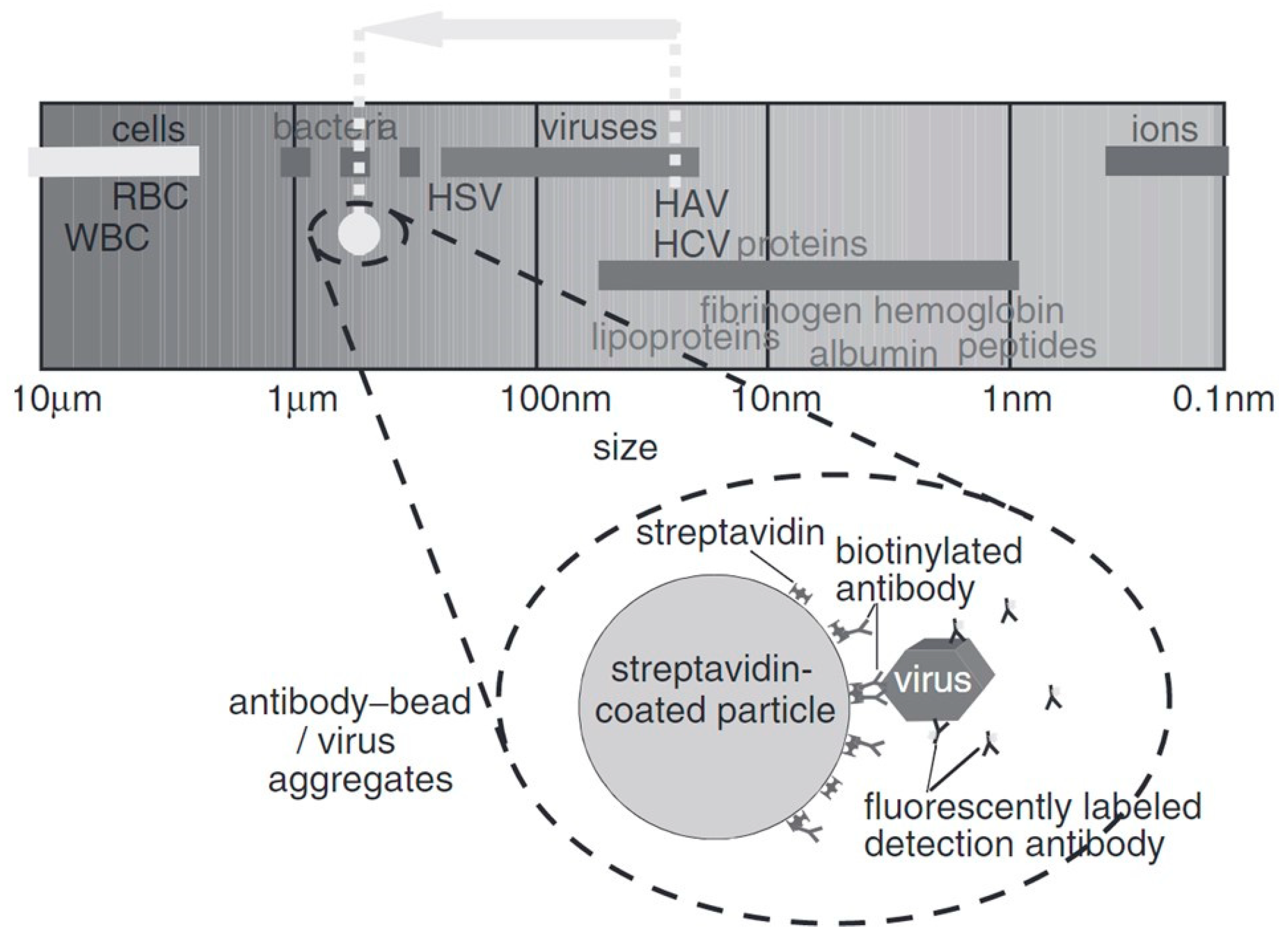
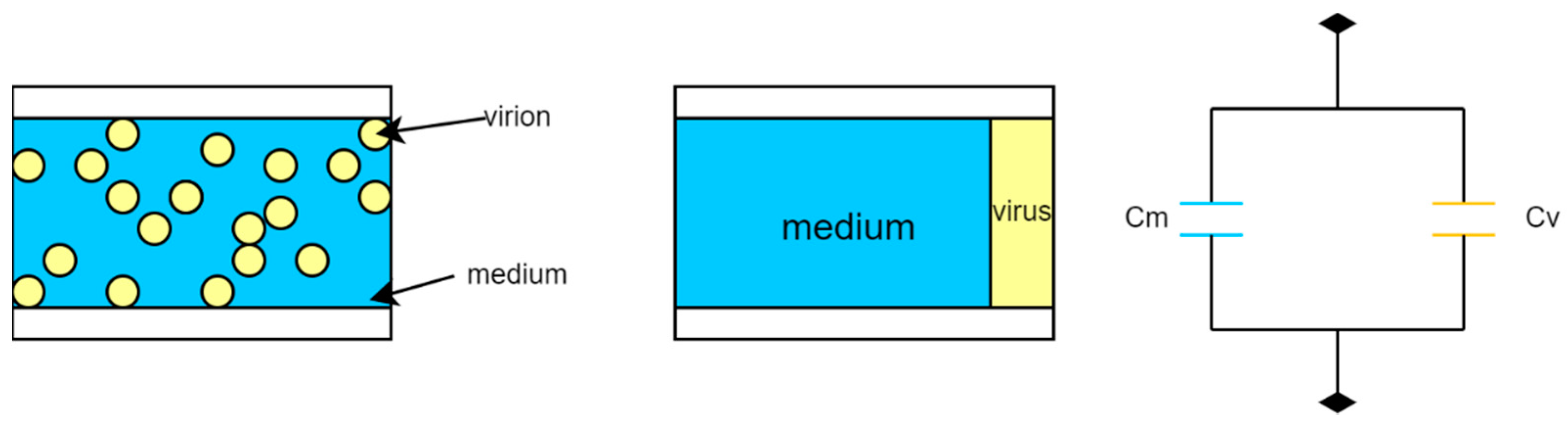
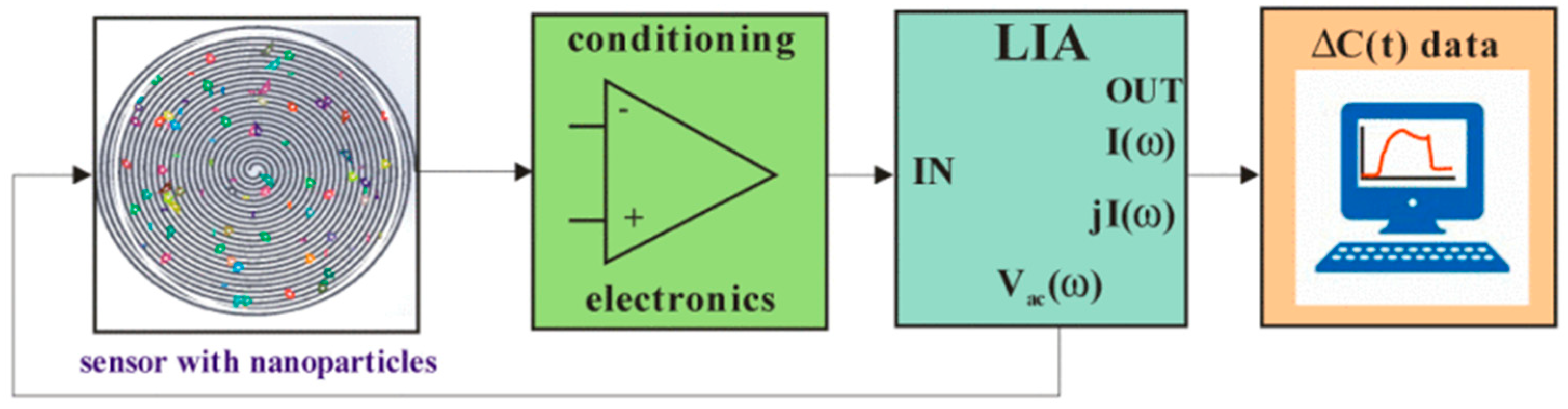
3. Electric Sampling
4. Electrical Inactivation
4.1. Inactivation by Irreversible Electroporation
Irreversible Electroporation of SARS-CoV-2
4.2. Short Pulse Effect
4.3. Damaging the Spike Protein
5. Electrode Configurations for Trapping, Sampling and Killing
