Low-Cost Optical pH Sensor with a Polyaniline (PANI)-Sensitive Layer Based on Commercial Off-the-Shelf (COTS) Components
Abstract
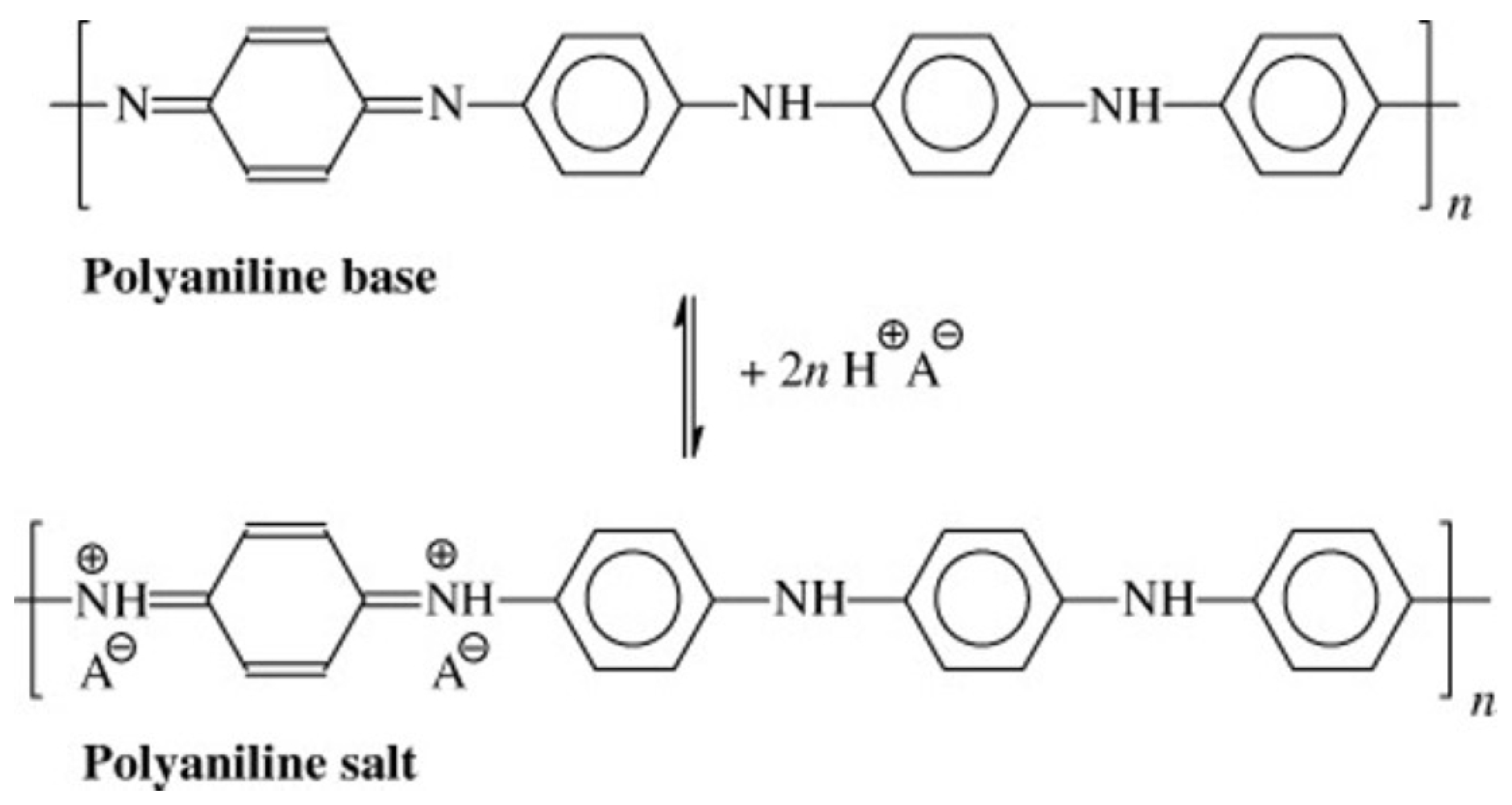
:1. Introduction
2. Materials and Methods
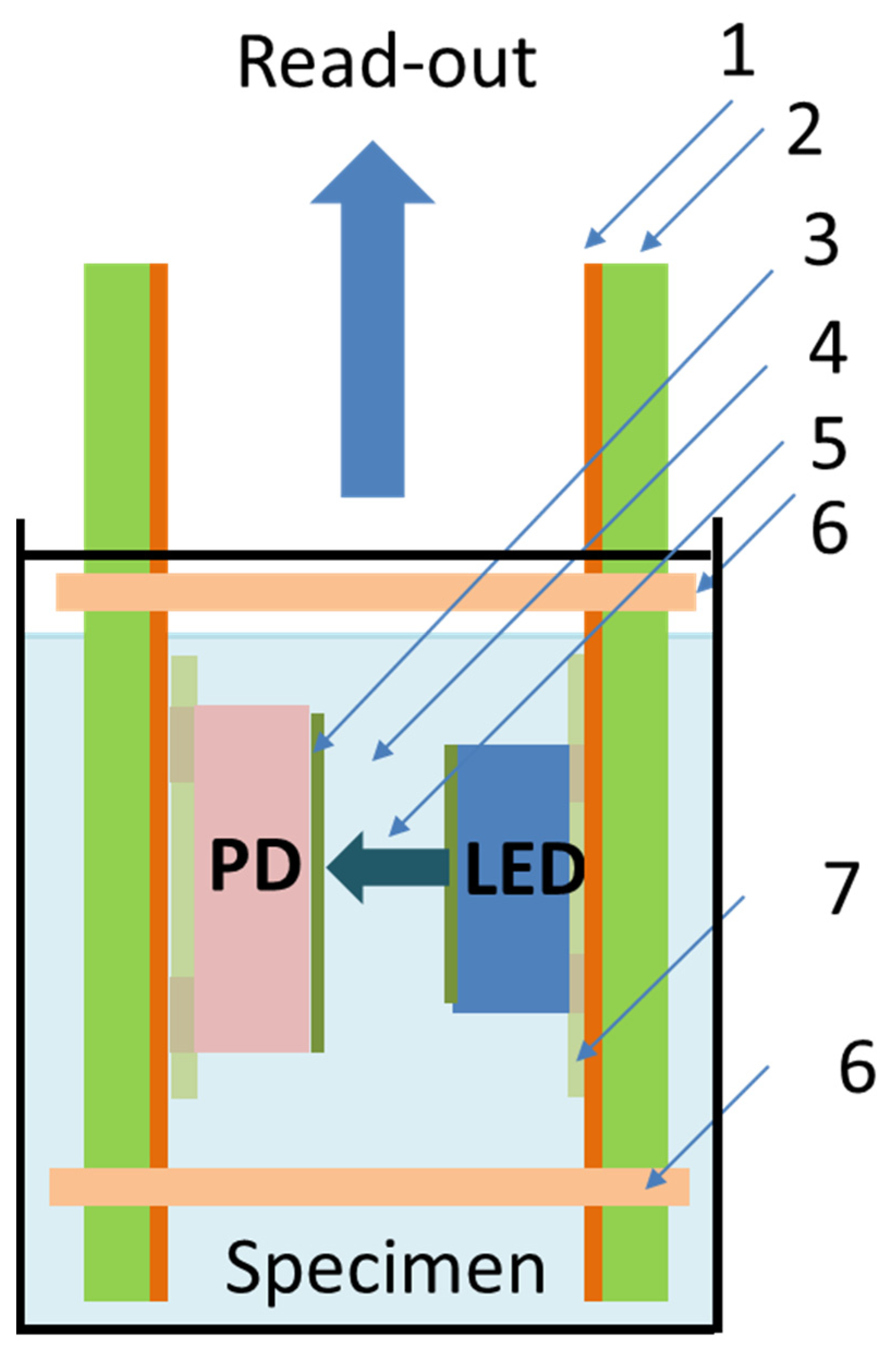
2.1. Concept and Architecture of the Optical pH Sensor
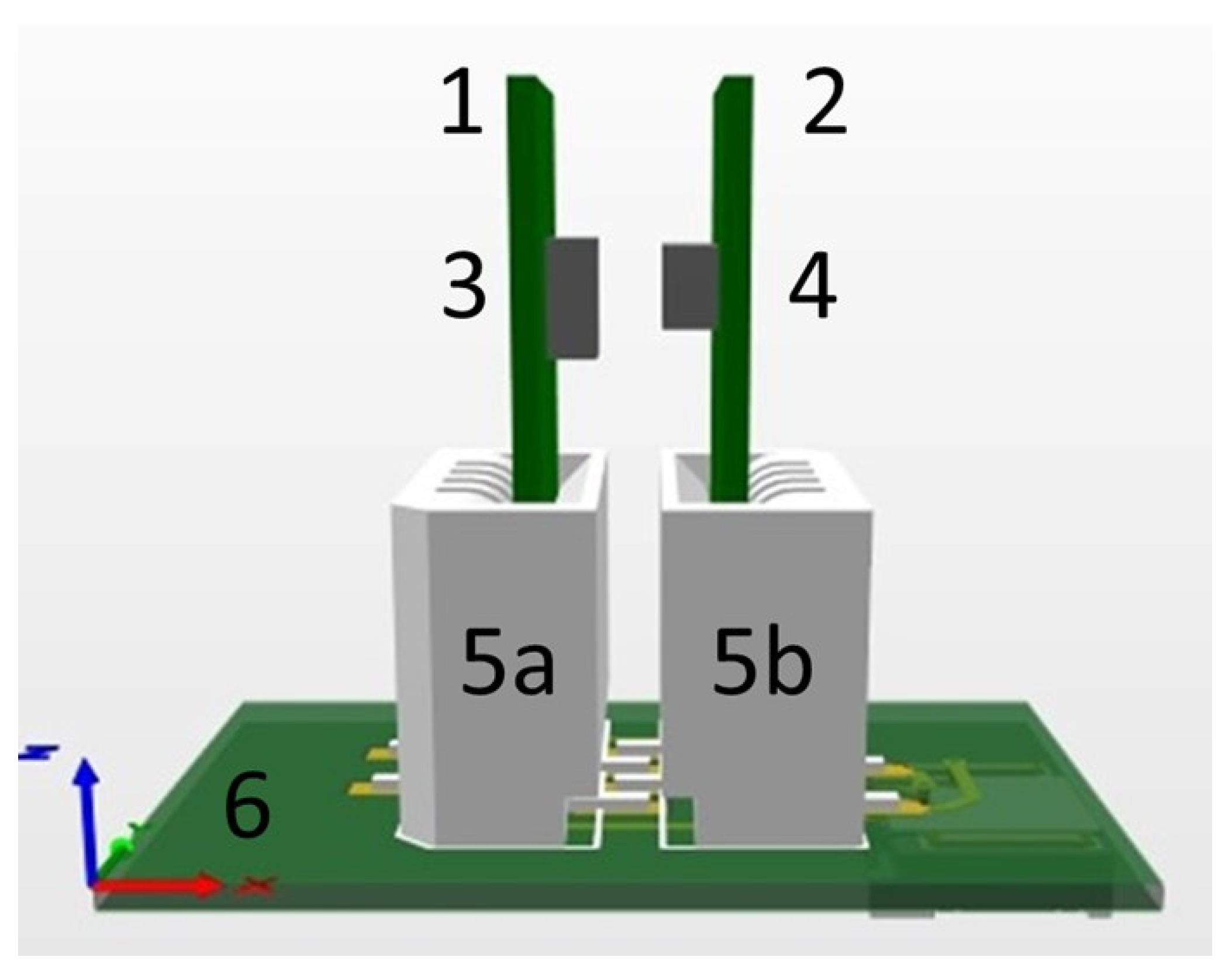
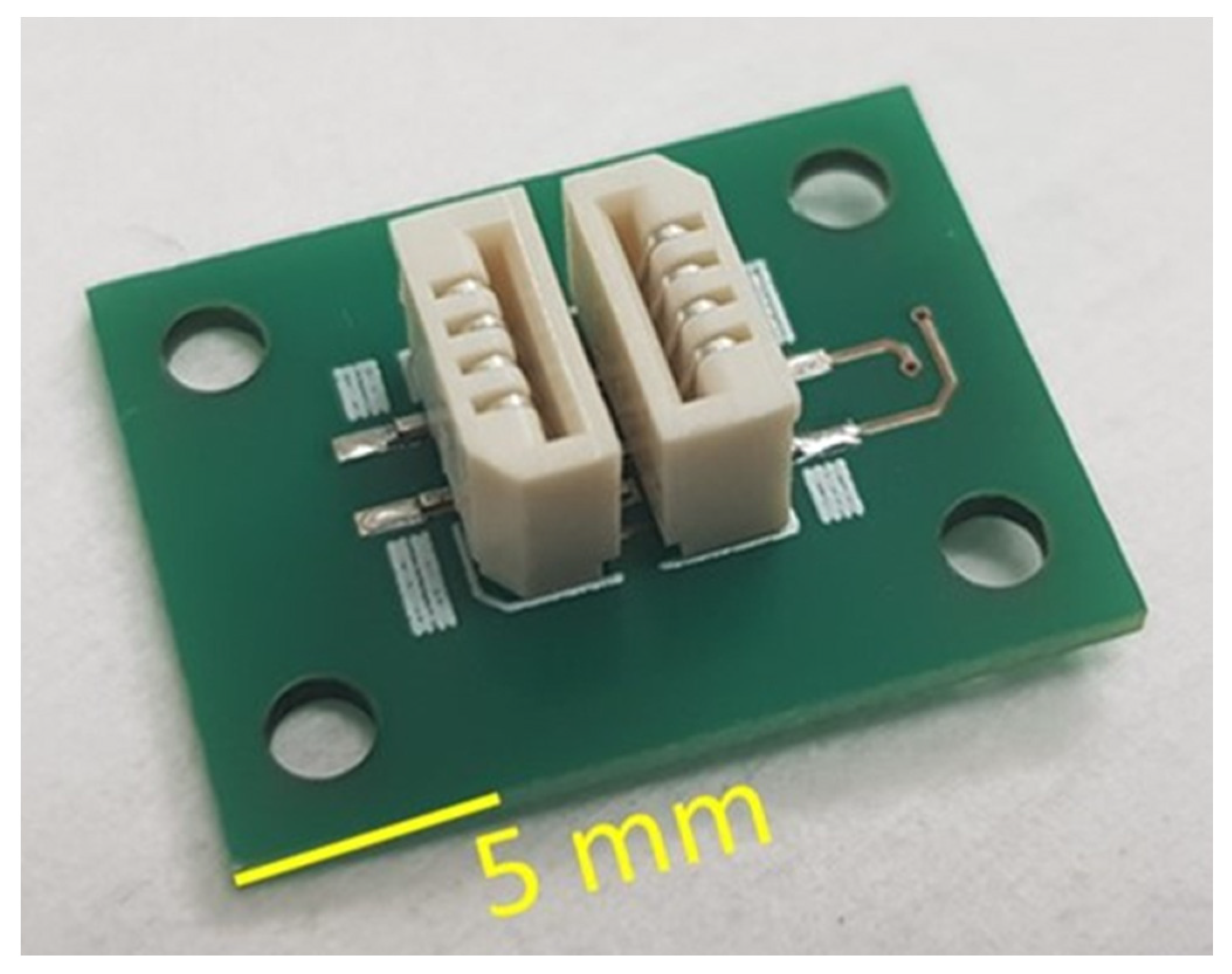
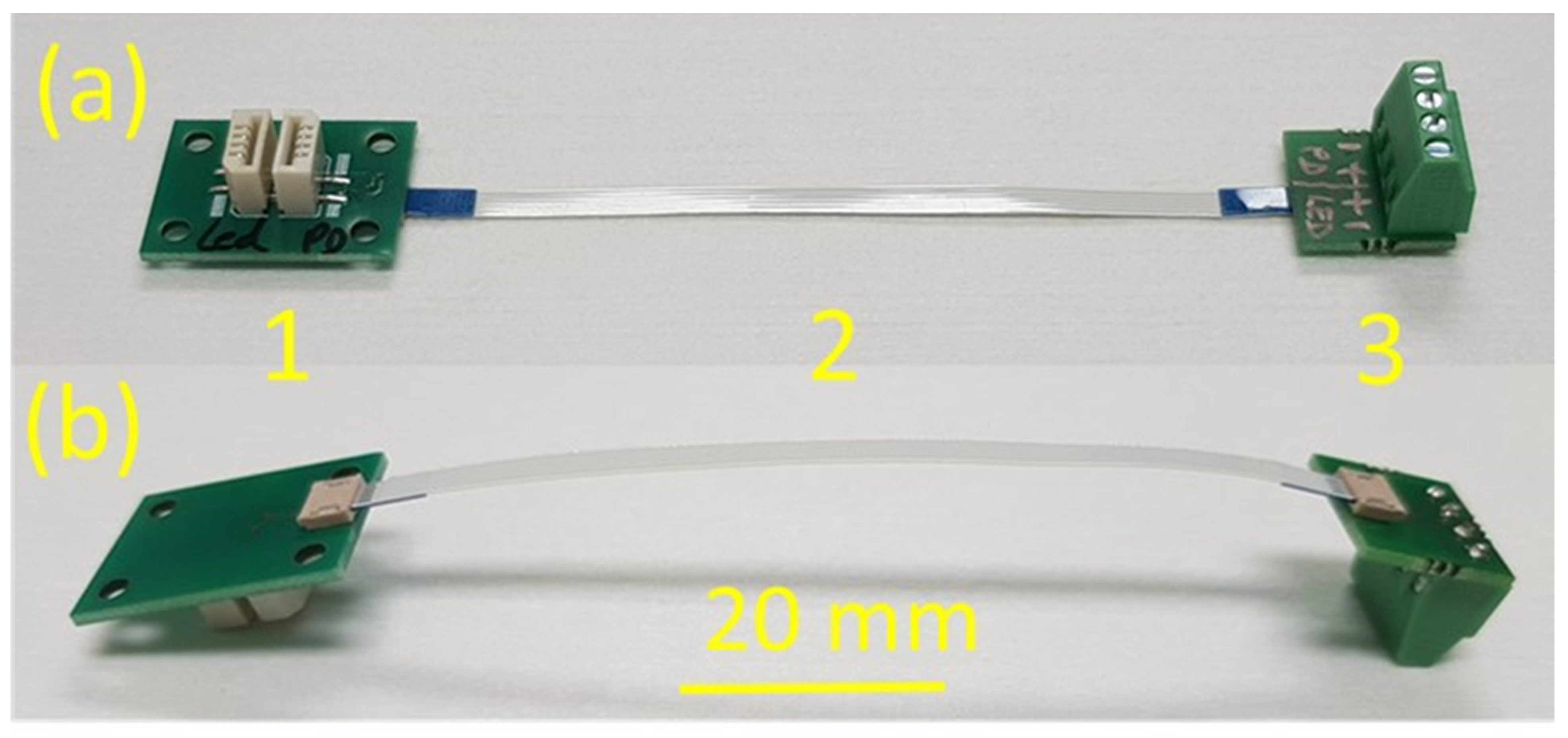
