Facile Route to Achieve a Hierarchical CuO/Nickel-Cobalt-Sulfide Electrode for Energy Storage
Abstract
:1. Introduction
2. Experimental
2.1. Materials
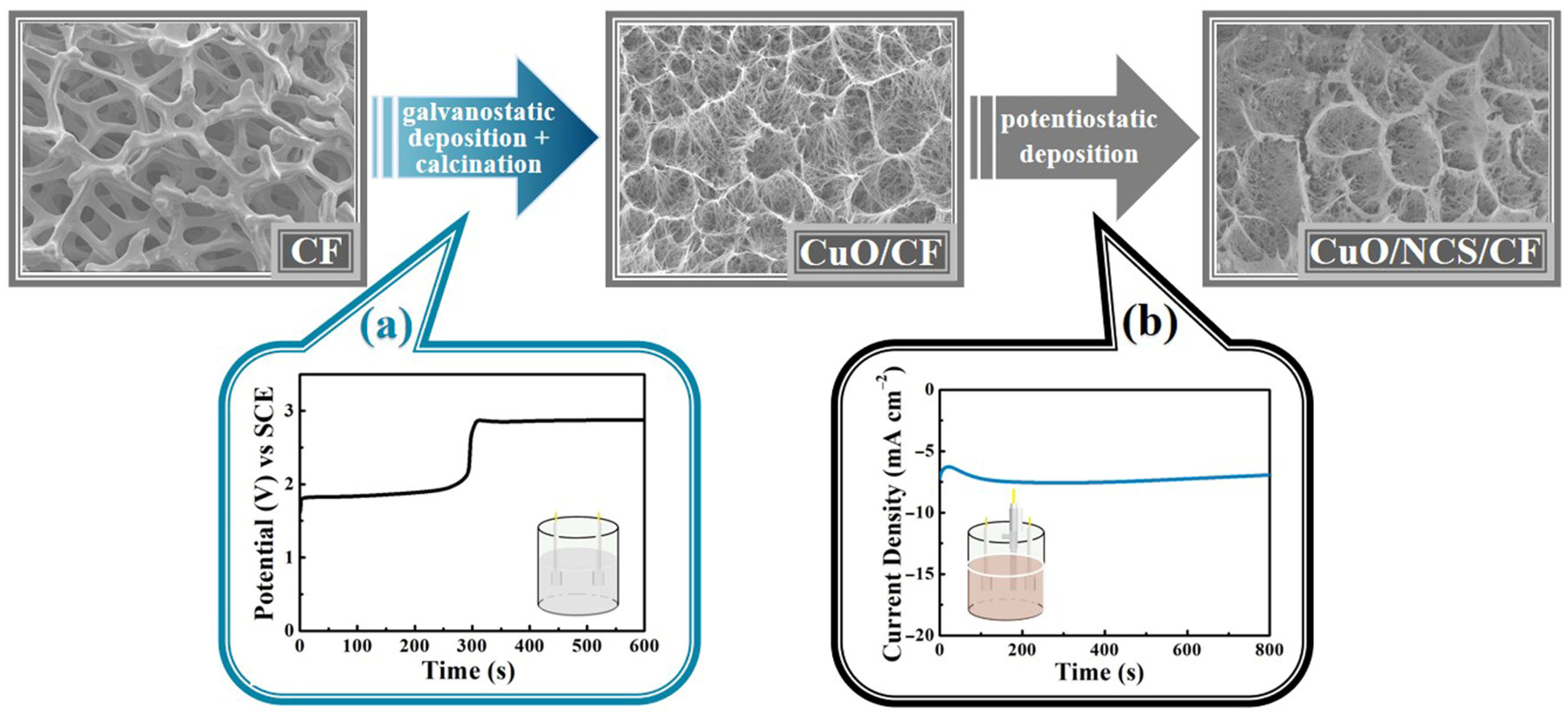
2.2. Stepwise Electrodeposition of CuO/NCS Electrode
2.3. Characterization
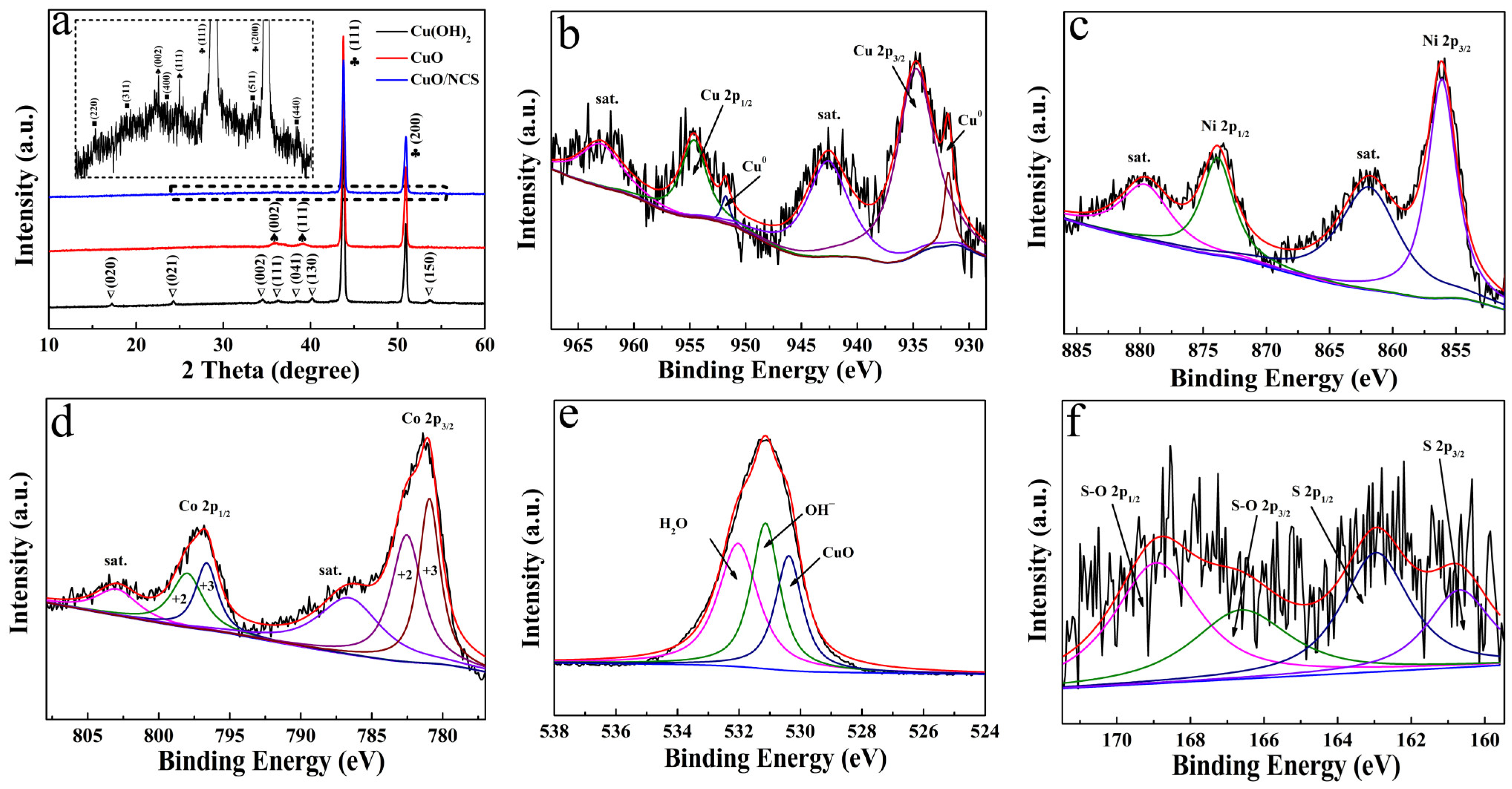
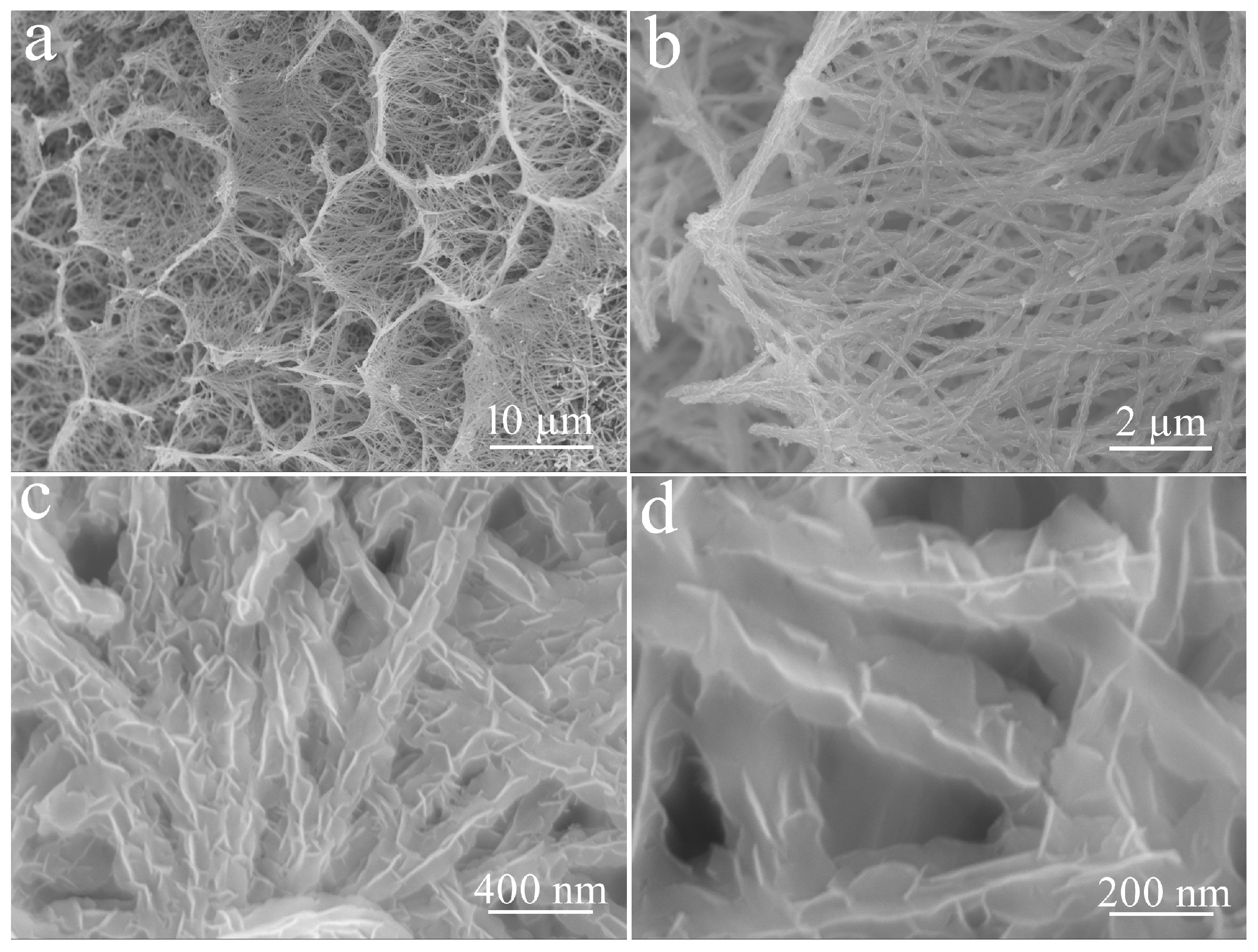
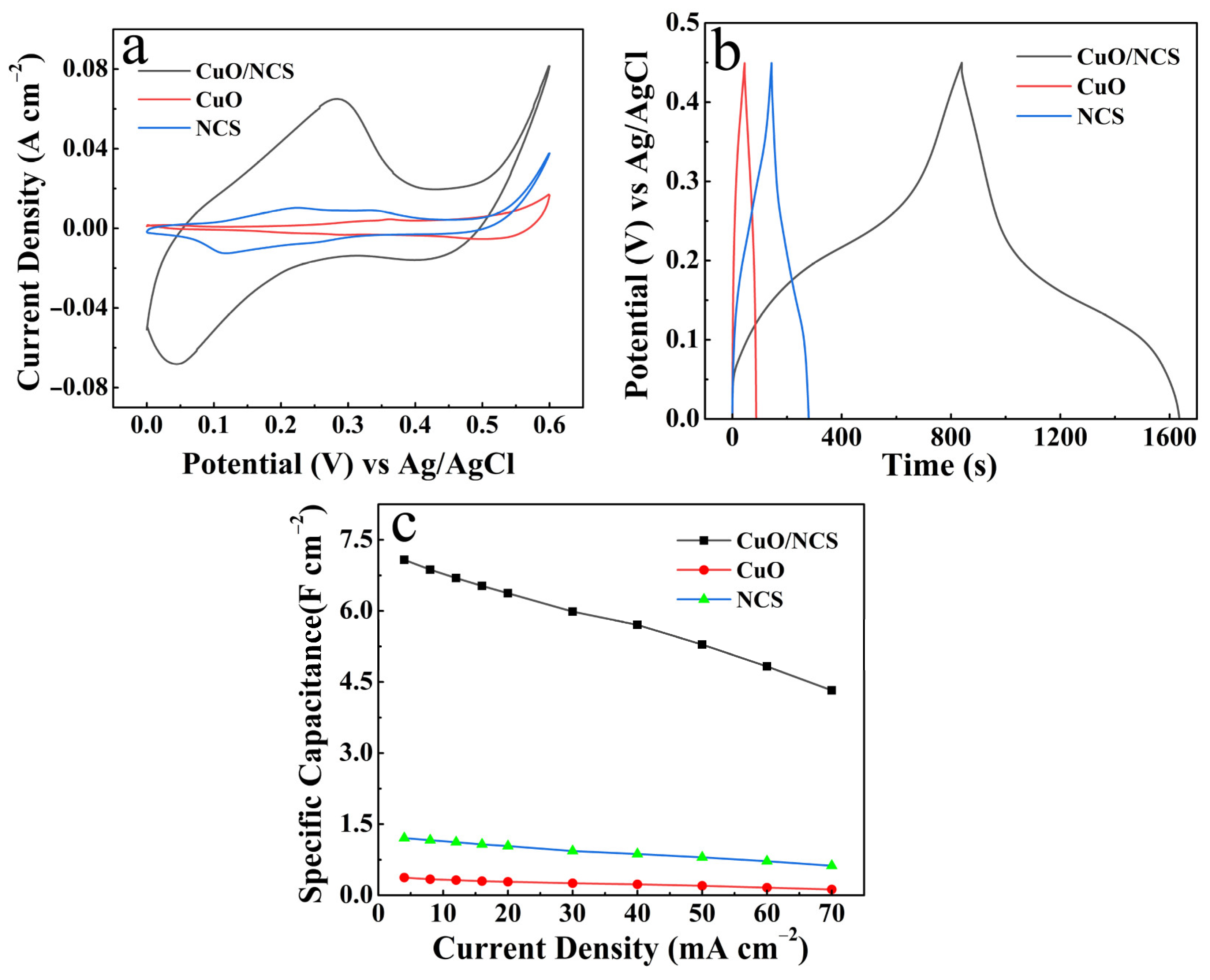
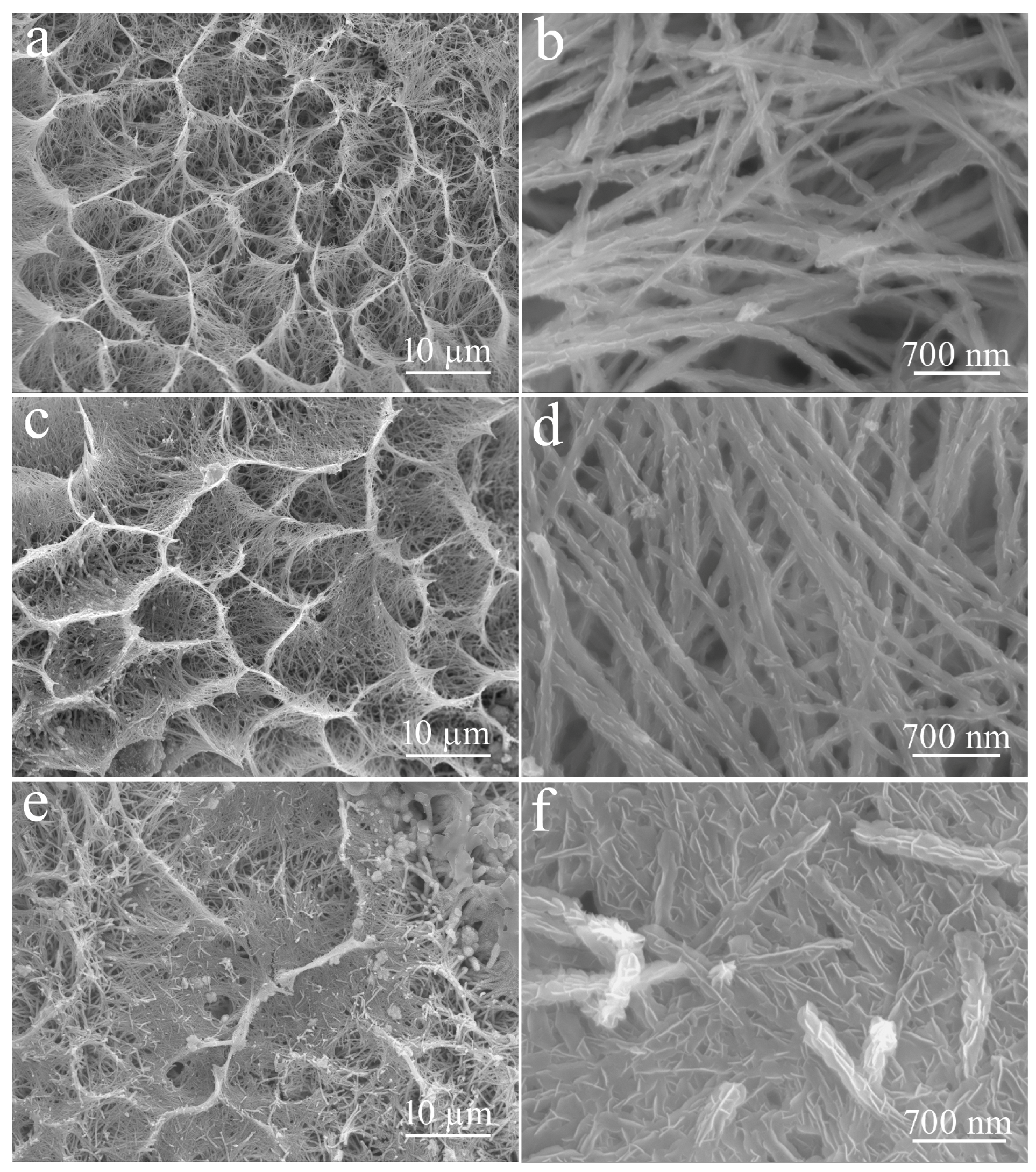
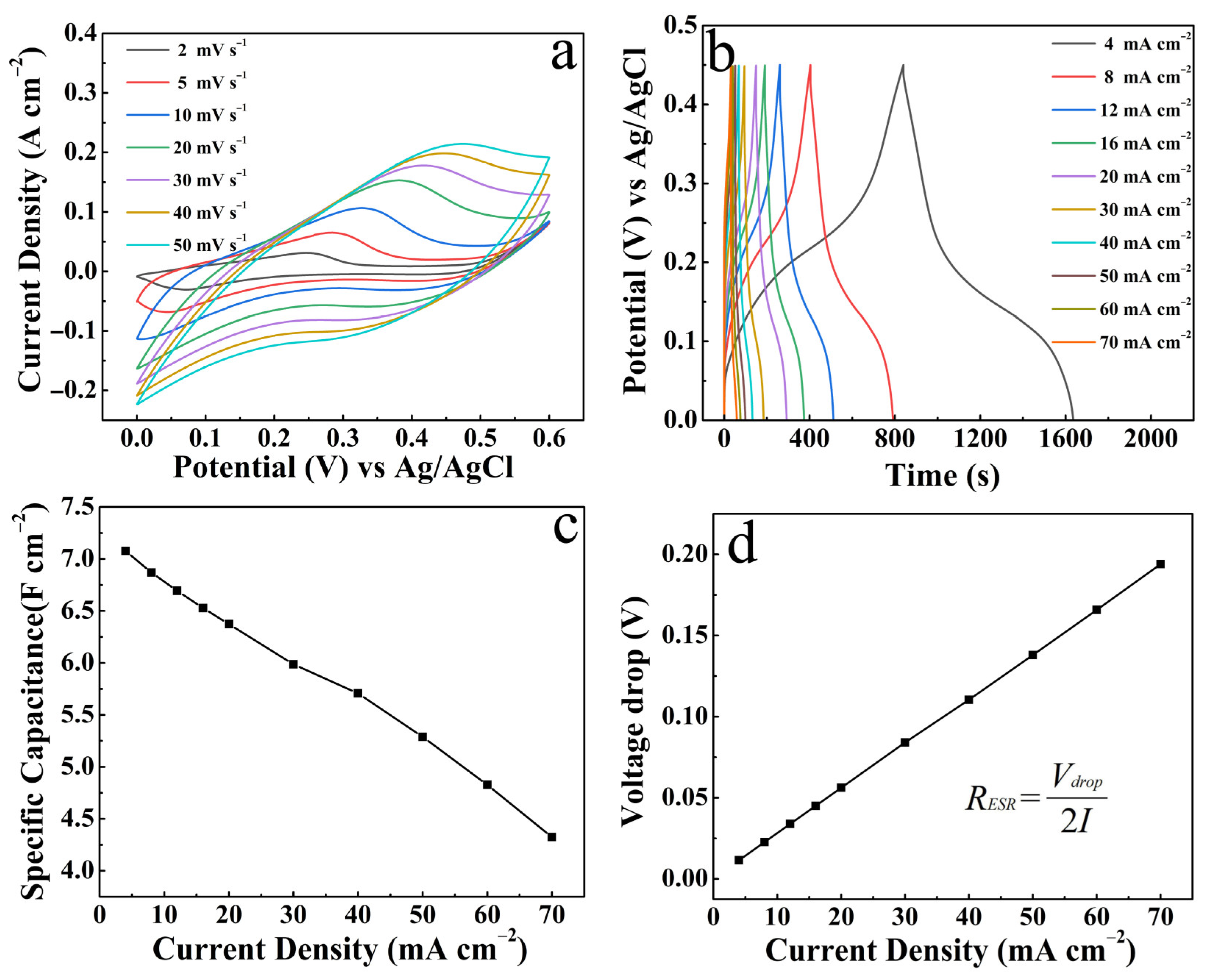
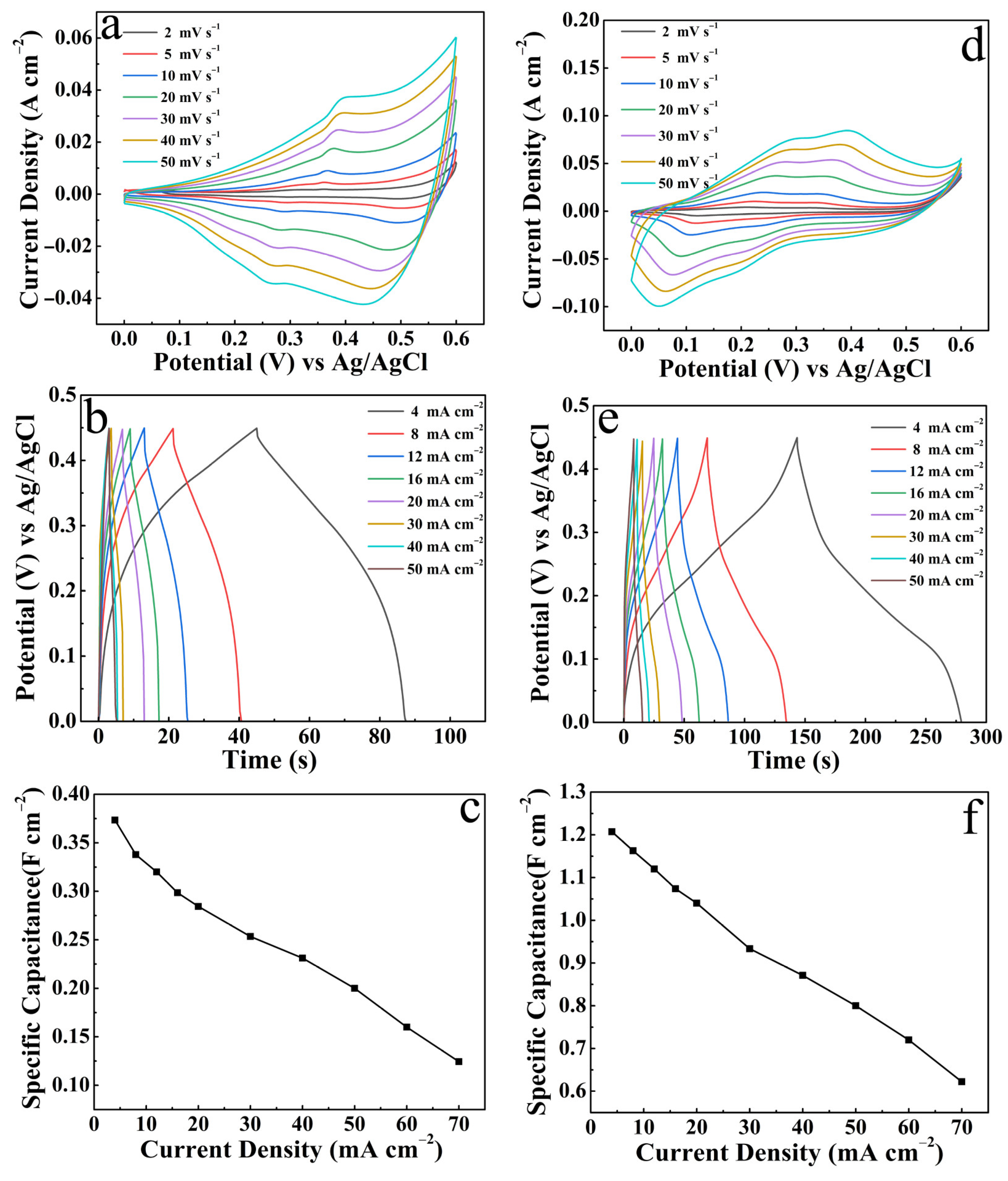
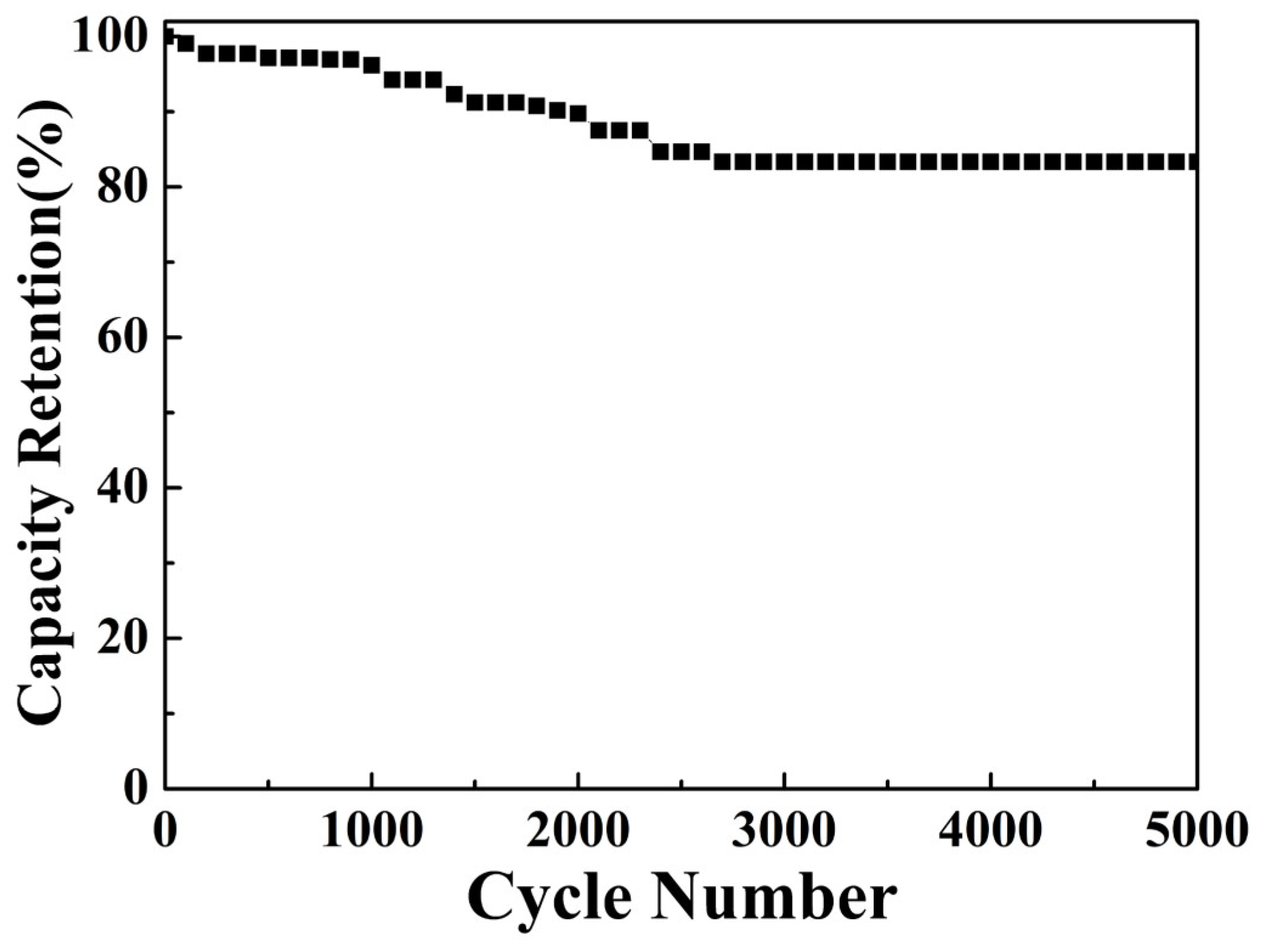
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hae, W.P.; Kwang, C.R. Recent advances in and perspectives on pseudocapacitive materials for Supercapacitors-A review. J. Power Sources 2023, 557, 232558. [Google Scholar]
- Xue Li, X.; Jianning Ren, J.N.; Deepak, S.; Xu, B.B.; Algadi, H.; Zeinhom, M.E.; Ma, Y.; Li, T.X.; Guo, Z.H. Progress of layered double hydroxides-based materials for supercapacitors. Mater. Chem. Front. 2023, 7, 1520–1561. [Google Scholar]