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Calculation CM-Factor vs. ω with Mathematica
Appendix A.2. How Should the DEP Force Be Calculated?
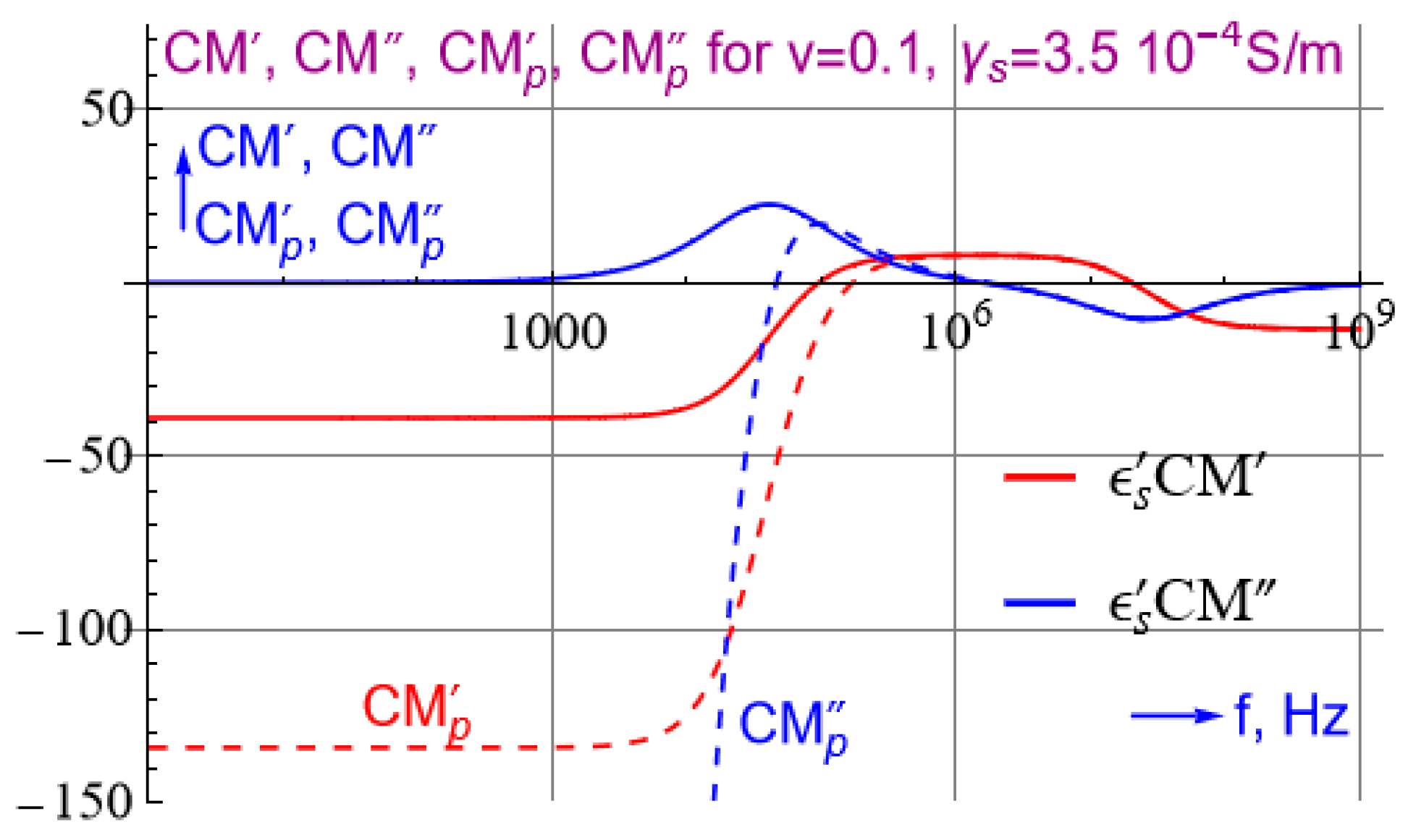
Appendix A.3. Simple Approximation for of the Virus
References
- World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 11 January 2023).