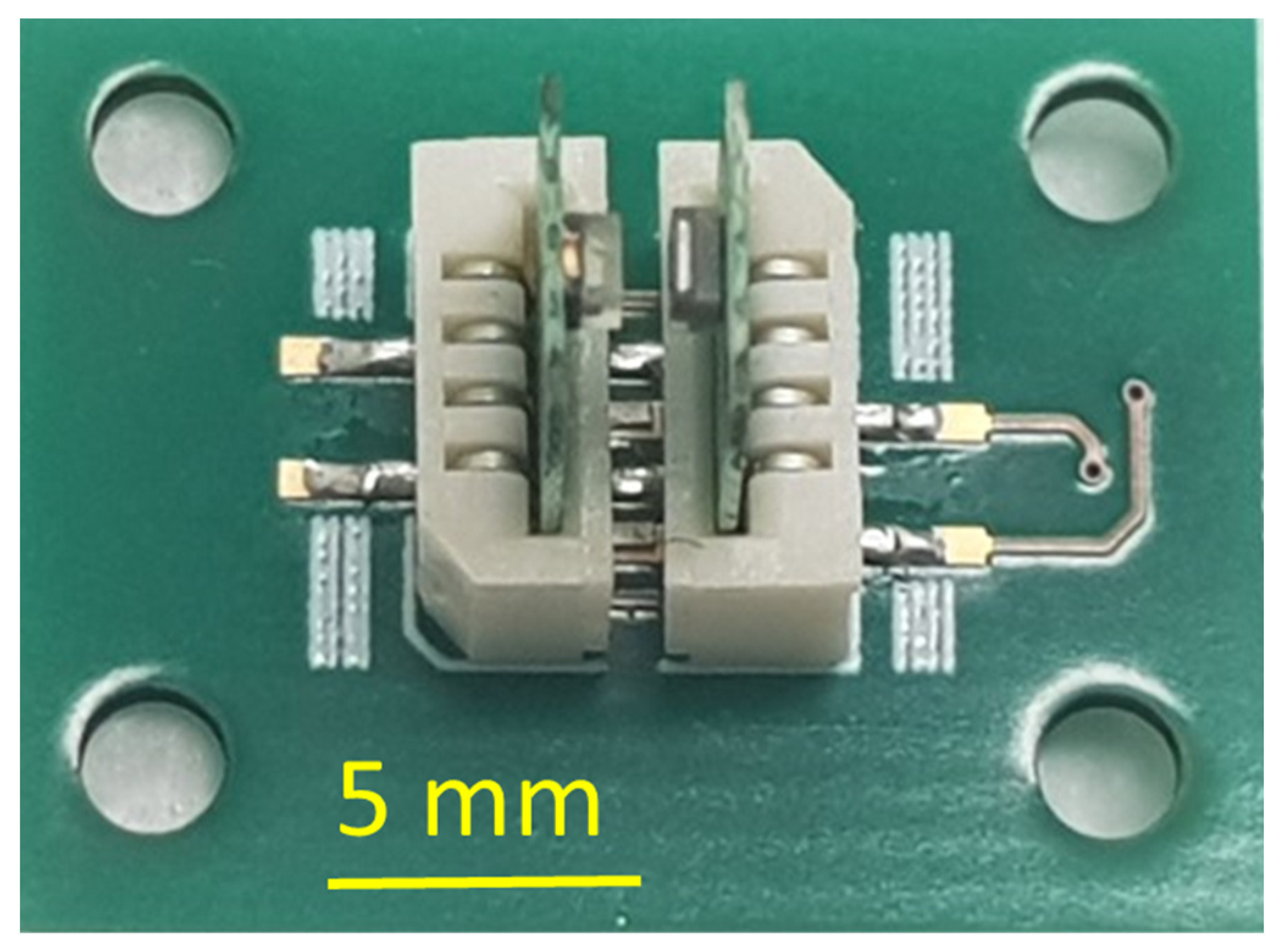
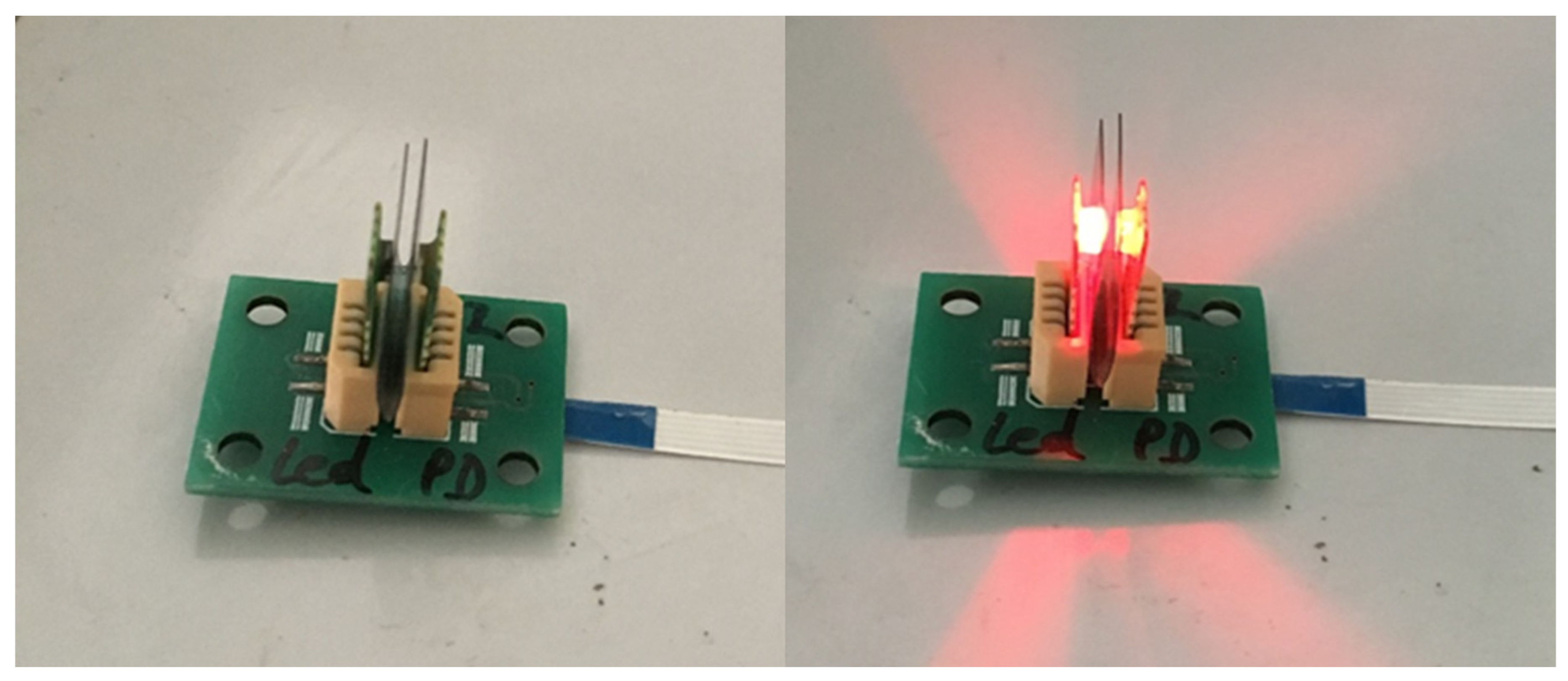
2.2. Assembly of the pH Sensor
3. Results
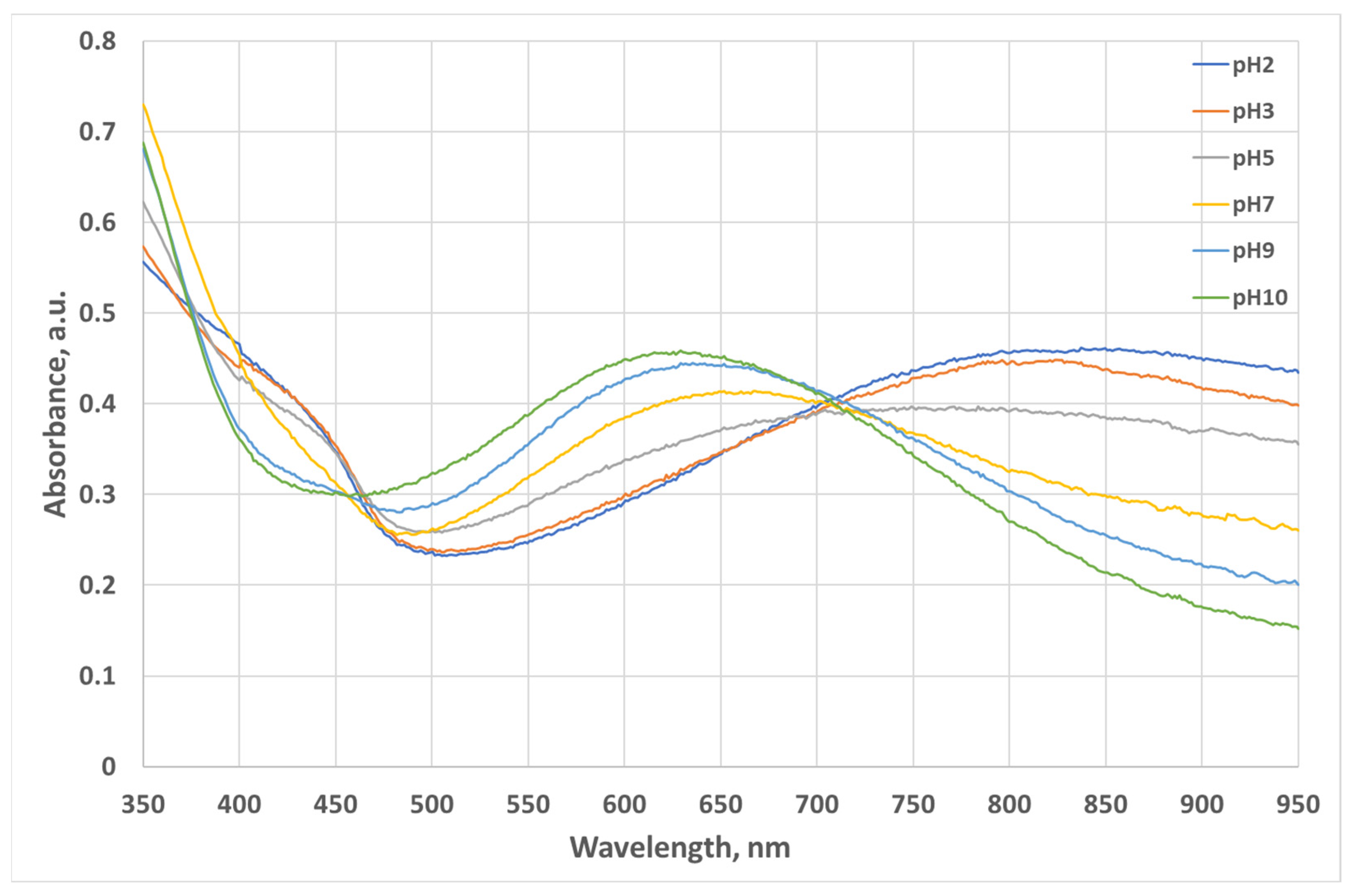
3.1. Optics Characterization
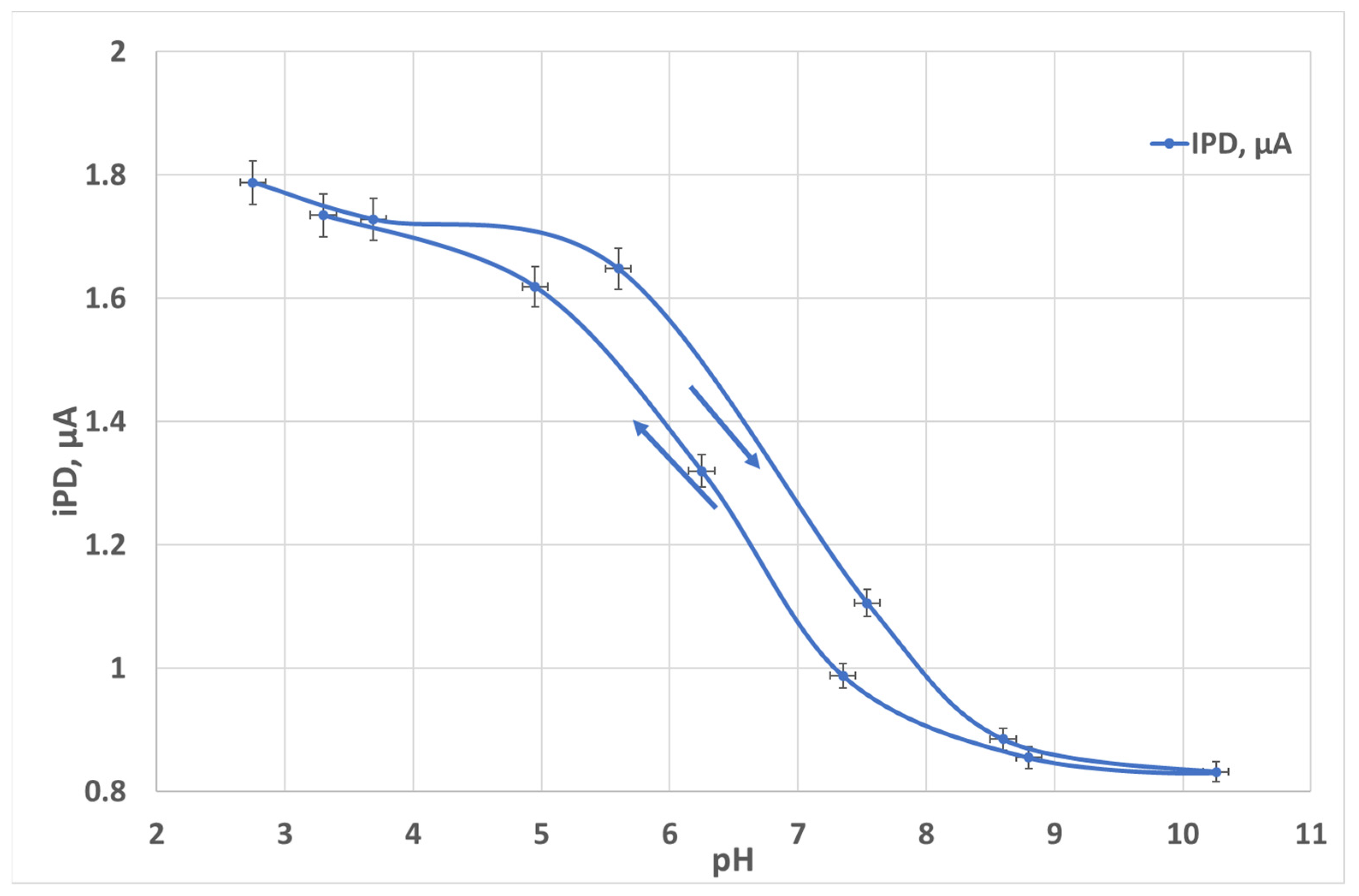
3.2. pH Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webster, D. pH—Principles and Measurement. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2003; pp. 4501–4507. [Google Scholar] [CrossRef]