- Liang, C.L.; Wang, S.Z.; Sha, S.Y.; Lv, S.Y.; Wang, G.D.; Wang, B.F.; Li, Q.B.; Yu, J.X.; Xu, X.G.; Zhang, L. Novel semiconductor materials for advanced supercapacitors. J. Mater. Chem. C 2023, 11, 4288–4317. [Google Scholar]
- Zhang, J.J.; Zhang, T.A.; Feng, S. Mechanism investigation of α-Ni(OH)2 electrodeposition from a NiCl2 solution. Int. J. Hydrogen Energy 2021, 46, 41–49. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Cheng, Z.X.; Wang, X.L.; Dou, S.X.; Kong, X.Y. High area-specific capacitance of Co(OH)2/hierarchical nickel/nickel foam supercapacitors and its increase with cycling. J. Mater. Chem. A 2017, 5, 7968–7978. [Google Scholar] [CrossRef]
- He, D.; Wang, G.D.; Liu, G.L.; Bai, J.H.; Suo, H.; Zhao, Z. Facile route to achieve mesoporous Cu(OH)2 nanorods on copper foam for high-performance supercapacitor electrode. J. Alloys Compd. 2017, 699, 706–712. [Google Scholar] [CrossRef]
- Chen, J.Z.; Xu, J.L.; Zhou, S.Z.; Zhao, N.; Wong, C.P. Facile and scalable fabrication of three-dimensional Cu(OH)2 nanoporous nanorods for solid-state supercapacitors. J. Mater. Chem. A 2015, 3, 17385–17391. [Google Scholar] [CrossRef]