- Aliabadi, H.A.M.; Eivazzadeh-Keihan, R.; Parikhani, A.B.; Mehraban, S.F.; Maleki, A.; Fereshteh, S.; Bazaz, M.; Zolriasatein, A.; Bozorgnia, B.; Rahmati, S.; et al. COVID-19: A systematic review and update on prevention, diagnosis, and treatment. Medcomm 2022, 3, e115. [Google Scholar] [CrossRef]
- Berkenbrock, J.A.; Grecco-Machado, R.; Achenbach, S. Microfluidic devices for the detection of viruses: Aspects of emergency fabrication during the COVID-19 pandemic and other outbreaks. Proc. R. Soc. A 2020, 476, 20200398. [Google Scholar] [CrossRef] [PubMed]
- Burkhartsmeyer, J.; Wang, Y.; Wong, K.S.; Gordon, R. Optical Trapping, Sizing, and Probing Acoustic Modes of a Small Virus. Appl. Sci. 2020, 10, 394. [Google Scholar] [CrossRef]
- Moshfegh, F.; Khosraviani, F.; Moghaddasi, N.; Limoodi, S.F.S.J.; Boluki, E. Antiviral optical techniques as a possible novel approach to COVID-19 treatment. J. Innov. Opt. Health Sci. 2021, 14, 2130002. [Google Scholar] [CrossRef]
- Yap, T.F.; Liu, Z.; Shveda, R.A.; Preston, D.J. A predictive model of the temperature-dependent inactivation of coronaviruses. Appl. Phys. Lett. 2020, 117, 060601. [Google Scholar] [CrossRef]
- Lukose, J.; Chidangil, S.; George, S.D. Optical technologies for the detection of viruses like COVID-19: Progress and prospects. Biosens. Bioelectron. 2021, 178, 113004. [Google Scholar] [CrossRef]
- Khadre, M.A.; Yousef, A.E. Susceptibility of Human Rotavirus to Ozone, High Pressure, and Pulsed Electric Field. J. Food Prot. 2002, 65, 1441–1446. [Google Scholar] [CrossRef]
- Ghatak, B.; Banerjee, S.; Ali, S.B.; Bandyopadhyay, R.; Das, N.; Mandal, D.; Tudu, B. Design of a self-powered triboelectric face mask. Nano Energy 2021, 79, 105387. [Google Scholar] [CrossRef]
- Nie, J.; Chen, X.; Wang, Z.L. Electrically Responsive Materials and Devices Directly Driven by the High Voltage of Triboelectric Nanogenerators. Adv. Funct. Mater. 2018, 29, 1806351. [Google Scholar] [CrossRef]
- Zhuang, J.; Yin, J.; Lv, S.; Wang, B.; Mu, Y. Advanced “lab-on-a-chip” to detect viruses–Current challenges and future perspectives. Biosens. Bioelectron. 2020, 163, 112291. [Google Scholar] [CrossRef]
- Lapizco-Encinas, B.H.; Rito-Palomares, M. Dielectrophoresis for the manipulation of nanobioparticles. Electrophoresis 2007, 28, 4521–4538. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.; Green, N.G. Dielectrophoretic manipulation of rod-shaped viral particles. J. Electrost. 1997, 42, 279–293. [Google Scholar] [CrossRef]
- Morgan, H.; Hughes, M.P.; Green, N.G. Separation of Submicron Bioparticles by Dielectrophoresis. Biophys. J. 1999, 77, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.; Morgan, H.; Rixon, F.J.; Burt, J.P.; Pethig, R. Manipulation of herpes simplex virus type 1 by dielectrophoresis. Biochim. Biophys. Acta 1998, 1425, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Akin, D.; Li, H.; Bashir, R. Real-Time Virus Trapping and Fluorescent Imaging in Microfluidic Devices. Nano Lett. 2004, 4, 257–259. [Google Scholar] [CrossRef]
- Madiyar, F.R.; Syed, L.U.; Culbertson, C.T.; Li, J. Manipulation of bacteriophages with dielectrophoresis on carbon nanofiber nanoelectrode array. Electrophoresis 2013, 34, 1123–1130. [Google Scholar] [CrossRef]
- Javidpour, L.; Božič, A.; Naji, A.; Podgornik, R. Electrostatic interactions between the SARS-CoV-2 virus and a charged electret fibre. Soft Matter 2021, 17, 4296–4303. [Google Scholar] [CrossRef]
- Leung, W.W.F.; Sun, Q. Electrostatic charged nanofiber filter for filtering airborne novel coronavirus (COVID-19) and nano-aerosols. Sep. Purif. Technol. 2020, 250, 116886. [Google Scholar] [CrossRef]
- Grom, F.; Kentsch, J.; Müller, T.; Schnelle, T.; Stelzle, M. Accumulation and trapping of hepatitis A virus particles by electrohydrodynamic flow and dielectrophoresis. Electrophoresis 2006, 27, 1386–1393. [Google Scholar] [CrossRef]
- Ermolina, I.; Milner, J.; Morgan, H. Dielectrophoretic investigation of plant virus particles: Cow Pea Mosaic Virus and Tobacco Mosaic Virus. Electrophoresis 2006, 27, 3939–3948. [Google Scholar] [CrossRef]
- Nakano, M.; Ding, Z.; Suehiro, J. Dielectrophoresis and dielectrophoretic impedance detection of adenovirus and rotavirus. Jpn. J. Appl. Phys. 2016, 55, 017001. [Google Scholar] [CrossRef]