- Buck, R.O.; Rondinini, S.; Covington, A.K.; Baucke, F.G.K.; Brett, C.M.A.; Camoes, M.F.; Milton, M.J.T.; Mussini, T.; Naumann, R.; Pratt, K.W.; et al. The measurement of pH: Definition, standards and procedures (IUPAC Recommendations 2002). Pure Appl. Chem. 2002, 74, 2169–2200. [Google Scholar] [CrossRef]
- Karastogianni, S.; Girousi, S.; Sotiropoulos, S. pH: Principles and Measurement. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2016; pp. 333–338. [Google Scholar] [CrossRef]
- Spitzer, P. Traceable measurements of pH. Accredit. Qual. Assur. 2001, 6, 55–60. [Google Scholar] [CrossRef]
- ISO 23496:2019; Determination of pH Value—Reference Buffer Solutions for the Calibration of pH Measuring Equipment. International Organization for Standardization (ISO): Geneva, Switzerland. Available online: https://www.iso.org/obp/ui/#iso:std:iso:23496:ed-1:v1:en (accessed on 16 November 2023).
- Stoukatch, S.; Dupont, F.; Redouté, J.-M. Device Processing Challenges for Miniaturized Sensing Systems Targeting Biological Fluids. Biomed. Mater. Devices Springer Nat. 2022, 1–17. [Google Scholar] [CrossRef]
- Dutta, S.; Sarma, D.; Patel, A.; Nath, P. Dye-Assisted pH Sensing Using a Smartphone. IEEE Photon-Technol. Lett. 2015, 27, 2363–2366. [Google Scholar] [CrossRef]
- Dupont, F.; Stoukatch, S.; Laurent, P.; Eersels, K.; van Grinsven, B.; Redouté, J.-M. Design Aspects for Portable LED-Based Colorimetric Characterization Systems Targeting Liquid Analytes, Optics and Lasers in Engineering; Elsevier: Amsterdam, The Netherlands, 2023; (submitted). [Google Scholar]
- Hamilton Company. Process Analytics. Available online: https://www.hamiltoncompany.com/process-analytics/ph-and-orp-knowledge/comparing-glass-membrane-versus-optical-ph-sensors#optical-p-h (accessed on 16 November 2023).
