Microfluidic Blood Separation: Key Technologies and Critical Figures of Merit
Abstract
:1. Introduction

2. Key Device Performance Figures of Merit: Definitions
2.1. Input and Output Concentrations
2.2. Efficiency
2.3. Purity
2.4. Flow Rate and Throughput
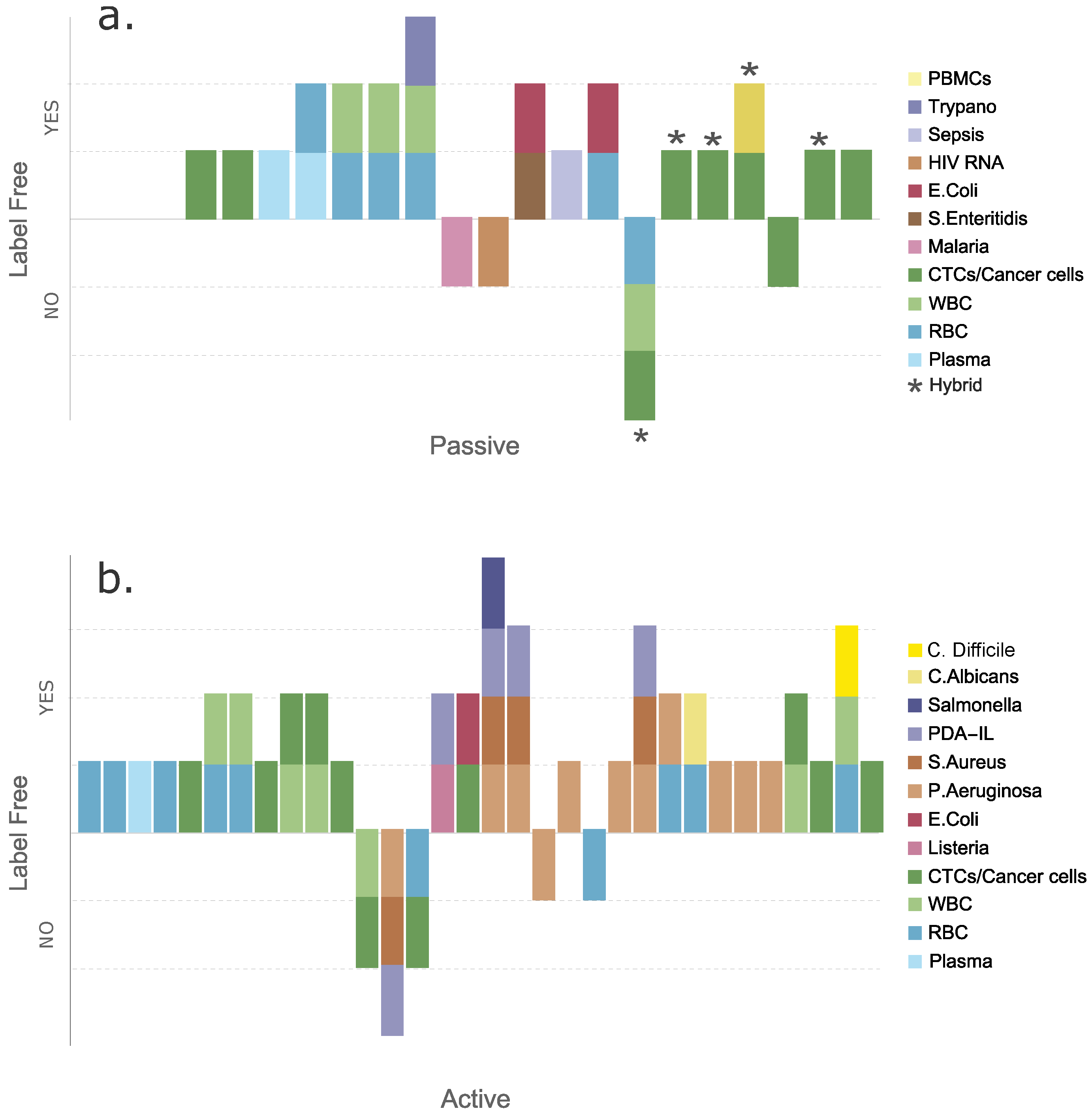
3. Passive Techniques
4. Passive Blood Separations
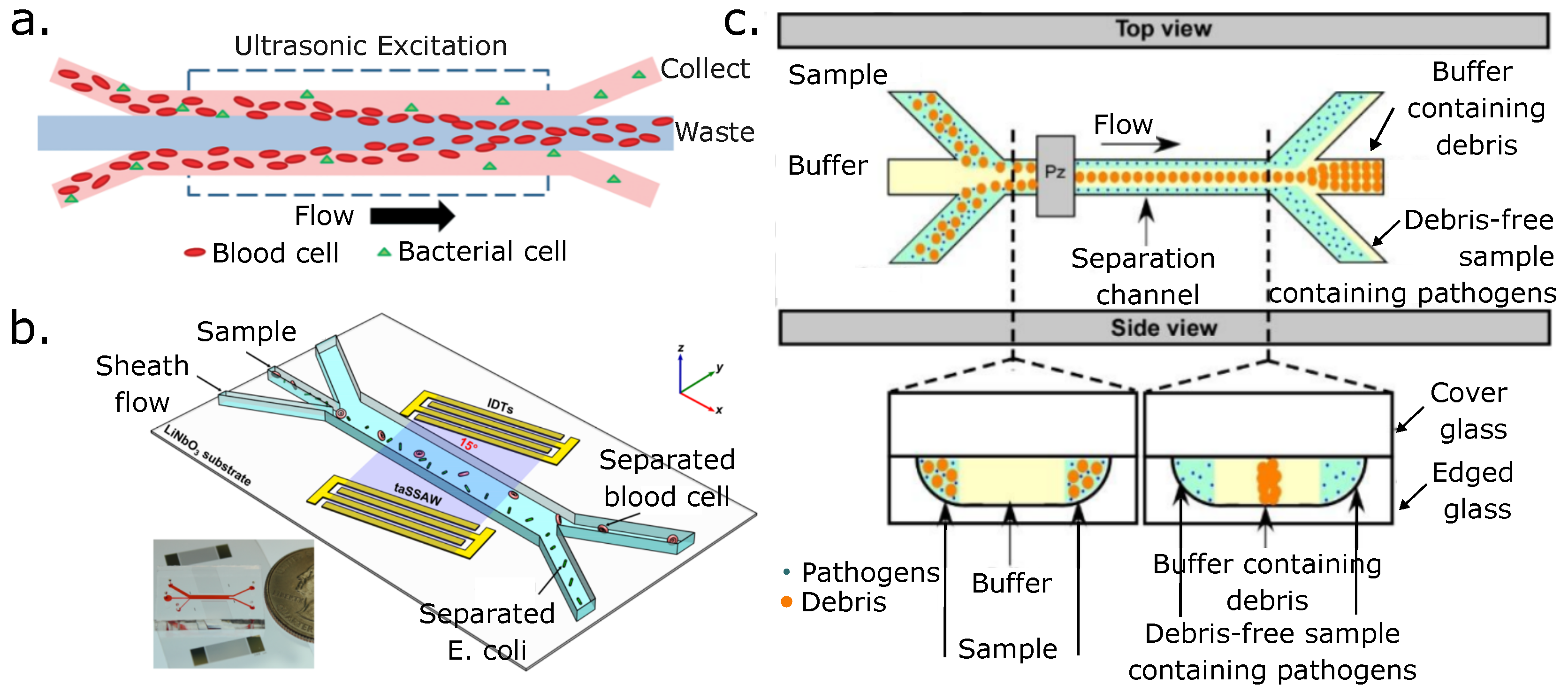
4.1. Passive Separation of Blood Cells
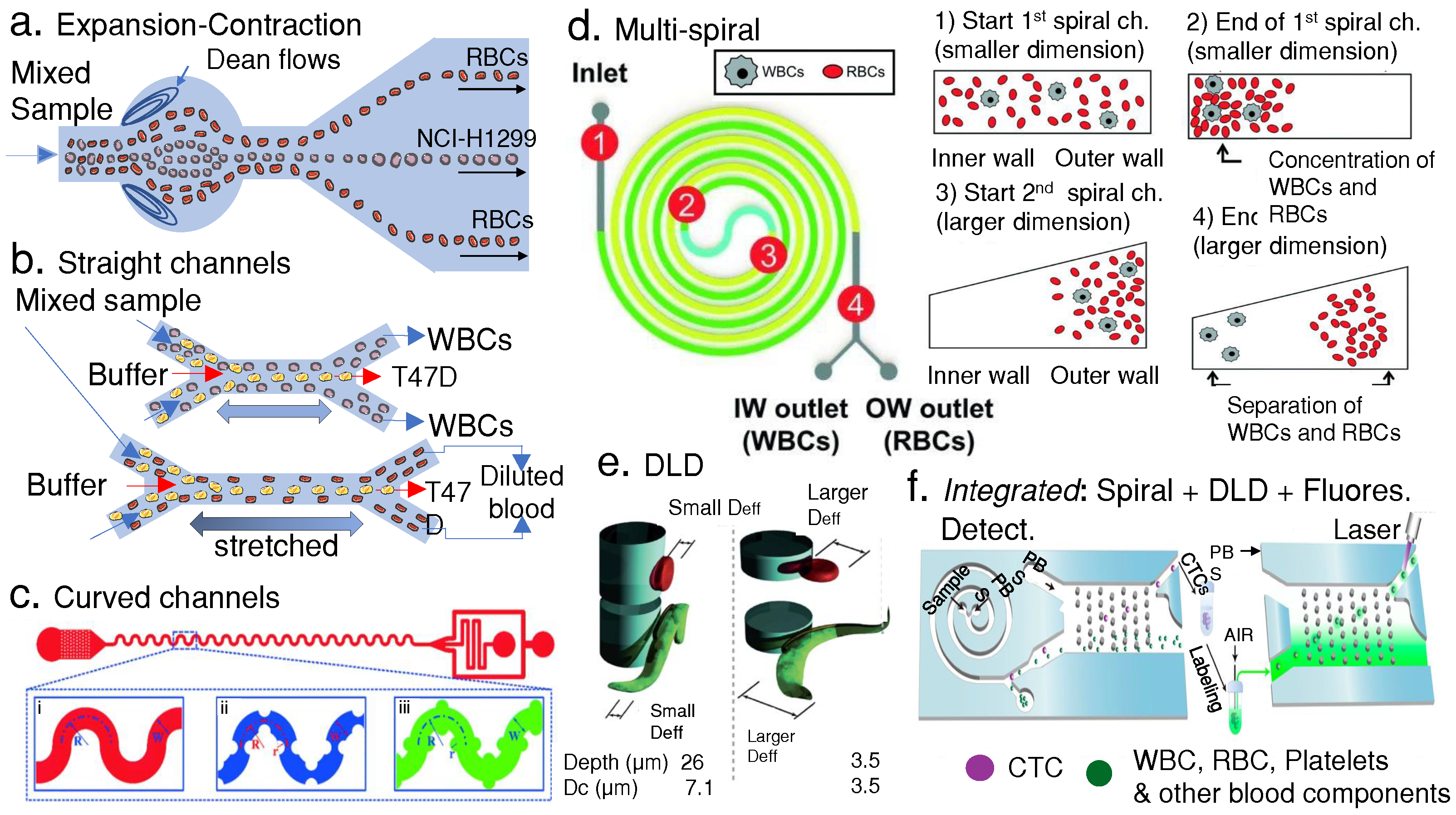
4.2. Passive Separation of Blood Pathogens
5. General Considerations and Parameters for Active Separations
Contrast Factor
6. Active Techniques
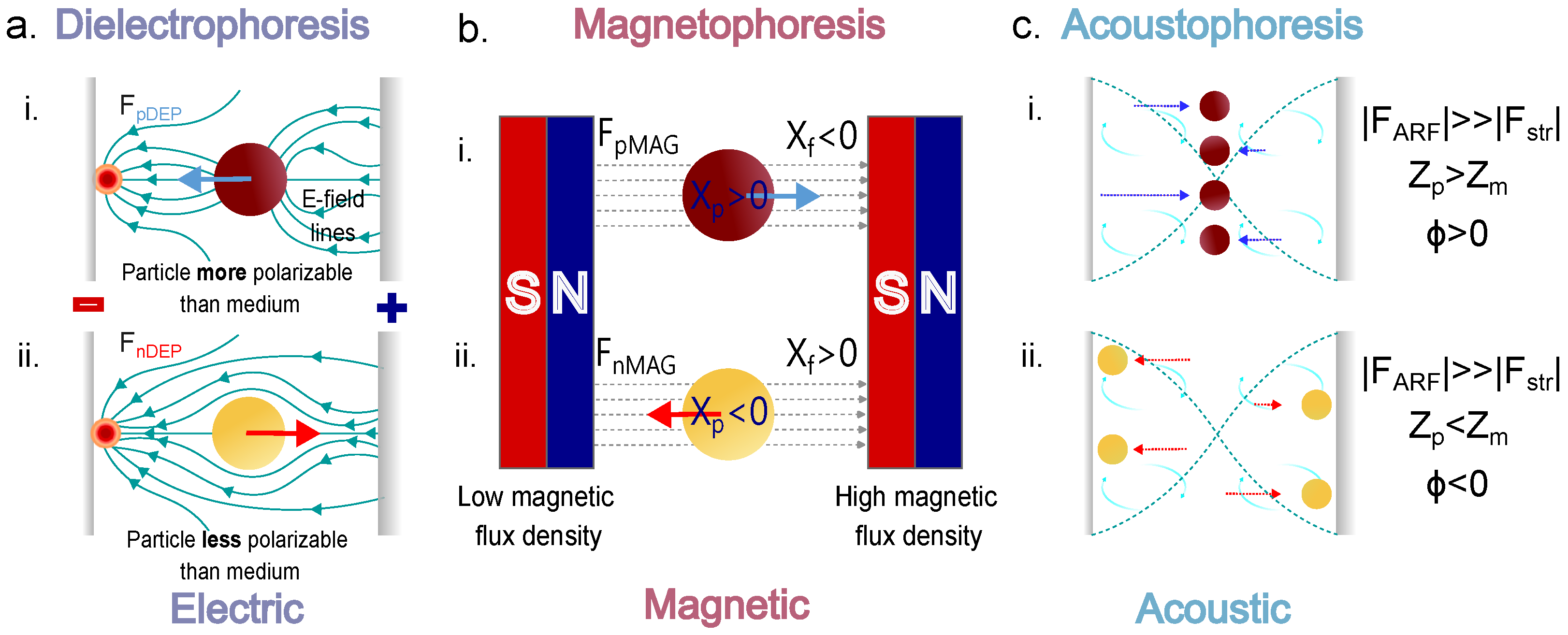
6.1. Electric Forces: Dielectrophoresis (DEP)
6.2. Magnetic Forces: Magnetophoresis
6.3. Acoustic Forces: Acoustophoresis
6.4. Thermal Forces: Thermophoresis
6.5. Optoelectronic Forces: Optoelectronic Tweezers (OETs)
7. Measurement and Quantification Techniques for Microfluidic Separated Blood Fractions
8. Active Blood Separation Applications
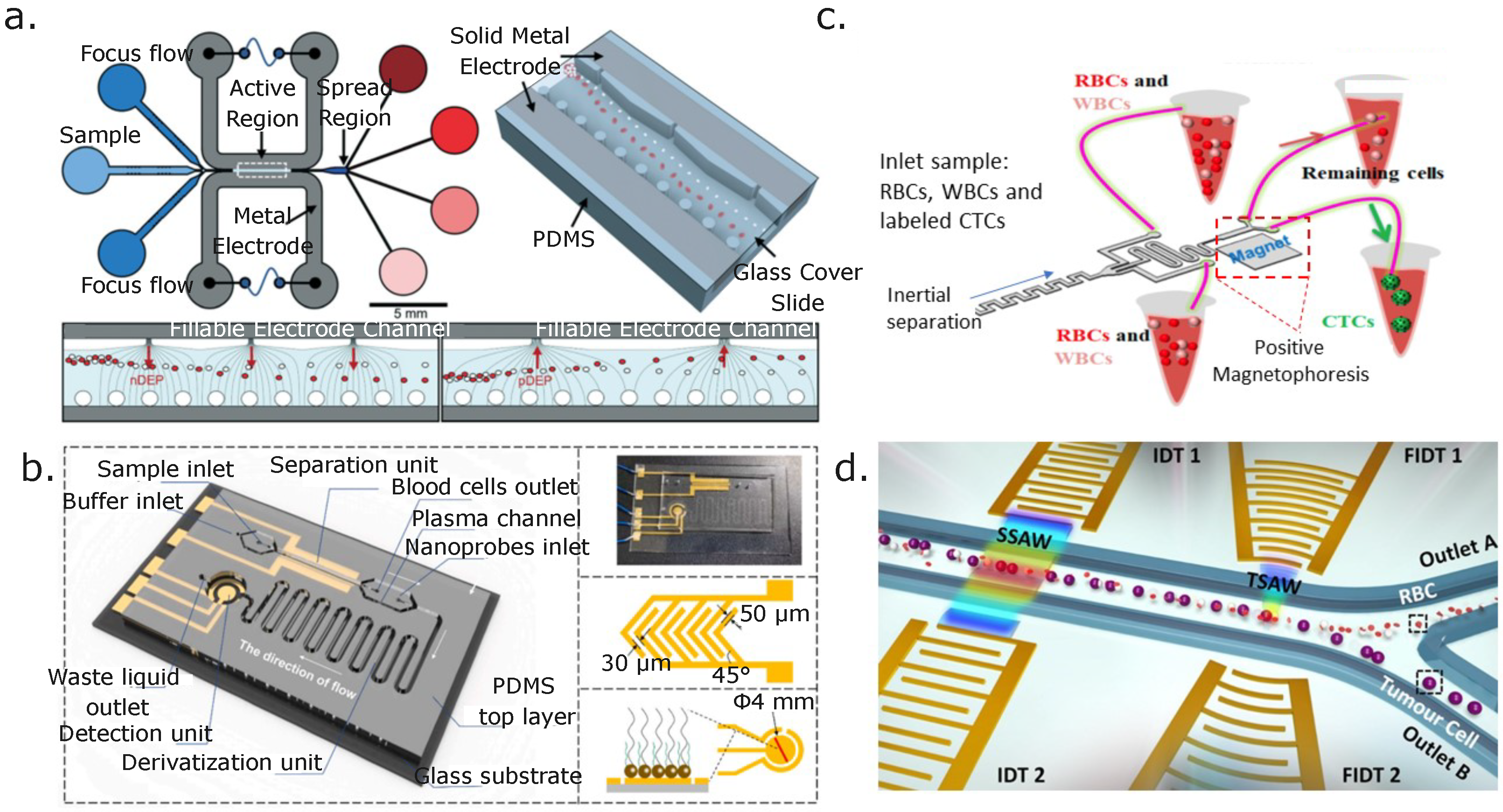
8.1. Active Separation of Blood Cells
8.2. Active Separation of Blood Pathogens
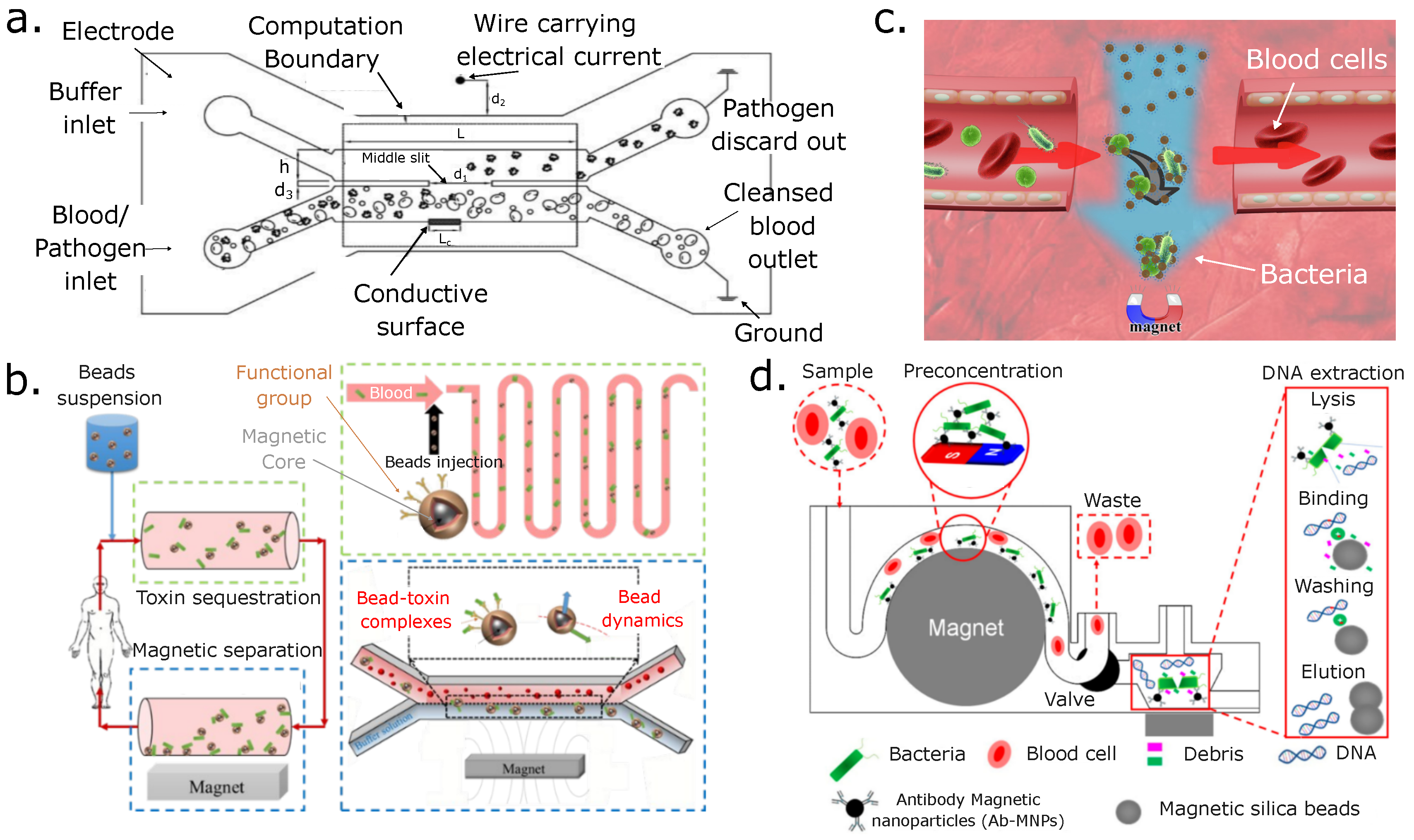
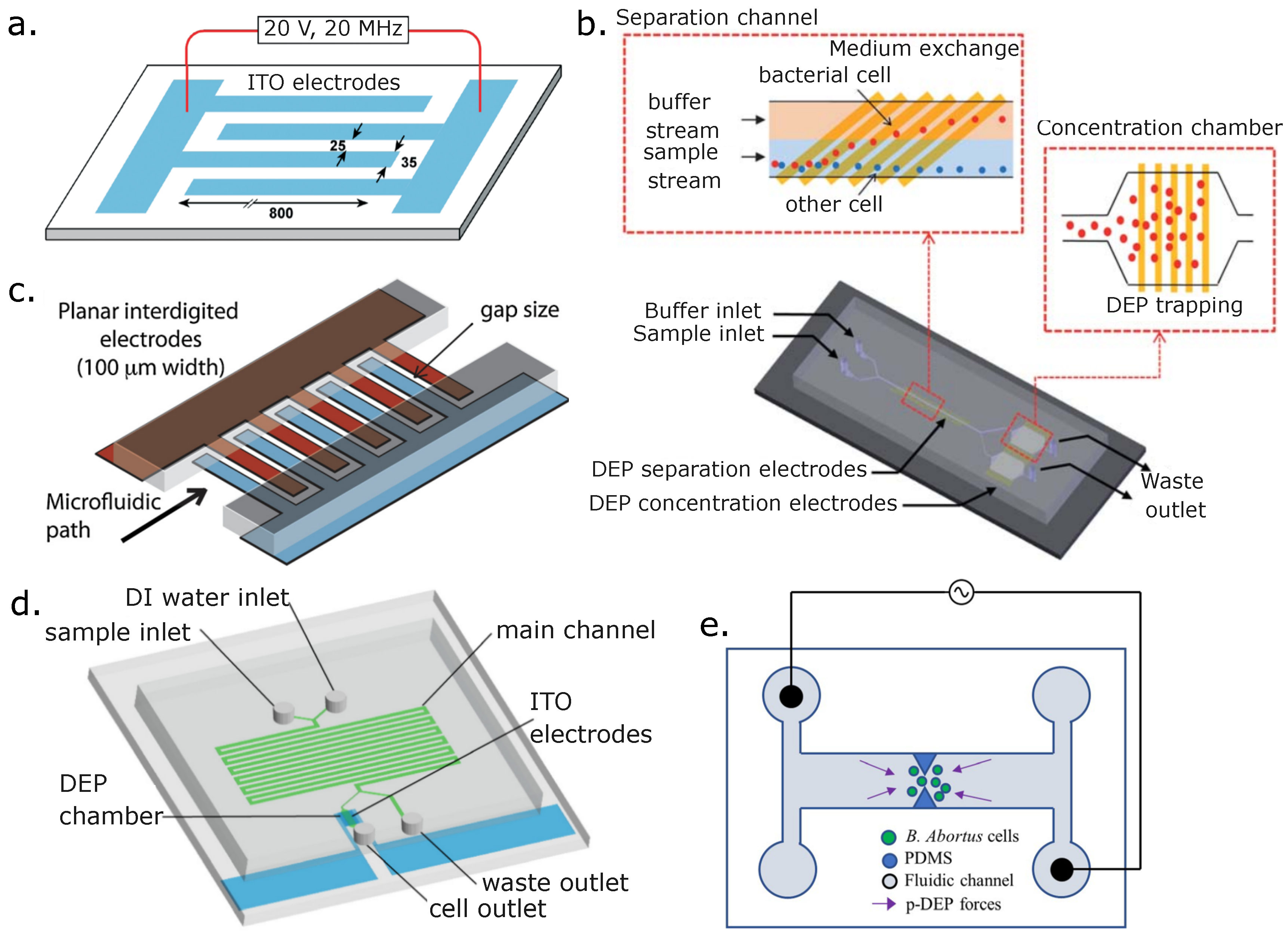
9. Discussion
10. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Afsaneh, H.; Mohammadi, R. Microfluidic platforms for the manipulation of cells and particles. Talanta Open 2022, 5, 100092. [Google Scholar] [CrossRef]
- Choe, S.W.; Kim, B.; Kim, M. Progress of Microfluidic Continuous Separation Techniques for Micro-/Nanoscale Bioparticles. Biosensors 2021, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, L.; Batte, K.E.; Trgovcich, J.; Wisler, J.; Marsh, C.B.; Piper, M. Methodological challenges in utilizing miRNAs as circulating biomarkers. J. Cell. Mol. Med. 2014, 18, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Westphal, M.; Lamszus, K. Circulating biomarkers for gliomas. Nat. Rev. Neurol. 2015, 11, 556–566. [Google Scholar] [CrossRef]
- Antfolk, M.; Laurell, T. Continuous flow microfluidic separation and processing of rare cells and bioparticles found in blood—A review. Anal. Chim. Acta 2017, 965, 9–35. [Google Scholar] [CrossRef]
- Narayana Iyengar, S.; Kumar, T.; Mårtensson, G.; Russom, A. High resolution and rapid separation of bacteria from blood using elasto-inertial microfluidics. Electrophoresis 2021, 42, 2538–2551. [Google Scholar] [CrossRef]
- Yoon, T.; Moon, H.S.; Song, J.W.; Hyun, K.A.; Jung, H.I. Automatically Controlled Microfluidic System for Continuous Separation of Rare Bacteria from Blood. Cytom. Part A 2019, 95, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Duffy, S.P.; Matthews, K.; Deng, X.; Santoso, A.T.; Islamzada, E.; Ma, H. Deformability-based sorting of red blood cells improves diagnostic sensitivity for malaria caused by Plasmodium falciparum. Lab Chip 2016, 16, 645–654. [Google Scholar] [CrossRef]
- Ashley, E.A.; White, N.J. Chapter 19: Malaria Diagnosis and Treatment. In The Travel and Tropical Medicine Manual; Saunders: Philadelphia, PA, USA, 2008. [Google Scholar]
- Costafreda, M.I.; Sauleda, S.; Rico, A.; Piron, M.; Bes, M. Detection of Nonenveloped Hepatitis E Virus in Plasma of Infected Blood Donors. J. Infect. Dis. 2021, 226, 1753–1760. [Google Scholar] [CrossRef]
- Narayanamurthy, V.; Jeroish, Z.E.; Bhuvaneshwari, K.S.; Samsuri, F. Hepatitis C virus (HCV) diagnosis via microfluidics. Anal. Methods 2021, 13, 740–763. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, M.; Lin, Y.; Xu, J. Acoustic Microfluidic Separation Techniques and Bioapplications: A Review. Micromachines 2020, 11, 921. [Google Scholar] [CrossRef]
- Basiri, A.; Heidari, A.; Nadi, M.F.; Fallahy, M.T.P.; Nezamabadi, S.S.; Sedighi, M.; Saghazadeh, A.; Rezaei, N. Microfluidic devices for detection of RNA viruses. Rev. Med. Virol. 2021, 31, e2154. [Google Scholar] [CrossRef]
- Tripathi, S.; Agrawal, A. Blood Plasma Microfluidic Device: Aiming for the detection of COVID-19 antibodies using an on-chip Elisa Platform. Trans. Indian Natl. Acad. Eng. 2020, 5, 217–220. [Google Scholar] [CrossRef]
- Berkenbrock, J.A.; Grecco-Machado, R.; Achenbach, S. Microfluidic devices for the detection of viruses: Aspects of emergency fabrication during the COVID-19 pandemic and other outbreaks. Proc. R. Soc. A Math. Phys. Eng. Sci. 2020, 476, 20200398. [Google Scholar] [CrossRef] [PubMed]
- Amasia, M.; Madou, M. Large-volume centrifugal microfluidic device for blood plasma separation. Bioanalysis 2010, 2, 1701–1710. [Google Scholar] [PubMed]
- Emmerich, M.E.; Sinningen, A.S.; Neubauer, P.; Birkholz, M. Dielectrophoretic separation of blood cells. Biomed. Microdevices 2022, 24, 33731–33738. [Google Scholar] [CrossRef]
- Kersaudy-Kerhoas, M.; Sollier, E. Micro-scale blood plasma separation: From acoustophoresis to egg-beaters. Lab Chip 2013, 13, 3323–3346. [Google Scholar] [CrossRef]
- Tetala, K.K.; Vijayalakshmi, M. A review on recent developments for biomolecule separation at analytical scale using microfluidic devices. Anal. Chim. Acta 2016, 906, 7–21. [Google Scholar]
- Burklund, A.; Tadimety, A.; Nie, Y.; Hao, N.; Zhang, J.X. Chapter One—Advances in diagnostic microfluidics. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 95, pp. 1–72. [Google Scholar] [CrossRef]
- Farahinia, A.; Zhang, W.; Badea, I. Novel microfluidic approaches to circulating tumor cell separation and sorting of blood cells: A review. J. Sci. Adv. Mater. Devices 2021, 6, 303–320. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Zhang, Y.; Liu, J. Advancing single-cell proteomics and metabolomics with microfluidic technologies. Analyst 2019, 144, 846–858. [Google Scholar]
- Mielczarek, W.S.; Obaje, E.A.; Bachmann, T.T.; Kersaudy-Kerhoas, M. Microfluidic blood plasma separation for medical diagnostics: Is it worth it? Lab Chip 2016, 16, 3441–3448. [Google Scholar] [CrossRef]
- Lapizco-Encinas, B.H.; Zhang, Y.V. Microfluidic systems in clinical diagnosis. Electrophoresis 2022, 44, 217–245. [Google Scholar] [CrossRef]