- Al Ahmad, M.; Mustafa, F.; Ali, L.M.; Karakkat, J.V.; Rizvi, T.A. Label-Free Capacitance-Based Identification of Viruses. Sci. Rep. 2015, 5, 9809. [Google Scholar] [CrossRef] [PubMed]
- Kentsch, J.; Dürr, M.; Schnelle, T.; Gradl, G.; Müller, T.; Jager, M.; Normann, A.; Stelzle, M. Microdevices for separation, accumulation, and analysis of biological micro- and nanoparticles. IEE Proc.-Nanobiotechnol. 2003, 150, 82–89. [Google Scholar] [CrossRef]
- Masuda, T.; Maruyama, H.; Honda, A.; Arai, F. Virus Enrichment for Single Virus Infection by Using 3D Insulator Based Dielectrophoresis. PLoS ONE 2014, 9, e94083. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Lawrence, R.M.; Jones, P.V.; Hogue, B.G.; Hayes, M.A. Concentration of Sindbis virus with optimized gradient insulator-based dielectrophoresis. Analyst 2016, 141, 1997–2008. [Google Scholar] [CrossRef]
- De Peña, A.C.; Redzuan, N.H.M.; Abajorga, M.K.; Hill, N.; Thomas, J.A.; Lapizco-Encinas, B.H. Analysis of Bacteriophages with Insulator-Based Dielectrophoresis. Micromachines 2019, 10, 450. [Google Scholar] [CrossRef]
- Lapizco-Encinas, B.; Davalos, R.V.; Simmons, B.; Cummings, E.B.; Fintschenko, Y. An insulator-based (electrodeless) dielectrophoretic concentrator for microbes in water. J. Microbiol. Methods 2005, 62, 317–326. [Google Scholar] [CrossRef]
- Gallo-Villanueva, R.C.; Perez-Gonzalez, V.H.; Cardenas-Benitez, B.; Jind, B.; Martinez-Chapa, S.O.; Lapizco-Encinas, B.H. Joule heating effects in optimized insulator-based dielectrophoretic devices: An interplay between post geometry and temperature rise. Electrophoresis 2019, 40, 1408–1416. [Google Scholar] [CrossRef]
- Johnson, M.W.; Wagner, G.W.; Bancroft, J.B. A Titrimetric and Electrophoretic Study of Cowpea Chlorotic Mottle Virus and its Protein. J. Gen. Virol. 1973, 19, 263–273. [Google Scholar] [CrossRef]
- Patolsky, F.; Zheng, G.; Hayden, O.; Lakadamyali, M.; Zhuang, X.; Lieber, C.M. Electrical detection of single viruses. Proc. Natl. Acad. Sci. USA 2004, 101, 14017–14022. [Google Scholar] [CrossRef]
- Milner, K.; Brown, A.; Allsopp, D.; Betts, W. Dielectrophoretic classification of bacteria using differential impedance measurements. Electron. Lett. 1998, 34, 66–68. [Google Scholar] [CrossRef]
- Kikutani, T.; Tamura, F.; Takahashi, Y.; Konishi, K.; Hamada, R. A novel rapid oral bacteria detection apparatus for effective oral care to prevent pneumonia. Gerodontology 2011, 29, e560–e565. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W. Monitoring of biological cell collection by dielectric spectroscopy. In Proceedings of the 2001 Annual Report Conference on Electrical Insulation and Dielectric Phenomena (Cat. No.01CH37225), Kitchener, ON, Canada, 14–17 October 2001; pp. 40–44. [Google Scholar] [CrossRef]
- Madiyar, F.R.; Haller, S.L.; Farooq, O.; Rothenburg, S.; Culbertson, C.; Li, J. AC dielectrophoretic manipulation and electroporation of vaccinia virus using carbon nanoelectrode arrays. Electrophoresis 2017, 38, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Al Ahmad, M.; Mustafa, F.; Ali, L.M.; Rizvi, T.A. Virus detection and quantification using electrical parameters. Sci. Rep. 2014, 4, 6831. [Google Scholar] [CrossRef] [PubMed]
- Hatsuki, R.; Honda, A.; Kajitani, M.; Yamamoto, T. Nonlinear electrical impedance spectroscopy of viruses using very high electric fields created by nanogap electrodes. Front. Microbiol. 2015, 6, 940. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Akin, D.; Bashir, R. Electrical capture and lysis of vaccinia virus particles using silicon nano-scale probe array. Biomed. Microdevices 2007, 9, 877–883. [Google Scholar] [CrossRef]
- Uc-Martin, J.; Guadarrama-Santana, A.; Garcia-Valenzuela, A. Detecting the presence of nanoparticles in suspension in droplets with a coplanar differential capacitive sensor. In Proceedings of the 2020 IEEE International Conference on Engineering Veracruz (ICEV), Boca del Rio, Mexico, 26–29 October 2020; pp. 1–5. [Google Scholar] [CrossRef]
- Scherlag, B.J.; Scherlag, R.A.; Po, S.S. Apparatus for generating negative air ions: Inactivation of coronaviruses. Lett. Health Biol. Sci. 2020, 5, 3. [Google Scholar] [CrossRef]
- Nishikawa, K.; Nojima, H. Airborne virus inactivation technology using cluster ions generated by discharge plasma. Sharp Tech. J. 2003, 86, 10–15. [Google Scholar]