- Boyou Wang, B.Y.; Cao, B.H.; Wang, C.; Zhang, Y.B.; Yao, H.F.; Wang, Y.Q. The Optical and Electrical Performance of CuO Synthesized by Anodic Oxidation Based on Copper Foam. Materials 2020, 13, 5411. [Google Scholar] [CrossRef]
- Ambrish, K.; Arpit, T.; Mayank, G.; Gopinath, P.; Harpreet, S.G.; Harpreet, S.A. High performance CuO@brass supercapacitor electrodes through surface activation. J. Mater. Chem. A 2021, 9, 9327–9336. [Google Scholar]
- Xu, L.N.; Zhang, H.; Li, J.; Guo, X.; Sun, H.B.; Li, Y.A.; Wu, T. Designing core-shell Ni(OH)2@CuO nanowire arrays on three dimensional copper foams for high-performance asymmetric supercapacitors. ChemElectroChem 2019, 6, 5462–5468. [Google Scholar] [CrossRef]
- Pei, N.B.; Liu, J.X.; Ma, H.S.; Chen, Z.Q.; Zhang, P.; Zhao, J.B. Silver Copper Oxide Nanowires by Electrodeposition for Stable Lithium Metal Anode in Carbonate-Based Electrolytes. ACS Sustain. Chem. Eng. 2022, 10, 7196–7204. [Google Scholar] [CrossRef]
- Chen, F.; Chen, C.; Hu, Q.; Xiang, B.; Song, T.T.; Zou, X.F.; Li, W.N.; Xiong, B.X.; Deng, M.S. Synthesis of CuO@CoNi LDH on Cu foam for high-performance supercapacitors. Chem. Eng. J. 2020, 401, 126145. [Google Scholar] [CrossRef]