- Yu, B.Y.; Elbuken, C.; Shen, C.; Huissoon, J.P.; Ren, C.L. An integrated microfluidic device for the sorting of yeast cells using image processing. Sci. Rep. 2018, 8, 3550. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, H.; Shu, W.; Tian, J.; Huang, Y.; Song, Y.; Wang, R.; Li, E.; Slamon, D.; Hou, D.; et al. An integrated microfluidic device for rapid and high-sensitivity analysis of circulating tumor cells. Sci. Rep. 2017, 7, 42612. [Google Scholar] [CrossRef]
- Tseng, C.C.; Lu, S.Y.; Chen, S.J.; Wang, J.M.; Fu, L.M.; Wu, Y.H. Microfluidic aptasensor POC device for determination of whole blood potassium. Anal. Chim. Acta 2022, 1203, 339722. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Albero, M.; Sebastian, V.; Sese, J.; Pazo-Cid, R.; Mendoza, G.; Arruebo, M.; Martin-Duque, P.; Santamaria, J. Isolation of exosomes from whole blood by a new microfluidic device: Proof of concept application in the diagnosis and monitoring of pancreatic. J. Nanobiotechnol. 2020, 18, 150. [Google Scholar] [CrossRef]
- Wang, X.; He, X.; He, Z.; Hou, L.; Ge, C.; Wang, L.; Li, S.; Xu, Y. Detection of prostate-specific antigen in whole blood by microfluidic chip integrated with dielectrophoretic separation and electrochemical sensing. Biosens. Bioelectron. 2022, 204, 114057. [Google Scholar] [CrossRef] [PubMed]
- Pinho, D.; Carvalho, V.; Gonçalves, I.M.; Teixeira, S.; Lima, R. Visualization and Measurements of Blood Cells Flowing in Microfluidic Systems and Blood Rheology: A Personalized Medicine Perspective. J. Pers. Med. 2020, 10, 249. [Google Scholar] [CrossRef]
- Bruus, H. Theoretical Microfluidics; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Mohammed, M.I.; Haswell, S.; Gibson, I. Lab-on-a-chip or Chip-in-a-lab: Challenges of Commercialization Lost in Translation. Procedia Technol. 2015, 20, 54–59. [Google Scholar] [CrossRef]
- Ahadian, S.; Civitarese, R.; Bannerman, D.; Mohammadi, M.H.; Lu, R.; Wang, E.; Davenport-Huyer, L.; Lai, B.; Zhang, B.; Zhao, Y.; et al. Organ-on-a-chip platforms: A convergence of advanced materials, cells, and microscale technologies. Adv. Healthc. Mater. 2018, 7, 1700506. [Google Scholar]
- Nguyen, N.T.; Wereley, S.T. Fundamentals and Applications of Microfluidics; Artech House: London, UK, 2019. [Google Scholar]
- Maria, M.S.; Chandra, T.; Sen, A. Capillary flow-driven blood plasma separation and on-chip analyte detection in microfluidic devices. Microfluid. Nanofluidics 2017, 21, 72. [Google Scholar]
- Liang, W.; Yang, X.; Wang, J.; Wang, Y.; Yang, W.; Liu, L. Determination of Dielectric Properties of Cells using AC Electrokinetic-based Microfluidic Platform: A Review of Recent Advances. Micromachines 2020, 11, 513. [Google Scholar] [CrossRef]
- Shiri, F.; Feng, H.; Gale, B.K. Passive and active microfluidic separation methods. In Particle Separation Techniques; Elsevier: Amsterdam, The Netherlands, 2022; pp. 449–484. [Google Scholar]
- Wang, Y.; Nunna, B.B.; Talukder, N.; Etienne, E.E.; Lee, E.S. Blood Plasma Self-Separation Technologies during the Self-Driven Flow in Microfluidic Platforms. Bioengineering 2021, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.A.; Bhole, K.S. A review on microfluidic devices for separation of blood constituents. IOP Conf. Ser. Mater. Sci. Eng. 2020, 810, 012024. [Google Scholar] [CrossRef]
- Tomlinson, M.J.; Tomlnson, S.; Yang, X.B.; Kirkham, J. Cell separation: Terminology and practical considerations. J. Tissue Eng. 2012, 4, 2041731412472690. [Google Scholar] [CrossRef] [PubMed]
- Laxmi, V.; Tripathi, S.; Joshi, S.S.; Agrawal, A. Separation and Enrichment of Platelets from Whole Blood Using a PDMS-Based Passive Microdevice. Ind. Eng. Chem. Res. 2020, 59, 4792–4801. [Google Scholar] [CrossRef]
- Bayareh, M. An updated review on particle separation in passive microfluidic devices. Chem. Eng. Process.-Process. Intensif. 2020, 153, 107984. [Google Scholar]
- Amini, H.; Lee, W.; Di Carlo, D. Inertial microfluidic physics. Lab Chip 2014, 14, 2739–2761. [Google Scholar] [CrossRef]
- Xiang, N.; Ni, Z. Portable Battery-Driven Microfluidic Cell Separation Instrument with Multiple Operational Modes. Anal. Chem. 2022, 94, 16813–16820. [Google Scholar] [CrossRef]
- Catarino, S.O.; Rodrigues, R.O.; Pinho, D.; Miranda, J.M.; Minas, G.; Lima, R. Blood cells separation and sorting techniques of passive microfluidic devices: From fabrication to applications. Micromachines 2019, 10, 593. [Google Scholar] [CrossRef]
- Nasiri, R.; Shamloo, A.; Ahadian, S.; Amirifar, L.; Akbari, J.; Goudie, M.J.; Lee, K.; Ashammakhi, N.; Dokmeci, M.R.; Di Carlo, D.; et al. Microfluidic-Based Approaches in Targeted Cell/Particle Separation Based on Physical Properties: Fundamentals and Applications. Small 2020, 16, 2000171. [Google Scholar] [CrossRef]
- Tang, H.; Niu, J.; Jin, H.; Lin, S.; Cui, D. Geometric structure design of passive label-free microfluidic systems for biological micro-object separation. Microsyst. Nanoeng. 2022, 8, 62. [Google Scholar]
- Kim, G.Y.; Han, J.I.; Park, J.K. Inertial Microfluidics-Based Cell Sorting. BioChip J. 2018, 12, 257–267. [Google Scholar] [CrossRef]
- Tang, W.; Zhu, S.; Jiang, D.; Zhu, L.; Yang, J.; Xiang, N. Channel innovations for inertial microfluidics. Lab Chip 2020, 20, 3485–3502. [Google Scholar] [CrossRef]
- Salafi, T.; Zhang, Y.; Zhang, Y. A Review on Deterministic Lateral Displacement for Particle Separation and Detection. Nano-Micro Lett. 2019, 11, 77. [Google Scholar] [CrossRef]
- Hochstetter, A.; Vernekar, R.; Austin, R.H.; Becker, H.; Beech, J.P.; Fedosov, D.A.; Gompper, G.; Kim, S.C.; Smith, J.T.; Stolovitzky, G.; et al. Deterministic Lateral Displacement: Challenges and Perspectives. ACS Nano 2020, 14, 10784–10795. [Google Scholar] [CrossRef]
- Stavrakis, S.; Holzner, G.; Choo, J.; deMello, A. High-throughput microfluidic imaging flow cytometry. Curr. Opin. Biotechnol. 2019, 55, 36–43. [Google Scholar] [CrossRef]
- Wang, M.; Liang, H.; Chen, X.; Chen, D.; Wang, J.; Zhang, Y.; Chen, J. Developments of Conventional and Microfluidic Flow Cytometry Enabling High-Throughput Characterization of Single Cells. Biosensors 2022, 12, 443. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Han, Y.; Xiang, N.; Ni, Z. Asymmetric serpentine microchannel based impedance cytometer enabling consistent transit and accurate characterization of tumor cells and blood cells. Sens. Actuators B Chem. 2021, 336, 129719. [Google Scholar] [CrossRef]