- Pyro Science Sensors. Available online: https://www.pyroscience.com/en/products/all-sensors/attributes/pH?gclid=CjwKCAjw_YShBhAiEiwAMomsEAHQmUtJJfRGGVIC9DGq_uSruIqpuAk8efnfWAc53-vOD9WTPOjeEBoCTckQAvD_BwE (accessed on 16 November 2023).
- Mahinnezhad, S.; Izquierdo, R.; Shih, A. Fully Printed pH Sensor based on Polyaniline/Graphite Nanocomposites. J. Electrochem. Soc. 2023, 170, 2. [Google Scholar] [CrossRef]
- Perrin, F.-X.; Oueiny, C. Chapter 5, Polyaniline-Based Thermoplastic Blends. In Polyaniline Blends, Composites, and Nanocomposites; Visakh, P.M., Pina, C.D., Falletta, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 117–147. [Google Scholar] [CrossRef]
- Abu-Thabit, N.; Umar, Y.; Ratemi, E.; Ahmad, A.; Ahmad Abuilaiwi, F. A Flexible Optical pH Sensor Based on Polysulfone Membranes Coated with pH-Responsive Polyaniline Nanofibers. Sensors 2016, 16, 986. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wang, Y.; Li, X.; Jiang, X.; Gao, P.; Sun, K.; Zhou, J.; Zhang, Z.; Jiang, Q. An Optical Sensor with Polyaniline-Gold Hybrid Nanostructures for Monitoring pH in Saliva. Nanomaterials 2017, 7, 67. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2020, 63, 1–22. [Google Scholar] [CrossRef]
- Aldaba, A.L.; González-Vila, Á.; Debliquy, M.; Lopez-Amo, M.; Caucheteur, C.; Lahem, D. Polyaniline deposition on tilted fiber Bragg grating for pH sensing. In Proceedings of the 2017, 25th Optical Fiber Sensors Conference (OFS), Jeju, Republic of Korea, 24–28 April 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Blinova, N.V.; Stejskal, J.; Trchová, M.; Prokeš, J. Control of polyaniline conductivity and contact angles by partial protonation. Polym. Int. 2008, 57, 66–69. [Google Scholar] [CrossRef]
- LAMBDA 365 UV/Vis Spectrophotometer. Available online: https://www.perkinelmer.com/product/lambda-365-spectrophotometer-uv-express-n4100020 (accessed on 16 November 2023).
- Stoukatch, S.; Fagnard, J.-F.; Dupont, F.; Laurent, P.; Debliquy, M.; Redouté, J.-M. Low Thermal Conductivity Adhesive as a Key Enabler for Compact, Low-Cost Packaging for Metal-Oxide Gas Sensors. IEEE Access 2022, 10, 19242–19253. [Google Scholar] [CrossRef]
- Molex 4 Way Straight Female FPC Connector. Available online: https://nl.rs-online.com/web/p/fpc-connectors/6706892 (accessed on 16 November 2023).
- Aldaba, A.L.; González-Vila, Á.; Debliquy, M.; Lopez-Amo, M.; Caucheteur, C.; Lahem, D. Polyaniline-coated tilted fiber Bragg gratings for pH sensing. Sens. Actuators B Chem. 2018, 254, 1087–1093. [Google Scholar] [CrossRef]
- Overview of Materials, Polyethylene Terephthalate (PET), Unreinforced. Available online: https://www.matweb.com/search/datasheet.aspx?MatGUID=a696bdcdff6f41dd98f8eec3599eaa20&ckck=1 (accessed on 27 October 2023).
- Szczurek, A.; Tran, T.N.L.; Kubacki, J.; Gąsiorek, A.; Startek, K.; Mazur-Nowacka, A.; Dell’Anna, R.; Armellini, C.; Varas, S.; Carlotto, A.; et al. Polyethylene terephthalate (PET) optical properties deterioration induced by temperature and protective effect of organically modified SiO2–TiO2 coating. Mater. Chem. Phys. 2023, 306, 128016. [Google Scholar] [CrossRef]
- Steinegger, A.; Wolfbeis, O.S.; Borisov, S.M. Optical Sensing and Imaging of pH Values: Spectroscopies, Materials, and Applications. Chem. Rev. 2020, 120, 12357–12489. [Google Scholar] [CrossRef] [PubMed]
- Light Sources. Available online: https://www.oceaninsight.com/products/light-sources/ (accessed on 16 November 2023).
- Analytics and Sample Preparation, Spectroquant Prove. Available online: https://www.merckmillipore.com/BE/en/analytics-and-sample-preparation/spectroquant-prove/nQib.qB.49QAAAFNP.EtMC17,na (accessed on 16 November 2023).
- UV-Vis-NIR, Spectrophotometers Comparison. Available online: https://www.ssi.shimadzu.com/products/molecular-spectroscopy/uv-vis/comparison/index.html (accessed on 16 October 2023).
- Mini-Spectrometers. Available online: https://www.hamamatsu.com/eu/en/product/optical-sensors/spectrometers/mini-spectrometer.html (accessed on 16 November 2023).
- LEDs. Available online: https://www.hamamatsu.com/eu/en/product/light-and-radiation-sources/led.html (accessed on 16 November 2023).
- LUXEON Rubix. Available online: https://lumileds.com/wp-content/uploads/2023/04/DS309-luxeon-rubix-datasheet.pdf (accessed on 27 October 2023).
- Photodiode. Available online: https://www.vishay.com/docs/81951/temd7000.pdf (accessed on 27 October 2023).
- KOKI Company Ltd. Providing Soldering Materials and Solutions Worldwide, Search Products, Solder Paste, S3X70-M500D. Available online: https://www.ko-ki.co.jp/en/products/detail/47/ (accessed on 16 November 2023).