- Liu, G.L.; He, X.; He, D.; Cui, B.Y.; Zhu, L.; Suo, H. Construction of CuO@Ni-Fe layered double hydroxide hierarchical core-shell nanorods arrays on copper foam for high-performance Supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 30, 2080–2088. [Google Scholar]
- Zheng, L.X.; Song, J.L.; Ye, X.Y.; Wang, Y.Z.; Shi, X.W.; Zheng, H.J. Construction of self-supported hierarchical NiCo-S nanosheet arrays for supercapacitors with ultrahigh specific capacitance. Nanoscale 2020, 12, 13811–13821. [Google Scholar] [CrossRef]
- Zhao, J.P.; Wang, Y.H.; Qian, Y.D.; Jin, H.L.; Tang, X.Y.; Huang, Z.M.; Lou, J.Y.; Zhang, Q.C.; Lei, Y.; Shun Wang, S. Hierarchical Design of Cross-Linked NiCo2S4 Nanowires Bridged NiCo-Hydrocarbonate Polyhedrons for High Performance Asymmetric Supercapacitor. Adv. Funct. Mater. 2022, 33, 2210238. [Google Scholar] [CrossRef]
- Liang, Y.T.; Zeng, Y.X.; Tang, X.F.; Xia, W.; Song, B.; Yao, F.B.; Yang, Y.; Chen, Y.S.; Peng, C.X.; Zhou, C.Y.; et al. One-step synthesis of Cu(OH)2-Cu/Ni foam cathode for electrochemical reduction of nitrate. Chem. Eng. J. 2023, 451, 138936. [Google Scholar]
- Tauquir, S.M.; Karnan, M.; Subramani, K.; Sathish, M. One-step superficial electrodeposition of nickel-cobalt-sulfide for high-energy hybrid asymmetric supercapacitor. Mater. Lett. 2022, 323, 132563. [Google Scholar] [CrossRef]
- Pu, X.L.; Ren, X.H.; Yin, H.F.; Tang, Y.; Yuan, H.D. One-step electrodeposition strategy for growing nickel cobalt hydroxysulfide nanosheets for supercapacitor application. J. Alloys Compd. 2021, 865, 158736. [Google Scholar] [CrossRef]
- Wang, X.M.; Deng, Y.Y.; Wang, Z.H.; Li, Z.W.; Wang, L.C.; Ouyang, J.; Zhou, C.; Luo, Y.F. Sophora-like Nickel-Cobalt Sulfifide and Carbon Nanotube Composites in Carbonized Wood Slice Electrodes for All-Solid-State Supercapacitors. ACS Appl. Energy Mater. 2022, 5, 7400–7407. [Google Scholar] [CrossRef]
- Dai, M.Z.; Zhao, D.P.; Liu, H.Q.; Zhu, X.F.; Wu, X.; Wang, B. Nanohybridization of Ni-Co-S Nanosheets with ZnCo2O4 Nanowires as Supercapacitor Electrodes with Long Cycling Stabilities. ACS Appl. Energy Mater. 2021, 4, 2637–2643. [Google Scholar] [CrossRef]