- Salahi, A.; Honrado, C.; Rane, A.; Caselli, F.; Swami, N.S. Modified Red Blood Cells as Multimodal Standards for Benchmarking Single-Cell Cytometry and Separation Based on Electrical Physiology. Anal. Chem. 2022, 94, 2865–2872. [Google Scholar] [CrossRef]
- Gnyawali, V.; Strohm, E.M.; Wang, J.Z.; Tsai, S.S.; Kolios, M.C. Simultaneous acoustic and photoacoustic microfluidic flow cytometry for label-free analysis. Sci. Rep. 2022, 9, 1585. [Google Scholar] [CrossRef]
- Huang, X.X.; Urosevic, N.; Inglis, T.J.J. Accelerated bacterial detection in blood culture by enhanced acoustic flow cytometry (AFC) following peptide nucleic acid fluorescence in situ hybridization (PNA-FISH). PLoS ONE 2019, 14, e0201332. [Google Scholar] [CrossRef] [PubMed]
- Mehran, A.; Rostami, P.; Saidi, M.S.; Firoozabadi, B.; Kashaninejad, N. HigH-Throughput, Label-Free Isolation of White Blood Cells from Whole Blood Using Parallel Spiral Microchannels with U-Shaped Cross-Section. Biosensors 2021, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, A.; Yazdani, A.; Saghafifar, F. Investigation of a two-step device implementing magnetophoresis and dielectrophoresis for separation of circulating tumor cells from blood cells. Eng. Life Sci. 2020, 20, 296–304. [Google Scholar] [CrossRef]
- Caffiyar, M.Y.; Lim, K.P.; Basha, I.H.K.; Hamid, N.H.; Cheong, S.C.; Ho, E.T.W. Label-Free, High-Throughput Assay of Human Dendritic Cells from Whole-Blood Samples with Microfluidic Inertial Separation Suitable for Resource-Limited Manufacturing. Micromachines 2020, 11, 514. [Google Scholar] [CrossRef]
- Di Carlo, D.; Irimia, D.; Tompkins, R.G.; Toner, M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Appl. Phys. Sci. 2007, 104, 18892–18897. [Google Scholar] [CrossRef]
- Amani, A.; Shamloo, A.; Vatani, P.; Ebrahimi, S. Particles Focusing and Separation by a Novel Inertial Microfluidic Device: Divergent Serpentine Microchannel. Ind. Eng. Chem. Res. 2022, 61, 14324–14333. [Google Scholar] [CrossRef]
- Huang, D.; Man, J.; Jiang, D.; Zhao, J.; Xiang, N. Inertial microfluidics: Recent advances. Electrophoresis 2020, 41, 2166–2187. [Google Scholar] [CrossRef]
- Ramya, S.; Kumar Praveen, S.; Dinesh Ram, G.; Lingaraja, D. A short review of spiral microfluidic devices with distinct cross-sectional geometries. Microfluid. Nanofluidics 2020, 26, 95. [Google Scholar] [CrossRef]
- Xiang, N.; Ni, Z. Inertial microfluidics: Current status, challenges, and future opportunities. Lab Chip 2022, 22, 4792–4804. [Google Scholar] [CrossRef]
- Zhou, J.; Papautsky, I. Fundamentals of inertial focusing in microchannels. Lab Chip 2013, 13, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Han, L.; Tan, W.; Cao, W.; Zhu, G. Hydrodynamic separation by changing equilibrium positions in contraction-expansion array channels. Microfluid Nanofluid 2019, 23, 52. [Google Scholar] [CrossRef]
- Mach, A.J.; Di Carlo, D. Continuous scalable blood filtration device using inertial microfluidics. Biotechnol. Bioeng. 2010, 107, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, H.; Yadav, S.; Phan, H.P.; Ta, H.; Zhang, J.; Nguyen, N.T. Size-tuneable isolation of cancer cells using stretchable inertial microfluidics. Lab Chip 2021, 21, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.M.; Toner, M. Inertial Focusing in Microfluidics. Annu. Rev. Biomed. Eng. 2014, 16, 371–396. [Google Scholar] [CrossRef]
- Jeon, H.; Jundi, B.; Choi, K.; Ryu, H.; Levy, B.D.; Lim, G.; Han, J. Fully-automated and field-deployable blood leukocyte separation platform using multi-dimensional double spiral (MDDS) inertial microfluidics. Lab Chip 2020, 20, 3612–3624. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, R.; Lai, L.; van Dalum, G.; Behrens, B.; Bhagat, A.; Knoefel, W.T.; Nevel, R.P.L.; Stoecklein, N.H. Technical Validation of a new microfluidic device for enrichment of CTCs from large volumes of blood by using buffy coats to mimic diagnostic leukapheresis products. Sci. Rep. 2020, 10, 20312. [Google Scholar] [CrossRef]
- Holm, S.H.; Beech, J.P.; Barrett, M.P.; Tegenfeldt, J.O. Correction: Simplifying microfluidic separation devices towards field-detection of blood parasites. Anal. Methods 2016, 8, 5726. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, D.; Han, Y.; Wang, C.; Xiang, N.; Ni, Z. Inertial microfluidic cube for automatic and fast extraction of white blood cells from whole blood. Lab Chip 2020, 20, 244–252. [Google Scholar] [CrossRef]
- Torres-Castro, K.; Jarmoshti, J.; Xiao, L.; Rane, A.; Salahi, A.; Jin, L.; Li, X.; Caselli, F.; Honrado, C.; Swami, N.S. Multichannel Impedance Cytometry Downstream of Cell Separation by Deterministic Lateral Displacement to Quantify Macrophage Enrichment in Heterogeneous Samples (Adv. Mater. Technol. 8/2023). Adv. Mater. Technol. 2023, 8, 2370037. [Google Scholar] [CrossRef]
- Cha, H.; Fallahi, H.; Dai, Y.; Yadav, S.; Hettiarachchi, S.; McNamee, A.; An, H.; Xiang, N.; Nguyen, N.T.; Zhang, J. Tuning particle inertial separation in sinusoidal channels by embedding periodic obstacle microstructures. Lab Chip 2022, 22, 2789–2800. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, R.; Shamloo, A.; Akbari, J. Design of two Inertial-based microfluidic devices for cancer cell separation from Blood: A serpentine inertial device and an integrated inertial and magnetophoretic device. Chem. Eng. Sci. 2022, 252, 117283. [Google Scholar] [CrossRef]
- Venugopal, D.; Kasani, N.; Manjunath, Y.; Li, G.; Jussuf T. Kaifi, J.T.; Kwon, J.W. Clog-free hig-throughput microfluidic cell isolation with multifunctional microposts. Sci. Rep. 2021, 11, 16685. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ge, C.; Yang, Y.; Gan, M.; Xu, Y.; Chen, L.; Li, S. Direct separation and enumeration of CTCs in viscous blood based on co-flow microchannel with tunable shear rate: A proof-of-principle study. Anal. Bioanal. Chem. 2022, 414, 7683–7694. [Google Scholar] [CrossRef]
- Xu, X.; Huang, X.; Sun, J.; Wang, R.; Yao, J.; Han, W.; Wei, M.; Chen, J.; Guo, J.; Sun, L.; et al. Recent progress of inertial microfluidic-based cell separation. Analyst 2021, 146, 7070–7086. [Google Scholar] [CrossRef]