- Kakutani, K.; Matsuda, Y.; Nonomura, T.; Takikawa, Y.; Takami, T.; Toyoda, H. A Simple Electrostatic Precipitator for Trapping Virus Particles Spread via Droplet Transmission. Int. J. Environ. Res. Public Health 2021, 18, 4934. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, N.; Fan, G.; Yu, J.; Gao, J.; Sun, G.; Ding, B. Electreted polyetherimide–silica fibrous membranes for enhanced filtration of fine particles. J. Colloid Interface Sci. 2015, 439, 12–20. [Google Scholar] [CrossRef]
- Huo, Z.-Y.; Kim, Y.-J.; Suh, I.-Y.; Lee, D.-M.; Lee, J.H.; Du, Y.; Wang, S.; Yoon, H.-J.; Kim, S.-W. Triboelectrification induced self-powered microbial disinfection using nanowire-enhanced localized electric field. Nat. Commun. 2021, 12, 3693. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Bhardwaj, J.; Han, C.-H.; Jang, J. Gentle Sampling of Submicrometer Airborne Virus Particles using a Personal Electrostatic Particle Concentrator. Environ. Sci. Technol. 2016, 50, 12365–12372. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; An, S.; Hwang, J. An integrated system of air sampling and simultaneous enrichment for rapid biosensing of airborne coronavirus and influenza virus. Biosens. Bioelectron. 2020, 170, 112656. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Sowers, A.E.; Jordan, C.A. (Eds.) Electroporation and Electrofusion in Cell Biology; Springer: New York, NY, USA, 1998. [Google Scholar] [CrossRef]
- Ivorra, A.; Villemejane, J.; Mir, L.M. Electrical modeling of the influence of medium conductivity on electroporation. Phys. Chem. Chem. Phys. 2010, 12, 10055–10064. [Google Scholar] [CrossRef]
- Lee, S.-W.; Tai, Y.-C. A micro cell lysis device. Sens. Actuators A Phys. 1999, 73, 74–79. [Google Scholar] [CrossRef]
- Schoenbach, K.; Joshi, R.; Stark, R.; Dobbs, F.; Beebe, S. Bacterial decontamination of liquids with pulsed electric fields. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 637–645. [Google Scholar] [CrossRef]
- de la Rosa, C.; Tilley, P.A.; Fox, J.D.; Kaler, K.V.I.S. Microfluidic Device for Dielectrophoresis Manipulation and Electrodisruption of Respiratory Pathogen Bordetella pertussis. IEEE Trans. Biomed. Eng. 2008, 55, 2426–2432. [Google Scholar] [CrossRef]
- Schoenbach, K.H.; Abou-Ghazala, A.; Vithoulkas, T.; Alden, R.W.; Turner, R.; Beebe, S. The effect of pulsed electrical fields on biological cells. In Proceedings of the Digest of Technical Papers. 11th IEEE International Pulsed Power Conference (Cat. No.97CH36127), Baltimore, MD, USA, 29 June–2 July 1997; Volume 1, pp. 73–78. [Google Scholar]
- Beveridge, J.; Wall, K.; MacGregor, S.; Anderson, J.; Rowan, N. Pulsed electric field inactivation of spoilage microorganisms in alcoholic beverages. Proc. IEEE 2004, 92, 1138–1143. [Google Scholar] [CrossRef]
- Beebe, S.; Fox, P.; Rec, L.; Somers, K.; Stark, R.; Schoenbach, K. Nanosecond pulsed electric field (nsPEF) effects on cells and tissues: Apoptosis induction and tumor growth inhibition. IEEE Trans. Plasma Sci. 2002, 30, 286–292. [Google Scholar] [CrossRef]
- Kotnik, T.; Bobanović, F.; Miklavčič, D. Sensitivity of transmembrane voltage induced by applied electric fields—A theoretical analysis. Bioelectrochem. Bioenerg. 1997, 43, 285–291. [Google Scholar] [CrossRef]
- Kotnik, T.; Mir, L.M.; Flisar, K.; Puc, M.; Miklavčič, D. Cell membrane electropermeabilization by symmetrical bipolar rectangular pulses: Part I. Increased efficiency of permeabilization. Bioelectrochemistry 2001, 54, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Church, C.; Zhu, J.; Huang, G.; Tzeng, T.-R.; Xuan, X. Integrated electrical concentration and lysis of cells in a microfluidic chip. Biomicrofluidics 2010, 4, 044101. [Google Scholar] [CrossRef] [PubMed]
- Vulto, P.; Dame, G.; Maier, U.; Makohliso, S.; Podszun, S.; Zahn, P.; Urban, G.A. A microfluidic approach for high efficiency extraction of low molecular weight RNA. Lab Chip 2010, 10, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.; Tanaka, N.; Yanagida, Y.; Hatsuzawa, T. On-chip single-cell lysis for extracting intracellular material. Jpn. J. Appl. Phys. 2007, 46, 6410. [Google Scholar] [CrossRef]
- Sholanov, K. Electrophysical Processes Effecting the COVID-19 Virus before It Enters Organism. 2020. Available online: https://www.researchgate.net/publication/343163569_ELECTROPHYSICAL_PROCESSES_EFFECTING_THE_COVID-19_VIRUS_BEFORE_IT_ENTERS_ORGANISM (accessed on 1 November 2022).