- Krishnan, S.A.; Su, Y.Z.; Wang, P.J.; Panitat, H.; Wu, J.H.; Hsieh, C.K.; Chang, J.K.; Lin, J.Y. Free-standing 3D core-shell architecture of Ni3S2@NiCoP as an efficient cathode material for hybrid supercapacitors. J. Colloid Interf. Sci. 2022, 625, 565–575. [Google Scholar]
- Fu, S.Q.; Yang, X.C.; Zhao, P.D.; Yao, X.; Jiao, Z.; Cheng, L.L. Regulable Electron Transfer on ZnS/CoS2/CC Prepared by an MOF-on-MOF Strategy for Robust LIB Performance. ACS Appl. Energy Mater. 2022, 5, 5159–5169. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Yu, Y.; Mu, Z.C.; Wang, Y.H.; Usman, A.; Jing, S.Y.; Xing, S.X. Urea-assisted enhanced electrocatalytic activity of MoS2-Ni3S2 for overall water splitting. Inorg. Chem. Front. 2020, 7, 3588–3597. [Google Scholar] [CrossRef]
- He, D.; Wan, J.N.; Liu, G.L.; Suo, H.; Zhao, C. Design and construction of hierarchical α-Co(OH)2-coated ultra-thin ZnO flower nanostructures on nickel foam for high performance supercapacitors. J. Alloys Compd. 2020, 838, 155556–155564. [Google Scholar] [CrossRef]