- Xiang, N.; Wang, J.; Li, Q.; Han, Y.; Huang, D.; Ni, Z. Precise Size-Based Cell Separation via the Coupling of Inertial Microfluidics and Deterministic Lateral Displacement. Anal. Chem. 2019, 91, 10328–10334. [Google Scholar] [CrossRef]
- Pei, H.; Li, L.; Wang, Y.; Sheng, R.; Wang, Y.; Xie, S.; Shui, L.; Si, H.; Tang, B. Single-Cell Phenotypic Profiling of CTCs in Whole Blood Using an Integrated Microfluidic Device. Anal. Chem. 2019, 91, 11078–11084. [Google Scholar] [CrossRef]
- Stemple, C.C.; Angus, S.V.; Park, T.S.; Yoon, J.Y. Smartphone-based OPTOFLUIDIC Lab-on-a-chip for detecting pathogens from blood. SLAS Technol. 2014, 19, 35–41. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef]
- Batcho, E.C.; Miller, S.; Cover, T.L.; McClain, M.S.; Marasco, C.; Bell, C.S.; Giorgio, T.D. Inertial-based Fluidic Platform for Rapid Isolation of Blood-borne Pathogens. Mil. Med. 2021, 186, 129–136. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, P.; Ngamsom, B.; Dyer, C.E.; Iles, A.; Pamme, N. Inertial focusing of microparticles, bacteria, and blood in serpentine glass channels. Electrophoresis 2021, 42, 2246–2255. [Google Scholar] [CrossRef]
- Phillips, E.A.; Moehling, T.J.; Ejendal, K.F.; Hoilett, O.S.; Byers, K.M.; Basing, L.A.; Jankowski, L.A.; Bennett, J.B.; Lin, L.K.; Stanciu, L.A.; et al. Microfluidic rapid and autonomous analytical device (microraad) to detect HIV from whole blood samples. Lab Chip 2019, 19, 3375–3386. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, J.; Kim, S.J.; Ko, D.H.; Lee, S.H.; Lee, S.J.; Park, J.K.; Lee, M.K. On-site extraction and purification of bacterial nucleic acids from blood samples using an unpowered microfluidic device. Sens. Actuators B Chem. 2020, 320, 128346. [Google Scholar] [CrossRef]
- Sivaramakrishnan, M.; Kothandan, R.; Govindarajan, D.K.; Meganathan, Y.; Kandaswamy, K. Active microfluidic systems for cell sorting and separation. Curr. Opin. Biomed. Eng. 2020, 13, 60–68. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, J.; Yuan, D.; Li, W. Hybrid microfluidics combined with active and passive approaches for continuous cell separation. Electrophoresis 2017, 38, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, A.; Parast, F.Y. Simulation of Blood Particle Separation in a Trapezoidal Microfluidic Device by Acoustic Force. IEEE Trans. Electron Devices 2019, 66, 1495–1503. [Google Scholar] [CrossRef]
- Liu, Y.; Lim, K.M. Particle separation in microfluidics using a switching ultrasonic field. Lab Chip 2011, 11, 3167–3173. [Google Scholar] [CrossRef] [PubMed]
- Sajeesh, P.; Sen, A.K. Particle separation and sorting in microfluidic devices: A review. Microfluid Nanofluidics 2014, 17, 1–52. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, M.; Ren, L.; Liu, J.; Whitley, P.H.; Wang, L.; Huang, T.J. High-throughput acoustic separation of platelets from whole blood. Lab Chip 2016, 16, 3466. [Google Scholar] [CrossRef]
- Tripathi, S.; Kumar, Y.B.V.; Prabhakar, A.; Joshi, S.S.; Agrawal, A. Passive blood plasma separation at the microscale: A review of design principles and microdevices. J. Micromech. Microeng. 2015, 25, 083001. [Google Scholar] [CrossRef]
- Md Ali, M.A.; Ostrikov, K.K.; Khalid, F.A.; Majlis, B.Y.; Kayani, A.A. Active bioparticle manipulation in microfluidic systems. RSC Adv. 2016, 6, 113066–113094. [Google Scholar] [CrossRef]
- Sarno, B.; Heineck, D.; Heller, M.J.; Ibsen, S.D. Dielectrophoresis: Developments and applications from 2010 to 2020. Electrophoresis 2021, 42, 539–564. [Google Scholar] [CrossRef]
- Lo, Y.; Lin, Y.; Lei, U.; Wu, M.; Yang, P. Measurement of the Clausius-Mossotti factor of generalized dielectrophoresis. Appl. Phys. Lett. 2014, 104, 083701. [Google Scholar] [CrossRef]
- Pethig, R. Limitations of the Clausius-Mossotti function used in dielectrophoresis and electrical impedance studies of biomacromolecules. Electrophoresis 2019, 40, 2575–2583. [Google Scholar] [CrossRef]
- Çetin, B.; Li, D. Dielectrophoresis in microfluidics technology. Electrophoresis 2011, 32, 2410–2427. [Google Scholar] [CrossRef]
- Pesch, G.R.; Du, F. A review of dielectrophoretic separation and classification of non-biological particles. Electrophoresis 2021, 42, 134–152. [Google Scholar] [CrossRef]
- Kwizera, E.A.; Sun, M.; White, A.M.; Li, J.; He, X. Methods of Generating Dielectrophoretic Force for Microfluidic Manipulation of Bioparticles. Acs Biomater. Sci. Eng. 2021, 7, 2043–2063. [Google Scholar] [CrossRef]
- Hölzel, R.; Pethig, R. Protein dielectrophoresis: Key dielectric parameters and evolving theory. Electrophoresis 2021, 42, 513–538. [Google Scholar] [CrossRef]
- Xie, C.; Chen, B.; Liu, L.; Chen, H.; Wu, J. Iterative dipole moment method for the interaction of multiple dielectrophoretic particles in an AC electrical field. Eur. J. Mech.-B/Fluids 2016, 58, 50–58. [Google Scholar] [CrossRef]
- Sun, T.; Morgan, H. Single-cell microfluidic impedance cytometry: A review. Microfluid. Nanofluid. 2010, 8, 423–443. [Google Scholar] [CrossRef]
- Torres-Castro, K.; Honrado, C.; Varhue, W.; Farmehini, V.; Swami, N.S. High-througput dynamical analysis of dielectrophoretic frequency dispersion of single cells based on deflected flow streamlines. Anal. Bioanal. Chem. 2020, 412, 3847–3857. [Google Scholar] [CrossRef]
- Huang, X.; Torres-Castro, K.; Varhue, W.; Salahi, A.; Rasin, A.; Honrado, C.; Brown, A.; Guler, J.; Swami, N.S. Self-aligned sequential lateral field non-uniformities over channel depth for high throughput dielectrophoretic cell deflection. Lab Chip 2021, 21, 835–843. [Google Scholar] [CrossRef]
- Lapizco-Encinas, B.H. Microscale nonlinear electrokinetics for analysis of cellular materials in clinical applications: A review. Microchim. Acta 2021, 188, 104. [Google Scholar] [CrossRef]
- Huang, X.; Torres-Castro, K.; Varhue, W.; Rane, A.; Rasin, A.; Swami, N.S. On-chip microfluidic buffer swap of biological samples in-line with downstream dielectrophoresis. Electrophoresis 2022, 43, 1275–1282. [Google Scholar] [CrossRef]
- Alnaimat, F.; Dagher, S.; Mathew, B.; Hilal-Alnqbi, A.; Khashan, S. Microfluidics Based Magnetophoresis: A Review. Chem. Rec. 2018, 18, 1596–1612. [Google Scholar] [CrossRef]