- Kotnik, T.; Miklavčič, D.; Slivnik, T. Time course of transmembrane voltage induced by time-varying electric fields—A method for theoretical analysis and its application. Bioelectrochem. Bioenerg. 1998, 45, 3–16. [Google Scholar] [CrossRef]
- Marszalek, P.; Liu, D.S.; Tsong, T.Y. Schwan equation and transmembrane potential induced by alternating electric field. Biophys. J. 1990, 58, 1053–1058. [Google Scholar] [CrossRef]
- Grosse, C.; Barchini, R. The influence of diffusion on the dielectric properties of suspensions of conductive spherical particles in an electrolyte. J. Phys. D Appl. Phys. 1992, 25, 508–515. [Google Scholar] [CrossRef]
- Schoenbach, K.; Katsuki, S.; Stark, R.; Buescher, E.; Beebe, S. Bioelectrics-new applications for pulsed power technology. IEEE Trans. Plasma Sci. 2002, 30, 293–300. [Google Scholar] [CrossRef]
- Amr, A.-G.; Schoenbach, K.H. Biofouling prevention with pulsed electric fields. IEEE Trans. Plasma Sci. 2000, 28, 115–121. [Google Scholar] [CrossRef]
- Gashimov, A.M.; Kurbanov, E.D. The influence of high-voltage impulse treatments on biological cells. Surf. Eng. Appl. Electrochem. 2009, 45, 411–414. [Google Scholar] [CrossRef]
- Arbeitman, C.R.; Rojas, P.; Ojeda-May, P.; Garcia, M.E. The SARS-CoV-2 spike protein is vulnerable to moderate electric fields. Nat. Commun. 2021, 12, 5407. [Google Scholar] [CrossRef] [PubMed]
- Osorio-González, D.; Muñiz-Orozco, V.J.; González, C.P.; Fuentes-Acosta, M.; Mulia-Rodríguez, J.; Mandujano-Rosas, L.A. Receptor Binding Domain (RBD) Structural Susceptibility in the SARS-CoV-2 Virus Spike Protein Exposed to a Pulsed Electric Field. J. Nucl. Phys. Mater. Sci. Radiat. Appl. 2021, 8, 177–182. [Google Scholar] [CrossRef]
- Wood, N. Trapping of Nanoparticles with Dielectrophoretic Nano-Probes. Ph.D. Thesis, University of Louisville, Louisville, KY, USA, 2012. [Google Scholar] [CrossRef]
- Lu, H.; Schmidt, M.A.; Jensen, K.F. A microfluidic electroporation device for cell lysis. Lab Chip 2005, 5, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-A.; Chen, C.-H.; Ghaemmaghami, A.M.; Fan, S.-K. Separation of dendritic and T cells using electrowetting and dielectrophoresis. In Proceedings of the 2012 7th IEEE International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Kyoto, Japan, 5–8 March 2012; pp. 183–186. [Google Scholar] [CrossRef]
- Lu, K.-Y.; Wo, A.M.; Lo, Y.-J.; Chen, K.-C.; Lin, C.-M.; Yang, C.-R. Three dimensional electrode array for cell lysis via electroporation. Biosens. Bioelectron. 2006, 22, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhao, D.; Li, J.; Wu, Y.; Zhou, W.; Wang, W.; Liang, Z.; Li, Z. High cell viability microfluidic electroporation in a curved channel. Sens. Actuators B Chem. 2017, 250, 703–711. [Google Scholar] [CrossRef]
- Auerswald, J.; Knapp, H.F. Quantitative assessment of dielectrophoresis as a micro fluidic retention and separation technique for beads and human blood erythrocytes. Microelectron. Eng. 2003, 67–68, 879–886. [Google Scholar] [CrossRef]
- Bakewell, D.; Morgan, H. Dielectrophoresis of DNA: Time- and Frequency-Dependent Collections on Microelectrodes. IEEE Trans. NanoBiosci. 2006, 5, 1–8. [Google Scholar] [CrossRef]
- Morshed, B.I.; Shams, M.; Mussivand, T. Investigation of Low-Voltage Pulse Parameters on Electroporation and Electrical Lysis Using a Microfluidic Device with Interdigitated Electrodes. IEEE Trans. Biomed. Eng. 2014, 61, 871–882. [Google Scholar] [CrossRef]