- Wang, L.; You, J.H.; Zhao, Y.; Bao, W.T. Core-shell CuO@NiCoMn-LDH supported by copper foam for high-performance supercapacitors. Dalton Trans. 2022, 51, 3314–3322. [Google Scholar] [CrossRef]
- He, D.; Wan, J.N.; Suo, H.; Zhao, C. In situ facile surface oxidation method prepared ball of yarn-like copper oxide hierarchical microstructures on copper foam for high performance supercapacitor. Mater. Lett. 2016, 185, 165–168. [Google Scholar] [CrossRef]
- Wen, Y.X.; Liu, Y.P.; Wang, T.; Wang, Z.L.; Zhang, Y.A.; Wu, X.G.; Chen, X.T.; Peng, S.L.; He, D.Y. High-Mass-Loading Ni-Co-S Electrodes with Unfading Electrochemical Performance for Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 6531–6541. [Google Scholar] [CrossRef]
- Maile, N.C.; Shinde, S.K.; Koli, R.R.; Fulari, A.V.; Kim, D.Y.; Fulari, V.J. Effect of different electrolytes and deposition time on the supercapacitor properties of nanoflake-like Co(OH)2 electrodes. Ultrason. Sonochem. 2019, 51, 49–57. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Cheng, Z.; Chi, Y.; Wang, H.; Chu, X.; Zhao, Y.; Wu, B.; Wang, R.; Zhang, Z.; Wang, C.; et al. Facile Route to Achieve a Hierarchical CuO/Nickel-Cobalt-Sulfide Electrode for Energy Storage. Micromachines 2023, 14, 2095. https://doi.org/10.3390/mi14112095
Lv S, Cheng Z, Chi Y, Wang H, Chu X, Zhao Y, Wu B, Wang R, Zhang Z, Wang C, et al. Facile Route to Achieve a Hierarchical CuO/Nickel-Cobalt-Sulfide Electrode for Energy Storage. Micromachines. 2023; 14(11):2095. https://doi.org/10.3390/mi14112095
Chicago/Turabian StyleLv, Sa, Zhifei Cheng, Yaodan Chi, Huan Wang, Xuefeng Chu, Yang Zhao, Boqi Wu, Runsheng Wang, Zhiwen Zhang, Chao Wang, and et al. 2023. "Facile Route to Achieve a Hierarchical CuO/Nickel-Cobalt-Sulfide Electrode for Energy Storage" Micromachines 14, no. 11: 2095. https://doi.org/10.3390/mi14112095
APA StyleLv, S., Cheng, Z., Chi, Y., Wang, H., Chu, X., Zhao, Y., Wu, B., Wang, R., Zhang, Z., Wang, C., Yang, J., & Yang, X. (2023). Facile Route to Achieve a Hierarchical CuO/Nickel-Cobalt-Sulfide Electrode for Energy Storage. Micromachines, 14(11), 2095. https://doi.org/10.3390/mi14112095