- Rodríguez-Villarreal, A.I.; Tarn, M.D.; Madden, L.A.; Lutz, J.B.; Greenman, J.; Samitier, J.; Pamme, N. Flow focussing of particles and cells based on their intrinsic properties using a simple diamagnetic repulsion setup. Lab Chip 2011, 11, 1240–1248. [Google Scholar] [CrossRef]
- Munaz, A.; Shiddiky, M.J.A.; Nguyen, N.T. Recent advances and current challenges in magnetophoresis based micro magnetofluidics. Biomicrofluidics 2018, 12, 031501. [Google Scholar] [CrossRef]
- Zhao, W.; Cheng, R.; Miller, J.R.; Mao, L. Label-free microfluidic manipulation of particles and cells in magnetic liquids. Adv. Funct. Mater. 2016, 26, 3916–3932. [Google Scholar] [CrossRef]
- Xue, C.D.; Sun, Z.P.; Li, Y.J.; Chen, J.F.; Liu, B.; Qin, K.R. Separation of micro and sub-micro diamagnetic particles in dual ferrofluid streams based on negative magnetophoresis. Electrophoresis 2020, 41, 909–916. [Google Scholar] [CrossRef]
- Shiriny, A.; Bayareh, M. On magnetophoretic separation of blood cells using Halbach array magnets. Meccanica 2020, 55, 1903–1916. [Google Scholar] [CrossRef]
- Descamps, L.; Le Roy, D.; Tomba, C.; Deman, A.l. Magnetic Polymers for Magnetophoretic Separation in Microfluidic Devices. Magnetochemistry 2021, 7, 100. [Google Scholar] [CrossRef]
- Hejazian, M.; Li, W.; Nguyen, N.T. Lab on a chip for continuous-flow magnetic cell separation. Lab Chip 2015, 15, 959–970. [Google Scholar] [CrossRef]
- Su, P.; Ren, C.; Fu, Y.; Guo, J.; Guo, J.; Yuan, Q. Magnetophoresis in microfluidic lab: Recent advance. Sens. Actuators A Phys. 2021, 332, 113180. [Google Scholar] [CrossRef]
- Barani, A.; Paktinat, H.; Janmaleki, M.; Mohammadi, A.; Mosaddegh, P.; Fadaei-Tehrani, A.; Sanati-Nezhad, A. Microfluidic integrated acoustic waving for manipulation of cells and molecules. Biosens. Bioelectron. 2016, 85, 714–725. [Google Scholar] [CrossRef]
- Quelennec, A.; Gorman, J.J.; Reyes, D.R. Amontons-Coulomb-like slip dynamics in acousto-microfluidics. Nat. Commun. 2022, 13, 1429. [Google Scholar] [CrossRef]
- Ozcelik, A. 3D Printed Device for Separation of Cells and Particles by Tilted Bulk Acoustic Wave Actuation. Actuators 2022, 11, 249. [Google Scholar] [CrossRef]
- Skov, N.R.; Sehgal, P.; Kirby, B.J.; Bruus, H. Three-dimensional numerical modeling of surface-acoustic-wave devices: Acoustophoresis of micro-and nanoparticles including streaming. Phys. Rev. Appl. 2019, 12, 044028. [Google Scholar] [CrossRef]
- Yu, G.; Chen, X.; Xu, J. Acoustophoresis in variously shaped liquid droplets. Soft Matter 2011, 7, 10063–10069. [Google Scholar] [CrossRef]
- Kothapalli, S.V.; Wiklund, M.; Janerot-Sjoberg, B.; Paradossi, G.; Grishenkov, D. Investigation of polymer-shelled microbubble motions in acoustophoresis. Ultrasonics 2016, 70, 275–283. [Google Scholar] [CrossRef]
- Hsu, J.C.; Chao, C.L. Acoustophoretic patterning of microparticles in a microfluidic chamber driven by standing Lamb waves. Appl. Phys. Lett. 2021, 119, 103504. [Google Scholar] [CrossRef]
- Laurell, T.; Petersson, F.; Nilsson, A. Chip integrated strategies for acoustic separation and manipulation of cells and particles. Chem. Soc. Rev. 2007, 36, 492–506. [Google Scholar] [CrossRef]
- Bruus, H. Acoustofluidics 7: The acoustic radiation force on small particles. Lab Chip 2012, 12, 1014–1021. [Google Scholar] [CrossRef]
- Lenshof, A.; Magnusson, C.; Laurell, T. Acoustofluidics 8: Applications of acoustophoresis in continuous flow microsystems. Lab Chip 2012, 12, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ahmed, D.; Mao, X.; Lin, S.C.S.; Lawit, A.; Huang, T.J. Acoustic tweezers: Patterning cells and microparticles using standing surface acoustic waves (SSAW). Lab Chip 2009, 9, 2890–2895. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Ghosh, R.; Ganguly, R. Acoustophoretic separation of infected erythrocytes from blood plasma in a microfluidic platform using biofunctionalized, matched-impedance layers. Int. J. Numer. Methods Biomed. Eng. 2018, 34, e2943. [Google Scholar] [CrossRef]
- Lee, N.; Wiegand, S. Thermophoretic micron-scale devices: Practical approach and review. Entropy 2020, 22, 950. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xiaolei, P.; Xiaoling, W.; zhangming, M.; Xie, C.; Zheng, Y. Thermophoretic Tweezers for Low-Power and Versatile Manipulation of biological cells. ACS Nano 2017, 11, 3147–3154. [Google Scholar] [CrossRef]
- Vigolo, D.; Rusconi, R.; Stone, H.A.; Piazza, R. Thermophoresis: Microfluidics characterization and separation. Soft Matter 2010, 6, 3489–3493. [Google Scholar] [CrossRef]
- Tsuji, T.; Kozai, K.; Ishino, H.; Kawano, S. Direct observations of thermophoresis in microfluidic systems. Micro Nano Lett. 2017, 12, 520–525. [Google Scholar] [CrossRef]
- Asiaei, S.; Darvishi, V.; Davari, M.H.; Zohrevandi, D.; Moghadasi, H. Thermophoretic isolation of circulating tumor cells, numerical simulation and design of a microfluidic chip. J. Therm. Anal. Calorim. 2019, 137, 831–839. [Google Scholar] [CrossRef]
- Baier, T.; Tiwari, S.; Shrestha, S.; Klar, A.; Hardt, S. Thermophoresis of Janus particles at large Knudsen numbers. Phys. Rev. Fluids 2018, 3, 094202. [Google Scholar] [CrossRef]
- Gossett, D.R.; Weaver, W.M.; Mach, A.J.; Hur, S.C.; Tat Kwong Tse, H.; Lee, W.; Amini, H.; Di Carlo, D. Label-free cell separation and sorting in microfluidic systems. Anal. Bioanal. Chem. 2020, 397, 3249–3267. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, B.; Elsayed, M.; Nan, F.; Liang, W.; Valley, J.K.; Liu, L.; Huang, Q.; Wu, M.C.; Wheeler, A.R. Optoelectronic tweezers: A versatile toolbox for nano-/micro-manipulation. Chem. Soc. Rev. 2022, 51, 9203–9242. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Zhang, C.; Liu, F.; Chen, X.; Liang, G.; Ren, F.; Liang, S.; Song, C.; Shi, J.; Qiu, W.; et al. On-Chip Multicolor Photoacoustic Imaging Flow Cytometry. Anal. Chem. 2021, 93, 8134–8142. [Google Scholar] [CrossRef]
- Mikami, H.; Kawaguchi, M.; Huang, C.E.A. Virtual-freezing flourescence imaging flow cytometry. Nat. Commun. 2020, 11, 1162. [Google Scholar] [CrossRef]
- Mikami, H.; Kawaguchi, M.; Huang, C.E.A. Real-time image processing for microscopy-based label-free imaging flow cytometry in microfluidic chip. Sci. Rep. 2017, 7, 11651. [Google Scholar]