- ASYMTEK Line, Conformal Coating and Fluid Dispensing, Nordson ASYMPTEK Spectrum S-820. Available online: https://www.nordson.com/en/divisions/electronics-solutions/about-electronics-solutions/nordson-asymtek-line (accessed on 16 November 2023).
- SMD—Pick and Place Machine: Autotronik SMT Pick & Place BS384V1. Available online: https://www.autotronik-smt.de/en/products/smd-bestueckungsautomat (accessed on 27 October 2023).
- LPKF, ProtoFlow S—Lead-Free Reflow Oven. Available online: https://www.lpkf.com/en/industries-technologies/research-in-house-pcb-prototyping/products/lpkf-protoflow-s4 (accessed on 16 November 2023).
- JEDEC, Global Standards for the Microelectronics Industry. Available online: https://www.jedec.org/sites/default/files/docs/jstd020d-01.pdf (accessed on 27 October 2023).
- Underfill Material. Available online: www.henkel-adhesives.com/be/en/product/underfills (accessed on 16 November 2023).
- Stetsiv, Y.; Yatsyshyn, M.; Nykypanchuk, D.; Korniy, S.; Saldan, I.; Reshetnyak, O.; Bednarchuk, T. Characterization of polyaniline thin films prepared on polyethylene terephthalate substrate. Polym. Bull. 2021, 78, 6251–6265. [Google Scholar] [CrossRef]
- Castrellon-Uribe, J.; Nicho, M.E.; Reyes-Merino, G. Remote optical detection of low concentrations of aqueous ammonia employing conductive polymers of polyaniline. Sens. Actuators B Chem. 2009, 141, 40–44. [Google Scholar] [CrossRef]
- Qin, T.; Deng, L.; Zhang, P.; Tang, M.; Li, C.; Xie, H.; Huang, S.; Gao, X. Enhancement of Electrochromic Properties of Polyaniline Induced by Copper Ions. Nanoscale Res. Lett. 2022, 17, 51. [Google Scholar] [CrossRef]
- Park, H.J.; Yoon, J.H.; Lee, K.G.; Choi, B.J. Potentiometric performance of flexible pH sensor based on polyaniline nanofiber arrays. Nano Converg. 2019, 6, 9. [Google Scholar] [CrossRef]
- Ball, D.W. Field Guide to Spectroscopy; SPIE-Intl Soc Optical Eng: Bellingham, WA, USA, 2006. [Google Scholar]
- Combined pH Glass Electrode. Available online: https://www.metrohm.com/en_be/products/6/0233/60233100.html (accessed on 16 November 2023).
- Debarshi, S.; Khan, M.M. Portable and low-cost LED based Spectrophotometer for the Detection of Nitrite in simulated-Urine. In Proceedings of the 2019 International Conference on Electrical, Electronics and Computer Engineering (UPCON), Aligarh, India, 8–10 November 2019; pp. 1–4. [Google Scholar] [CrossRef]
- De Oliveira, H.J.S.; de Almeida, P.L., Jr.; Sampaio, B.A.; Fernandes, J.P.A.; Pessoa-Neto, O.D.; de Lima, E.A.; de Almeida, L.F. A handheld smartphone-controlled spectrophotometer based on hue to wavelength conversion for molecular absorption and emission measurements. Sens. Actuators B Chem. 2017, 238, 1084–1091. [Google Scholar] [CrossRef]
- Stoukatch, S. Chapter 2. Low-Temperature Microassembly Methods and Integration Techniques for Biomedical Applications. In Book Wireless Medical Systems and Algorithms; Design and Applications; Salvo, P., Hernandez-Silveira, M.l., Eds.; CRC Press: Boca Raton, FL, USA; Press Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 21–42. ISBN 978-1-4987-0076-4. [Google Scholar]
- Stoukatch, S.; Francis, L.A.; Dupont, F.; Kraft, M. Low-cost microfluidic device micromachining and sequential integration with SAW sensor intended for biomedical applications. Sens. Actuators A Phys. 2021, 319, 112526. [Google Scholar] [CrossRef]
- Vivaldi, F.; Santalucia, D.; Poma, N.; Bonini, A.; Salvo, P.; Del Noce, L.; Melai, B.; Kirchhain, A.; Kolivoška, V.; Sokolová, R.; et al. A voltammetric pH sensor for food and biological matrices. Sens. Actuators B Chem. 2020, 322, 128650. [Google Scholar] [CrossRef]
- Poma, N.; Vivaldi, F.; Bonini, A.; Carbonaro, N.; Di Rienzo, F.; Melai, B.; Kirchhain, A.; Salvo, P.; Tognetti, A.; Di Francesco, F. Remote monitoring of seawater temperature and pH by low cost sensors. Microchem. J. 2019, 148, 248–252. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoukatch, S.; Debliquy, M.; Dupont, F.; Redouté, J.-M. Low-Cost Optical pH Sensor with a Polyaniline (PANI)-Sensitive Layer Based on Commercial Off-the-Shelf (COTS) Components. Micromachines 2023, 14, 2197. https://doi.org/10.3390/mi14122197
Stoukatch S, Debliquy M, Dupont F, Redouté J-M. Low-Cost Optical pH Sensor with a Polyaniline (PANI)-Sensitive Layer Based on Commercial Off-the-Shelf (COTS) Components. Micromachines. 2023; 14(12):2197. https://doi.org/10.3390/mi14122197
Chicago/Turabian StyleStoukatch, Serguei, Marc Debliquy, Francois Dupont, and Jean-Michel Redouté. 2023. "Low-Cost Optical pH Sensor with a Polyaniline (PANI)-Sensitive Layer Based on Commercial Off-the-Shelf (COTS) Components" Micromachines 14, no. 12: 2197. https://doi.org/10.3390/mi14122197
APA StyleStoukatch, S., Debliquy, M., Dupont, F., & Redouté, J.-M. (2023). Low-Cost Optical pH Sensor with a Polyaniline (PANI)-Sensitive Layer Based on Commercial Off-the-Shelf (COTS) Components. Micromachines, 14(12), 2197. https://doi.org/10.3390/mi14122197