- Asokan, S.B.; Jawerth, L.; Carroll, R.L.; Cheney, R.E.; Washburn, S.; Superfine, R. Two-Dimensional Manipulation and Orientation of Actin−Myosin Systems with Dielectrophoresis. Nano Lett. 2003, 3, 431–437. [Google Scholar] [CrossRef]
- Jang, L.-S.; Huang, P.-H.; Lan, K.-C. Single-cell trapping utilizing negative dielectrophoretic quadrupole and microwell electrodes. Biosens. Bioelectron. 2009, 24, 3637–3644. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Rosenthal, A.; Voldman, J. nDEP microwells for single-cell patterning in physiological media. Lab Chip 2007, 7, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.S.; Morgan, H.; Green, N.G. Negative DEP traps for single cell immobilisation. Lab Chip 2009, 9, 1534–1540. [Google Scholar] [CrossRef]
- Krishnan, R.; Sullivan, B.D.; Mifflin, R.L.; Esener, S.C.; Heller, M.J. Alternating current electrokinetic separation and detection of DNA nanoparticles in high-conductance solutions. Electrophoresis 2008, 29, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T.P.; Lee, H.; Westervelt, R.M. Addressable micropost array for the dielectrophoretic manipulation of particles in fluid. Appl. Phys. Lett. 2004, 85, 6421–6423. [Google Scholar] [CrossRef]
- Cottet, J.; Fabregue, O.; Berger, C.; Buret, F.; Renaud, P.; Frénéa-Robin, M. MyDEP: A New Computational Tool for Dielectric Modeling of Particles and Cells. Biophys. J. 2018, 116, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.R.; Sauer, F.A.; Schwan, H.P. Electrorotation and levitation of cells and colloidal particles. Biophys. J. 1992, 63, 180–190. [Google Scholar] [CrossRef]
- Pohl, H.A.; Kaler, K.; Pollock, K. The continuous positive and negative dielectrophoresis of microorganisms. J. Biol. Phys. 1981, 9, 67–86. [Google Scholar] [CrossRef]
- Marszałek, P.; Zieliński, J.J.; Fikus, M. Experimental verification of a theoretical treatment of the mechanism of dielectrophoresis. Bioelectrochem. Bioenerg. 1989, 22, 289–298. [Google Scholar] [CrossRef]
- Kaler, K.V.; Jones, T.B. Dielectrophoretic spectra of single cells determined by feedback-controlled levitation. Biophys. J. 1990, 57, 173–182. [Google Scholar] [CrossRef]
- Gascoyne, P.R.C.; Becker, F.F.; Wang, X.-B. Numerical analysis of the influence of experimental conditions on the accuracy of dielectric parameters derived from electrorotation measurements. Bioelectrochem. Bioenerg. 1995, 36, 115–125. [Google Scholar] [CrossRef]
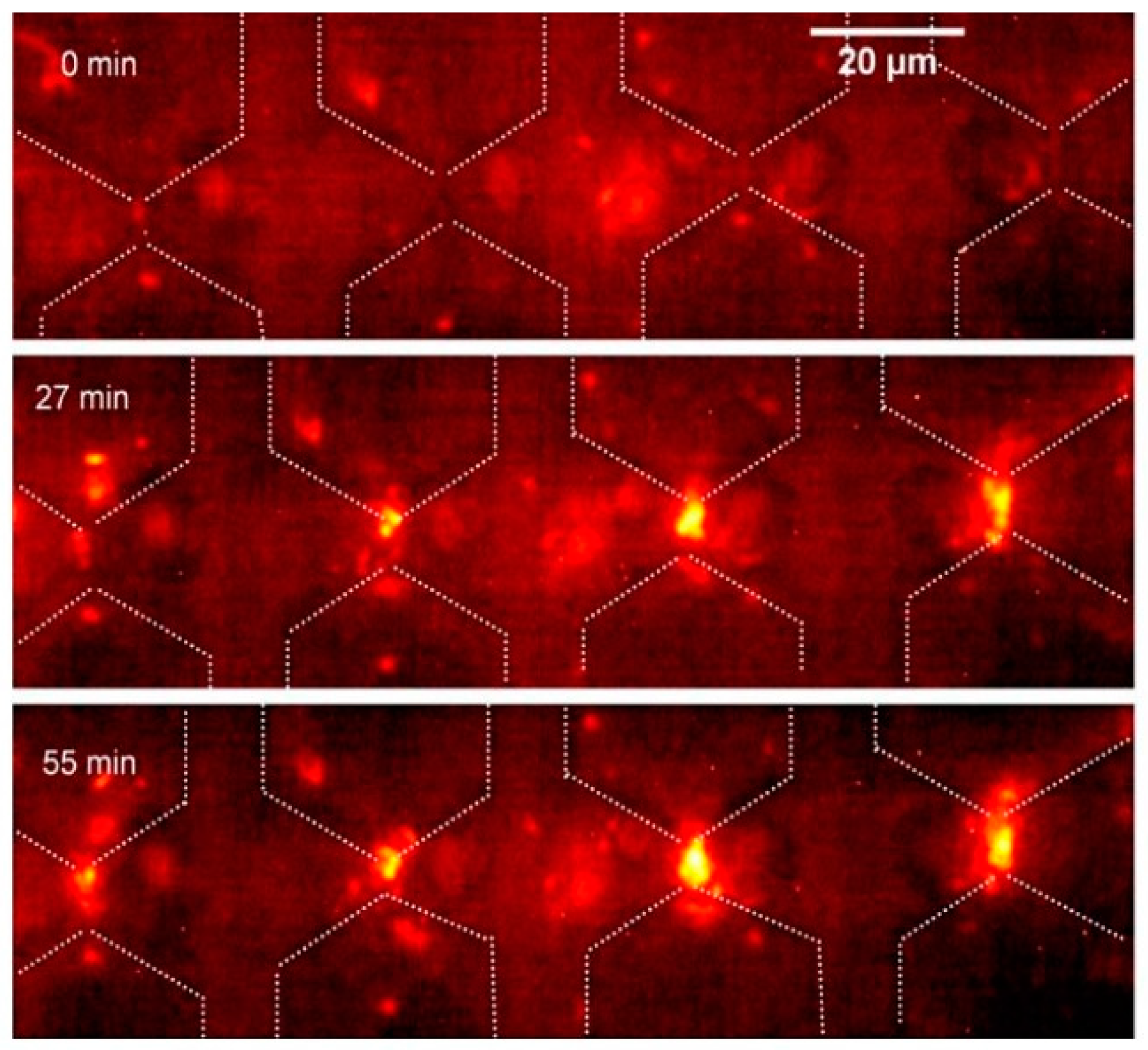


| Name | Size | Type | Capsulation | Trapping | Electrodes |
|---|---|---|---|---|---|
| Tobacco Mosaic | 280 nm | RNA virus | Non-enveloped | pDEP | Sawtooth (6 µm) [13] |
| Herpes simplex | 240 nm | DNA virus | Enveloped | pDEP | Quadrupole (6 µm) [15] |
| Vaccinia | 360 × 270 × 250 nm | DNA virus | Enveloped | pDEP | Interdigitated (10 µm) [16] |
| Influenza | 90 nm | RNA virus | Enveloped | nDEP | Quadrupole, Interdigitated (6 µm), (40 µm) [20] |
| Hepatitis A | 27 nm | RNA virus | Non-enveloped | nDEP/pDEP | Quadrupole Octrupole (2 µm) [24] |
| Cowpea Mosaic | 30 nm | RNA virus | Non-enveloped | pDEP | Castellated (2 µm) [21] |
| Adeno | 90 nm | DNA virus | Non-enveloped | iDEP | Castellated/Interdigitated (10 µm) [22] |