- Fang, Y.; Yu, N.; Jiang, Y.; Dang, C. High-Precision Lens-Less Flow Cytometer on a Chip. Micromachines 2018, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- DiSalvo, M.; Patrone, P.N.; Kearsley, A.J.; Cooksey, G.A. Serial flow cytometry in an inertial focusing optofluidic microchip for direct assessment of measurement variations. Lab Chip 2022, 22, 3217–3228. [Google Scholar] [CrossRef] [PubMed]
- Honrado, C.; Bisegna, P.; Swami, N.S.; Caselli, F. Single-cell microfluidic impedance cytometry: From raw signals to cell phenotypes using data analytics. Lab Chip 2021, 21, 22–54. [Google Scholar] [CrossRef]
- Petchakup, C.; Tay, H.M.; Li, K.H.H.; Hou, H.W. Integrated inertial-impedance cytometry for rapid label-free leukocyte isolation and profiling of neutrophil extracellular traps (NETs). Lab Chip 2019, 19, 1736–1746. [Google Scholar] [CrossRef]
- Lu, N.; Tay, H.M.; Petchakup, C.; He, L.; Gong, L.; Maw, K.K.; Leong, S.Y.; Lok, W.W.; Ong, H.B.; Guo, R.; et al. Label-free microfluidic cell sorting and detection for rapid blood analysis. Lab Chip 2023, 23, 1226–1257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X. Dielectrophoretic microfluidic device for separation of red blood cells and platelets: A model-based study. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 89. [Google Scholar] [CrossRef]
- Ertugrul, I.; Ulkir, O. Dielectrophoretic separation of platelet cells in a microfluidic channel and optimization with fuzzy logic. RSC Adv. 2020, 10, 33731–33738. [Google Scholar] [CrossRef] [PubMed]
- Elitas, M.; Yildizhan, Y.; Islam, M.; Martinez-Duarte, R.; Ozkazanc, D. Dielectrophoretic characterization and separation of monocytes and macrophages using 3D carbon-electrodes. Electrophoresis 2019, 40, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Yildizhan, Y.; Erdem, N.; Islam, M.; Martinez-Duarte, R.; Elitas, M. Dielectrophoretic Separation of Live and Dead Monocytes Using 3D Carbon-Electrodes. Sensors 2017, 17, 2691. [Google Scholar] [CrossRef]
- Sharbati, P.; Sadaghiani, A.K.; Koşar, A. New Generation Dielectrophoretic-Based Microfluidic Device for Multi-Type Cell Separation. Biosensors 2023, 13, 418. [Google Scholar] [CrossRef]
- Jiang, L.; Liang, F.; Huo, M.; Ju, M.; Xu, J.; Ju, S.; Jin, L.; Shen, B. Study on three-dimensional dielectrophoresis microfluidic chip for separation and enrichment of circulating tumor cells. Microelectron. Eng. 2023, 282, 112100. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Y.; Cui, X.; Fan, Y.; Xue, Y.; Miao, H.; Li, G. Extraction of Cell-Free Whole Blood Plasma Using a Dielectrophoresis-Based Microfluidic Device. Biotechnol. J. 2019, 14, 1800181. [Google Scholar] [CrossRef]
- Dudaie, M.; Nissim, N.; Barnea, I.; Gerling, T.; Duschl, C.; Kirschbaum, M.; Shaked, N.T. Label-free discrimination and selection of cancer cells from blood during flow using holography-induced dielectrophoresis. J. Biophotonics 2020, 13, e202000151. [Google Scholar] [CrossRef]
- Noosawat, P.; Srituravanich, W.; Damrongplasit, N.; Suzuki, Y.; Kaewthamasorn, M.; Pimpin, A. A microfluidic device for capturing malaria–infected red blood cells by magnetophoretic force using an array of V- and W-shaped nickel microstructures. Microfluid Nanofluidics 2022, 26, 78. [Google Scholar] [CrossRef]
- Nasiri, R.; Shamloo, A.; Akbari, J. Design of a Hybrid Inertial and Magnetophoretic Microfluidic Device for CTCs Separation from Blood. Micromachines 2021, 12, 877. [Google Scholar] [CrossRef]
- Jung, Y.; Choi, Y.; Han, K.-H.; Frazier, B.A. Six-stage cascade paramagnetic mode magnetophoretic separation system for human blood samples. Biomed. Microdevices 2010, 12, 637–645. [Google Scholar] [CrossRef]
- Karthick, S.; Pradeep, P.N.; Kanchana, P.; Sen, A.K. Acoustic impedance-based size-independent isolation of circulating tumour cells from blood using acoustophoresis. Lab Chip 2018, 18, 3802–3813. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Huang, P.H.; Zhang, R.; Mao, Z.; Chen, C.; Kemeny, G.; Li, P.; Lee, A.V.; Gyanchandani, R.; Armstrong, A.J.; et al. Circulating Tumor Cell Phenotyping via High-Throughput Acoustic Separation. Small 2018, 14, 1801131. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Mao, Z.; Peng, Z.; Zhou, L.; Chen, Y.; Huang, P.H.; Truica, C.I.; Drabick, J.J.; El-Deiry, W.S.; Dao, M.; et al. Acoustic separation of circulating tumor cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4970–4975. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhou, W.; Lin, Z.; Cai, F.; Li, F.; Wu, J.; Meng, L.; Niu, L.; Zheng, H. Sorting of tumour cells in a microfluidic device by multi-stage surface acoustic waves. Sens. Actuators B Chem. 2018, 258, 1174–1183. [Google Scholar] [CrossRef]
- Shi, Z.; Jin, L.; He, C.; Li, Y.; Jiang, C.; Wang, H.; Zhang, J.; Wang, J.; Zhao, W.; Zhao, C.; et al. Hemocompatible magnetic particles with broad-spectrum bacteria capture capability for blood purification. J. Colloid Interface Sci. 2020, 576, 1–9. [Google Scholar] [CrossRef]
- Abafogi, A.T.; Kim, J.; Lee, J.; Mohammed, M.O.; van Noort, D.; Park, S. 3D-printed modular microfluidic device enabling preconcentrating bacteria and purifying bacterial DNA in blood for improving the sensitivity of molecular diagnostics. Sensors 2020, 20, 1202. [Google Scholar] [CrossRef]
- Alipanah, M.; Hafttananian, M.; Hedayati, N.; Ramiar, A.; Alipanah, M. Microfluidic on-demand particle separation using induced charged electroosmotic flow and magnetic field. J. Magn. Magn. Mater. 2021, 537, 168156. [Google Scholar] [CrossRef]
- Gómez-Pastora, J.; González-Fernández, C.; Real, E.; Iles, A.; Bringas, E.; Furlani, E.P.; Ortiz, I. Computational modeling and fluorescence microscopy characterization of a two-phase magnetophoretic microsystem for continuous-flow blood detoxification. Lab Chip 2018, 18, 1593–1606. [Google Scholar] [CrossRef]
- Cai, D.; Xiao, M.; Xu, P.; Xu, Y.C.; Du, W. An integrated microfluidic device utilizing dielectrophoresis and multiplex array PCR for point-of-care detection of pathogens. Lab Chip 2014, 14, 3917–3924. [Google Scholar] [CrossRef]
- Park, S.; Zhang, Y.; Wang, T.H.; Yang, S. Continuous dielectrophoretic bacterial separation and concentration from physiological media of high conductivity. Lab Chip 2011, 11, 2893–2900. [Google Scholar] [CrossRef]
- Bisceglia, E.; Cubizolles, M.; Mallard, F.; Vinet, F.; Francais, O.; Le Pioufle, B. Micro-organism extraction from biological samples using DEP forces enhanced by osmotic shock. Lab Chip 2013, 13, 901–909. [Google Scholar] [CrossRef]
- Bisceglia, E.; Cubizolles, M.; Trainito, C.I.; Berthier, J.; Pudda, C.; Français, O.; Mallard, F.; Le Pioufle, B. A generic and label free method based on dielectrophoresis for the continuous separation of microorganism from whole blood samples. Sens. Actuators B Chem. 2015, 212, 335–343. [Google Scholar] [CrossRef]
- Breslauer, D.N.; Lee, P.J.; Lee, L.P. Microfluidics-based systems biology. Mol. BioSyst. 2006, 2, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Yi, Q.; Shen, C.; Lan, Y.; Urban, G.; Du, W. Direct enrichment of pathogens from physiological samples of high conductivity and viscosity using H-filter and positive dielectrophoresis. Biomicrofluidics 2018, 12, 014109. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Brenes, A.; Torres-Castro, K.; Marín-Benavides, R.; Acuña-Umaña, K.; Espinoza-Araya, C.; Ramírez-Carranza, R.; González-Espinoza, G.; Rojas-Campos, N.; Guzmán-Verri, C.; Sáenz-Arce, G.; et al. Combined electrokinetic manipulations of pathogenic bacterial samples in low-cost fabricated dielectrophoretic devices. AIP Adv. 2019, 9, 115303. [Google Scholar] [CrossRef]
- Gutiérrez-Jiménez, C.; Mora-Cartín, R.; Altamirano-Silva, P.; Chacón-Díaz, C.; Chaves-Olarte, E.; Moreno, E.; Barquero-Calvo, E. Neutrophils as trojan horse vehicles for Brucella abortus macrophage infection. Front. Immunol. 2019, 10, 1012. [Google Scholar] [CrossRef]
- Dash, S.; Mohanty, S. Dielectrophoretic separation of Micron and submicron particles: A Review. Electrophoresis 2014, 35, 2656–2672. [Google Scholar] [CrossRef]
- Abd Rahman, N.; Ibrahim, F.; Yafouz, B. Dielectrophoresis for Biomedical Sciences Applications: A Review. Sensors 2017, 17, 449. [Google Scholar] [CrossRef]
- Dow, P.; Kotz, K.; Gruszka, S.; Holder, J.; Fiering, J. Acoustic separation in plastic microfluidics for rapid detection of bacteria in blood using engineered bacteriophage. Lab Chip 2018, 18, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, F.; Bachman, H.; Cameron, C.E.; Zeng, X.; Huang, T.J. Acoustofluidic bacteria separation. J. Micromech. Microeng. 2016, 27, 015031. [Google Scholar] [CrossRef]
- Ngamsom, B.; Lopez-Martinez, M.J.; Raymond, J.C.; Broyer, P.; Patel, P.; Pamme, N. On-chip acoustophoretic isolation of microflora including S. typhimurium from raw chicken, beef and blood samples. J. Microbiol. Methods 2016, 123, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Perveen, S.; Sharma, R.; Singh, K. Combination of Subtherapeutic Doses of Tretazicar and Liposomal Amphotericin B Suppresses and Cures Leishmania major-Induced Cutaneous Lesions in Murine Models. J. ACS Infect. Dis. 2021, 7, 506–517. [Google Scholar]
- Park, S.; Shabani, R.; Akbari, J.; Tebon, P.; Dokmeci, M.R.; Ahadian, S. On-chip whole blood plasma separator based on microfiltration, sedimentation and wetting contrast. Microsyst. Technol. 2020, 11, 2077–2085. [Google Scholar] [CrossRef]
- Lin, S.; Zhi, X.; Chen, D.; Xia, F.; Shen, Y.; Niu, J.; Huang, S.; Song, J.; Miao, J.; Cui, D.; et al. A flyover style microfluidic chip for highly purified magnetic cell separation. Biosens. Bioelectron. 2019, 129, 175–181. [Google Scholar] [CrossRef]
- Urbansky, A.; Ohlsson, P.; Lenshof, A.; Garofalo, F.; Scheding, S.; Laurell, T. Rapid and effective enrichment of mononuclear cells from blood using acoustophoresis. Sci. Rep. 2017, 7, 17161. [Google Scholar] [CrossRef]
- Urbansky, A.; Olm, F.; Scheding, S.; Laurell, T.; Lenshof, A. Label-free separation of leukocyte subpopulations using high throughput multiplex acoustophoresis. Lab Chip 2019, 19, 1406–1416. [Google Scholar] [CrossRef]
- Undvall Anand, E.; Magnusson, C.; Lenshof, A.; Ceder, Y.; Lilja, H.; Laurell, T. Two-Step Acoustophoresis Separation of Live Tumor Cells from Whole Blood. Anal. Chem. 2021, 93, 17076–17085. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, V.; Dabbi, J.M.; Walker, J.L.; Mertiri, A.; Christianson, R.J.; Fiering, J. Rosette-induced separation of T cells by acoustophoresis. Biomicrofluidics 2022, 16, 054107. [Google Scholar] [CrossRef]
- Feng, X.; Meng, X.; Xiao, F.; Aguilar, Z.P.; Xu, H. Vancomycin-dendrimer based multivalent magnetic separation nanoplatforms combined with multiplex quantitative PCR assay for detecting pathogenic bacteria in human blood. Talanta 2021, 225, 121953. [Google Scholar] [CrossRef] [PubMed]
- De Plano, L.M.; Fazio, E.; Rizzo, M.G.; Franco, D.; Carnazza, S.; Trusso, S.; Neri, F.; Guglielmino, S.P. Phage-based assay for rapid detection of bacterial pathogens in blood by Raman spectroscopy. J. Immunol. Methods 2019, 465, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Sandetskaya, N.; Moos, D.; Pötter, H.; Seifert, S.; Jenerowicz, M.; Becker, H.; Zilch, C.; Kuhlmeier, D. An integrated versatile lab-on-a-chip platform for the isolation and nucleic acid-based detection of pathogens. Future Sci. OA 2017, 3, FSO177. [Google Scholar] [CrossRef] [PubMed]
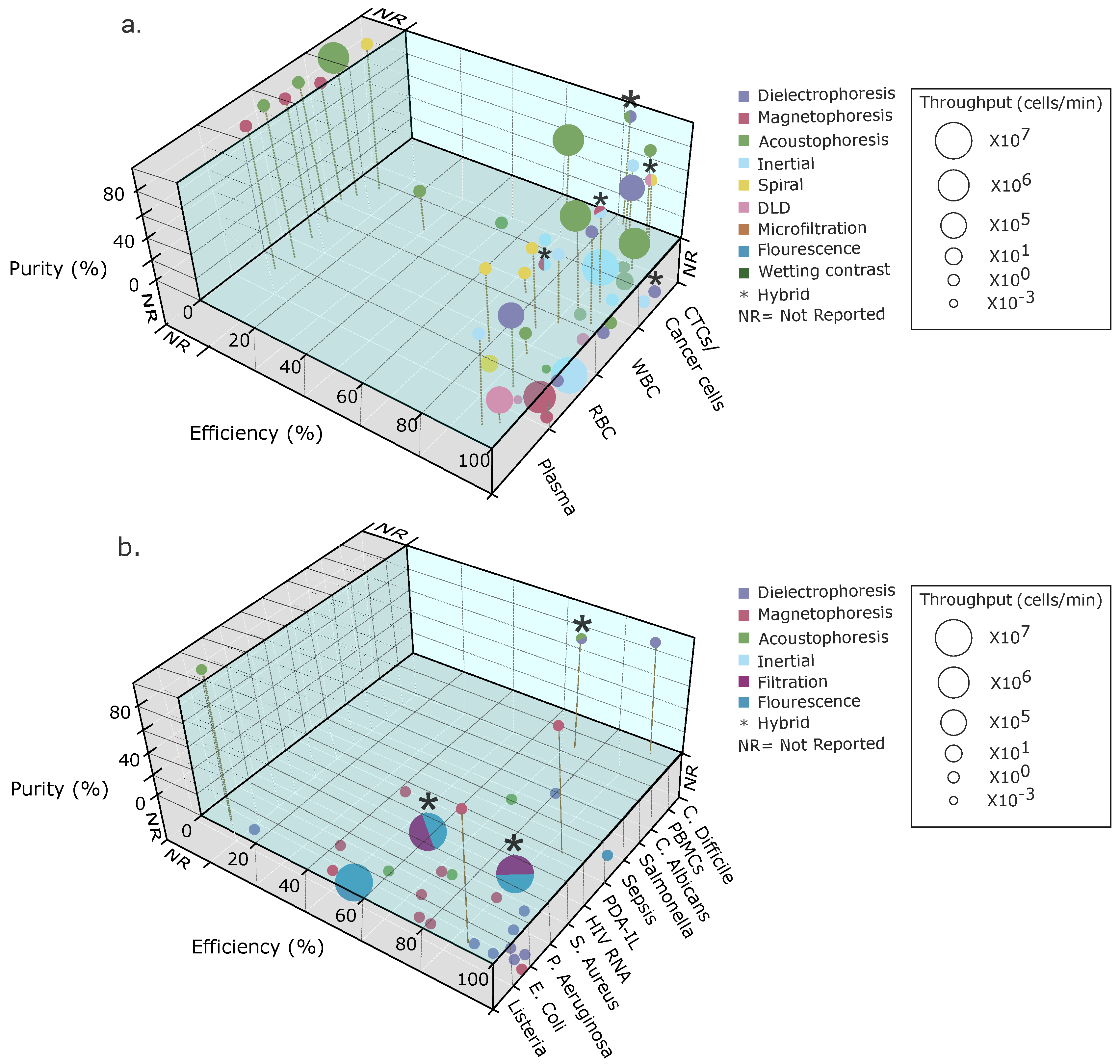
| Blood Contents to Separate | Sample Initial Concentration Volume (Cells/mL) | Separation Method | Target Sample | Separation Efficiency (%) | Purity (%) | Flow Rate (mL/min) | Cell Throughput (Cells/min) | Final Conc. (Cells/mL) | Label-Free (Y/N) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Plasma and NCI-H1299 from RBCs | Inertial: contraction expansions | Plasma and NCI-H1299 lung cancer cells | 96 and 95 | 70–80 | - | - | Yes | [179] | ||
| WBCs from RBCs | 500× dilution | Multidimensional double spiral | WBC (polymorphonuclear leucocytes) | >80 | >85 | 9.2 | − | 12× compared with input | Yes | [72] |
| RBCs, WBCs, and dendritic cells | Whole blood | Inertial: spiral | Dendritic cells | 77 | − | − | − | − | Yes | [60] |
| MCF-7,Hela, and PC-3 from diluted blood | Inertial: spiral with sheath flow | MFC-7 Hela PC-3 | >90 | - | 1.4 total flowrate for separation mode | >80 | - | Yes | [44] | |