| Sindbis | 130 nm | RNA virus | Enveloped | iDEP | Sawtooth gradient (0–700 V) [26] |
| T4 bacteriophage | 90 nm | DNA virus | Non-enveloped | iDEP | Circular and oval (80 µm) [27] |
| Virus Sampled | Virus Size | Sampling Mechanism | Sampling Time/Sampling Rate | Electrodes Used |
|---|---|---|---|---|
| Adenovirus, Rotavirus | 90 nm, 70 nm | DEPIM (5 Vpp, 100 kHz) | 60 s | Castellated and interdigitated (10 µm) [22] |
| Vaccinia virus | 360 × 270 × 250 nm | DEPIM (8 Vpp, 1 kHz) | 54 s 0.401 mm/s | Nanoelectrode array [35] |
| HIV, FIV | 100 nm, 100 nm | Dopant concentration | <15 min | Co-axial resonator [36] |
| HIV, FIV, MPMV | 100 nm, 100 nm, 250 nm | Capacitance measurement for electrically polarizable virus | NA | Co-axial resonator [23] |
| SDS micelle, copper nanoparticles | 4 nm, 500 nm | Differential capacitance | 200 s | Spiral electrode (120 µm) [39] |
| Influenza, TMV, Baculovirus | 100 nm, 20 × 300 nm, 30 × 360 nm | DEPIM | Sampling time—a few minutes | Nano-gap electrodes (510 nm) [37] |
| Sample | Excitation | Results | Electrode Design |
|---|---|---|---|
| Escherichia coli | Pulsed excitation | Lysis observed at 3.5 V and 500 µs pulse | Spike electrodes [49] |
| B. pertussis | Pulsed excitation | Lysed with 300 V and 50 µs pulse | Matrix electrodes (15 µm) [51] |
| Leukemia, Red blood cells | DC biased AC excitation | Electrokinetic lysis reported due to forces caused by 145 Vrms and 1 kHz frequency across the microchannel | External electric field across a micro-channel [57] |
| S. thermophilus, Escherichia coli | AC excitation | Thermo-electric lysis reported caused by 240–280 Vrms and 20 kHz | Two electrophoresis electrodes and one lysis electrode [58] |
| Plant protoplast | AC excitation | Lysis observed at 10 Vpp and 10 MHz | Two electrodes across a trapezoid channel [59] |
| Vaccinia Virus | AC excitation | Lysis observed at 20 Vpp and 100 kHz | Spike electrodes [38] |
| Vaccinia virus | AC excitation | Electroporation observed at 8 Vpp and 1 kHz at a reduced flow velocity of 0.05 mm/s with a few particles irreversibly electroporated | Carbon nanoelectrode arrays [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantri, D.; Wymenga, L.; van Turnhout, J.; van Zeijl, H.; Zhang, G. Manipulation, Sampling and Inactivation of the SARS-CoV-2 Virus Using Nonuniform Electric Fields on Micro-Fabricated Platforms: A Review. Micromachines 2023, 14, 345. https://doi.org/10.3390/mi14020345
Mantri D, Wymenga L, van Turnhout J, van Zeijl H, Zhang G. Manipulation, Sampling and Inactivation of the SARS-CoV-2 Virus Using Nonuniform Electric Fields on Micro-Fabricated Platforms: A Review. Micromachines. 2023; 14(2):345. https://doi.org/10.3390/mi14020345
Chicago/Turabian StyleMantri, Devashish, Luutzen Wymenga, Jan van Turnhout, Henk van Zeijl, and Guoqi Zhang. 2023. "Manipulation, Sampling and Inactivation of the SARS-CoV-2 Virus Using Nonuniform Electric Fields on Micro-Fabricated Platforms: A Review" Micromachines 14, no. 2: 345. https://doi.org/10.3390/mi14020345
APA StyleMantri, D., Wymenga, L., van Turnhout, J., van Zeijl, H., & Zhang, G. (2023). Manipulation, Sampling and Inactivation of the SARS-CoV-2 Virus Using Nonuniform Electric Fields on Micro-Fabricated Platforms: A Review. Micromachines, 14(2), 345. https://doi.org/10.3390/mi14020345