| Breast cancer cells (T47D) from blood | Diluted blood (1:50) | Inertial: cascaded sinusoidal | T47D | ∼56 | 1 | − | Yes | [76] | ||
| Plasma from blood | Whole blood | Passive: microfiltration, sedimentation, and wetting contrast | Plasma | ∼100 | ∼15 μL | - | - | - | Yes | [180] |
| RBCs from whole blood | Diluted whole blood () | Dielectrophoresis | RBC | - | - | − | Yes | [106] | ||
| Plasma from blood | Whole blood | Dielectrophoresis | Plasma | ∼98 | ∼100 | − | − | Yes | [153] | |
| RBCs from fixed RBCs | Diluted whole blood | Dielectrophoresis | RBCs and fixed RBCs | − | − | − | Yes | [107] | ||
| Live from dead monocytes | Dielectrophoresis | Live monocytes | 90 | − | − | − | − | Yes | [150] | |
| WBCs, RBCs, and CTCs | Diluted blood | Hybrid: inertial and magnetophoresis | CTCs | ∼95 | 93 | 1 | - | - | No | [156] |
| WBCs from RBCs | Whole blood | Whole blood magnetophoresis | WBCs and RBCs | 89.5 | − | − | − | Yes | [157] | |
| Malaria-infected RBCs from magnetic beads | 80% parasitemia | Magnetophoresis | − | 25 | − | 0.4 | − | − | Yes | [155] |
| CTC (MCF-7) from whole blood | − | Hybrid: inertial spiral, DLD, and fluourescence flow cytometry | CTCs | − | 92 | − | − | − | Separation yes, cytometry no | [82] |
| Tumor cells, RBCs, and WBCs | Diluted blood () and spiked tumor cells () | Hybrid: inertial spiral and DLD | CTCs | 99.9 | 93.59 | − | − | Yes | [81] | |
| WBCs, RBCs, and trypanosomes (T. cyclops) | Diluted blood sample (10x) with spiked parasite | Inertial: DLD | WBCs, RBCs, and trypanosomes | >94 (WBCs) >90 (RBCs) >80 (trypanosomes) | − | − | − | Yes | [73] | |
| RBCs from whole blood | Diluted whole blood | DEP | RBCs | − | − | − | Yes | [106] | ||
| Plasma from blood | Whole blood | DEP | Plasma | ∼98 | ∼100 | − | − | Yes | [153] | |
| RBCs from fixed RBCs | Diluted whole blood | DEP | RBCs and fixed RBCs | − | − | − | Yes | [107] | ||
| Monocytes (U937) and macrophages (U937) | DEP | Monocytes | 69.43 | − | to | − | Yes | [149] | ||
| WBCs from RBCs | Diluted blood | Magnetophoresis | WBCs | − | 93 | − | No | [181] | ||
| Mononuclear cells (MNCs) | Diluted blood (20x) | Acoustophoresis | WBCs | >43 (WBCs) and >87 (MNCs) | 54 (WBCs) 55 (MNCs) | − | Yes | [182] | ||
| WBCs, lymphocytes, monocytes, and granulocytes | − | Acoustophoresis | − | 96.8 (WBCs) 66.7 (lynphocytes) 99 (monocytes and granulocytes) | 96.5 (WBCs) 71.8 (lynphocytes) 10.1 (monocytes) 98.8 (granulocytes) | 0.1 | − | Yes | [183] | |
| Tumor cells (U87 glioma cells) from RBCs | Diluted blood spiked with tumor cells/mL | Acoustophoresis | Glioma tumor cells | 90 | − | − | − | − | [161] | |
| CTCs, WBCs from lysed blood | Lysed RBCs from whole blood with WBCs spiked with CTCs | Acoustophoresis | DU145 (prostate cancer cells) and MCF7 (breast cancer cells) | 42 (CTCs) | 99.75 (WBC depletion efficiency) | − | − | − | No | [184] |
| RBCs from bacteria | Diluted blood (20%) | Acoustophoresis | Bacteria | >40 (E. coli) >60 (S. aureus) >50 (P. aeruginosa) | − | 0.01 | − | – CFU/mL | No | [176] |
| T cells from RBCs | Diluted blood | Acoustophoresis | T cells | 90.5 | 99 | 0.4 | − | − | No for T cells and yes for RBCs | [185] |
| Microorganism Separated from Blood | Sample Initial Concentration Volume (CFU/mL) | Separation Method | Separation Efficiency (%) | Purity (%) | Flow Rate (μL/min) | Cell Throughput (Cells/mL) | Label-Free (Y/N) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Malaria | − | Passive: detecting by light | − | − | − | − | No | [83] |
| Listeria monocytogenes Staphylococcus aureus (Quickly sepsis clinical diagnostics) | from to | Active: Magnetic separation | 93.14 | 90 | − | − | Yes | [186] |
| Sepsis S. aureus, E. coli, P. aeruginosa, and Methicillin-resistant S. aureus | Electrostatic and hydrophobic interactions | from to | − | − | − | Yes | [162] | |
| Sepsis S. aureus, P. aeruginosa, and E. coli | 1.42 | Active: Magnetic separation and micro-Raman spectroscopy | 60–70 | − | − | Yes | [187] | |
| E. coli | Active: dielectrophoresis | 97 | − | from to | No | [169] | ||
| S. enteritidis and E. coli | and | Electrophoresis | Reduce time and equipment | − | − | − | Yes | [188] |
| HIV RNA | Whole blood | Passive: filtration and flourescent | Depends on particle size, from 47.6% to 81.9% | virus copies per mL | − | No | [87] | |
| E. coli | – | Electrophoresis by gel | 0.3–0.6 | − | − | − | Yes | [88] |
| RBC | − | Magnetophoresis | 100 | − | − | − | No | [164] |
| E. coli | – | Magnetophoresis | − | − | from to | − | Yes | [163] |
| Sepsis | Fluorescence | 100 | − | − | Yes | [85] | ||
| E. coli and RBCs | and | Flourescence | 54 and 97 | − | Yes | [86] | ||
| P. aeruginosa, S. aureus, and E. coli | DEP | 94.8 | − | 1 | − | Yes | [166] | |
| E. coli and RBCs | and | Acousto-phoresis | − | 96.0 and 95.85 | 9 and 7 | − | Yes | [177] |
| Salmonella and RBCs | Acoustophoresis | 60 and 90 | − | 10–30 and 10–70 | − | Yes | [178] | |
| E. coli | DEP | milk: 90 buffer: 70.7 human blood: 80.2 | − | 5 | − | Yes | [171] | |
| E. coli | DEP | 87.2 | − | 1.8 | − | Yes | [167] | |
| E. coli and C. albicans | DEP (+/−) | 97 (DEP+), 94 (DEP−) and 92 (DEP+), 56 (DEP−) | − | − | − | Yes | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Castro, K.; Acuña-Umaña, K.; Lesser-Rojas, L.; Reyes, D.R. Microfluidic Blood Separation: Key Technologies and Critical Figures of Merit. Micromachines 2023, 14, 2117. https://doi.org/10.3390/mi14112117
Torres-Castro K, Acuña-Umaña K, Lesser-Rojas L, Reyes DR. Microfluidic Blood Separation: Key Technologies and Critical Figures of Merit. Micromachines. 2023; 14(11):2117. https://doi.org/10.3390/mi14112117
Chicago/Turabian StyleTorres-Castro, Karina, Katherine Acuña-Umaña, Leonardo Lesser-Rojas, and Darwin R. Reyes. 2023. "Microfluidic Blood Separation: Key Technologies and Critical Figures of Merit" Micromachines 14, no. 11: 2117. https://doi.org/10.3390/mi14112117
APA StyleTorres-Castro, K., Acuña-Umaña, K., Lesser-Rojas, L., & Reyes, D. R. (2023). Microfluidic Blood Separation: Key Technologies and Critical Figures of Merit. Micromachines, 14(11), 2117. https://doi.org/10.3390/mi14112117





