High-Temperature Failure Evolution Analysis of K-Type Film Thermocouples
Abstract
:1. Introduction
2. Materials and Methods
3. Results
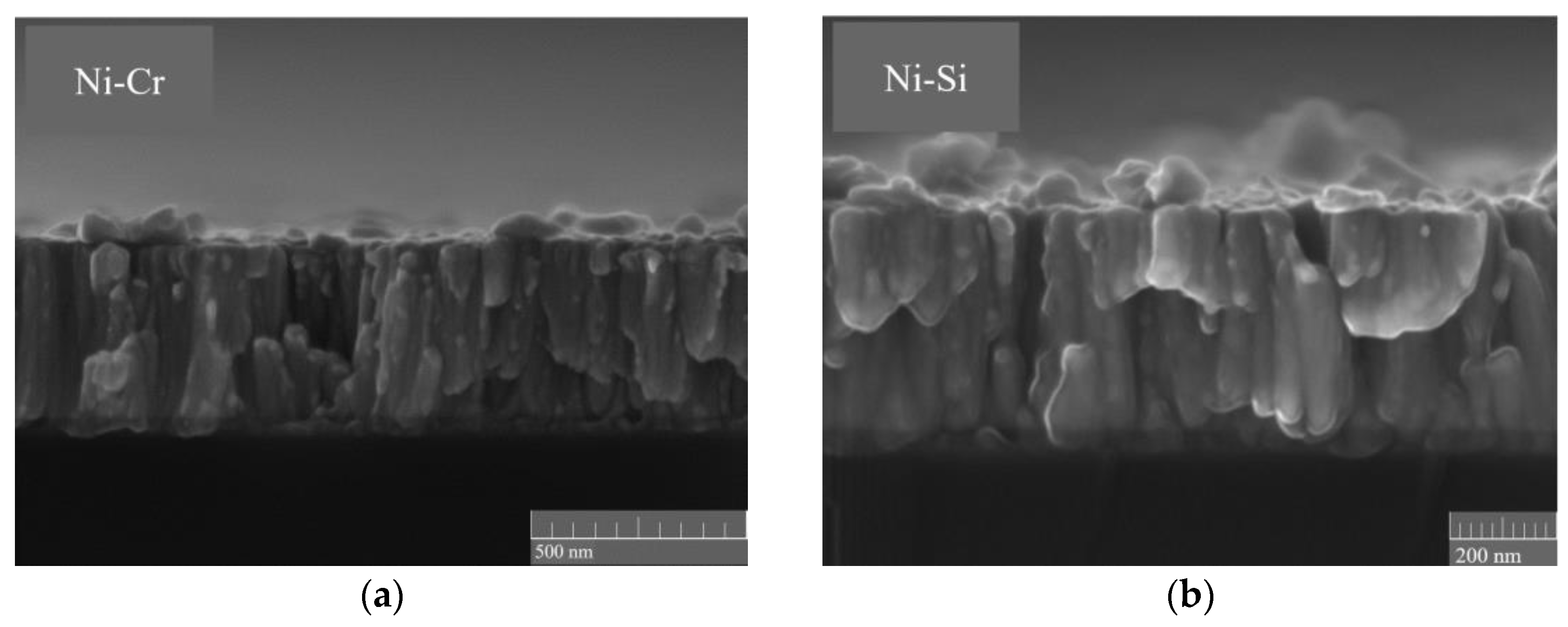
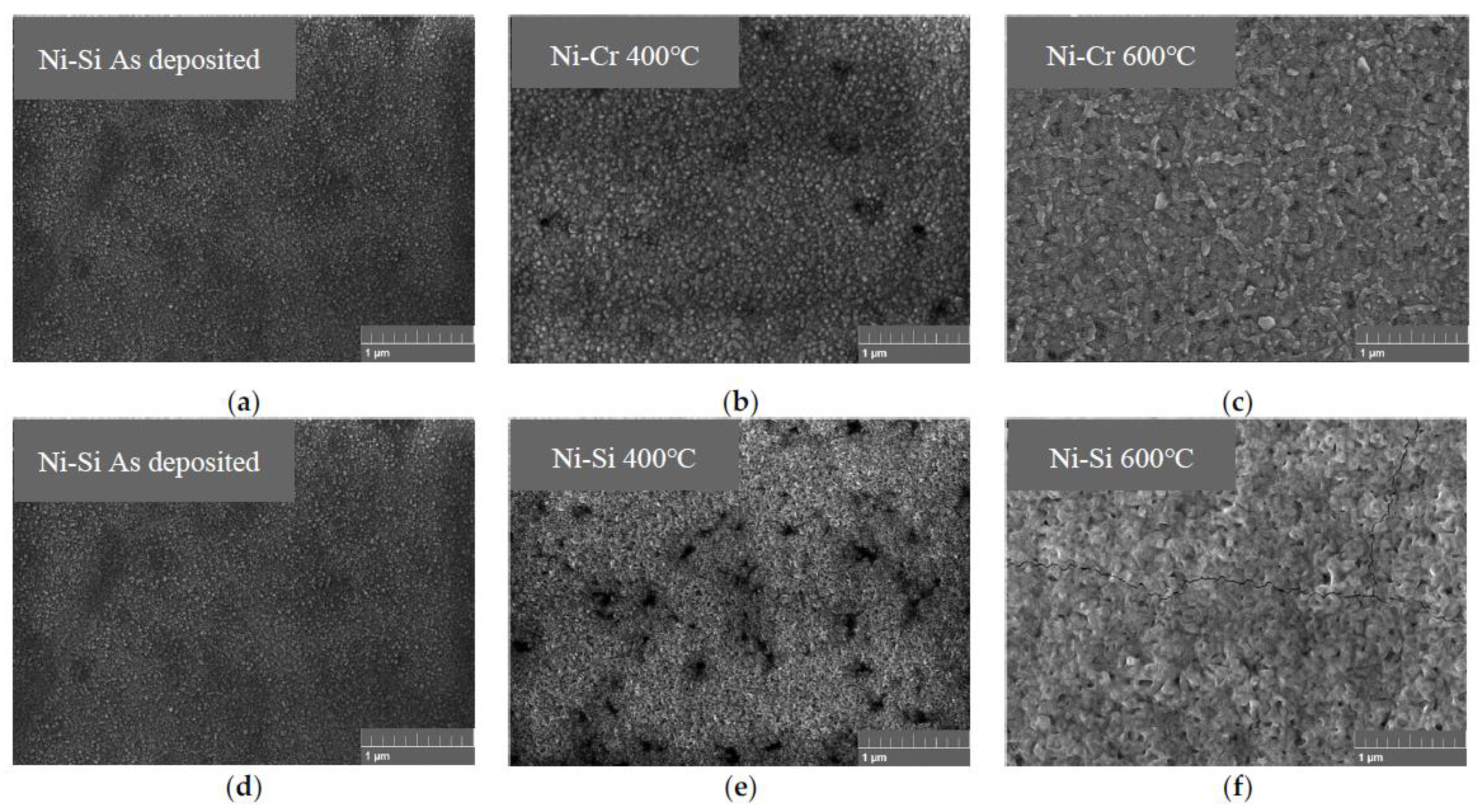
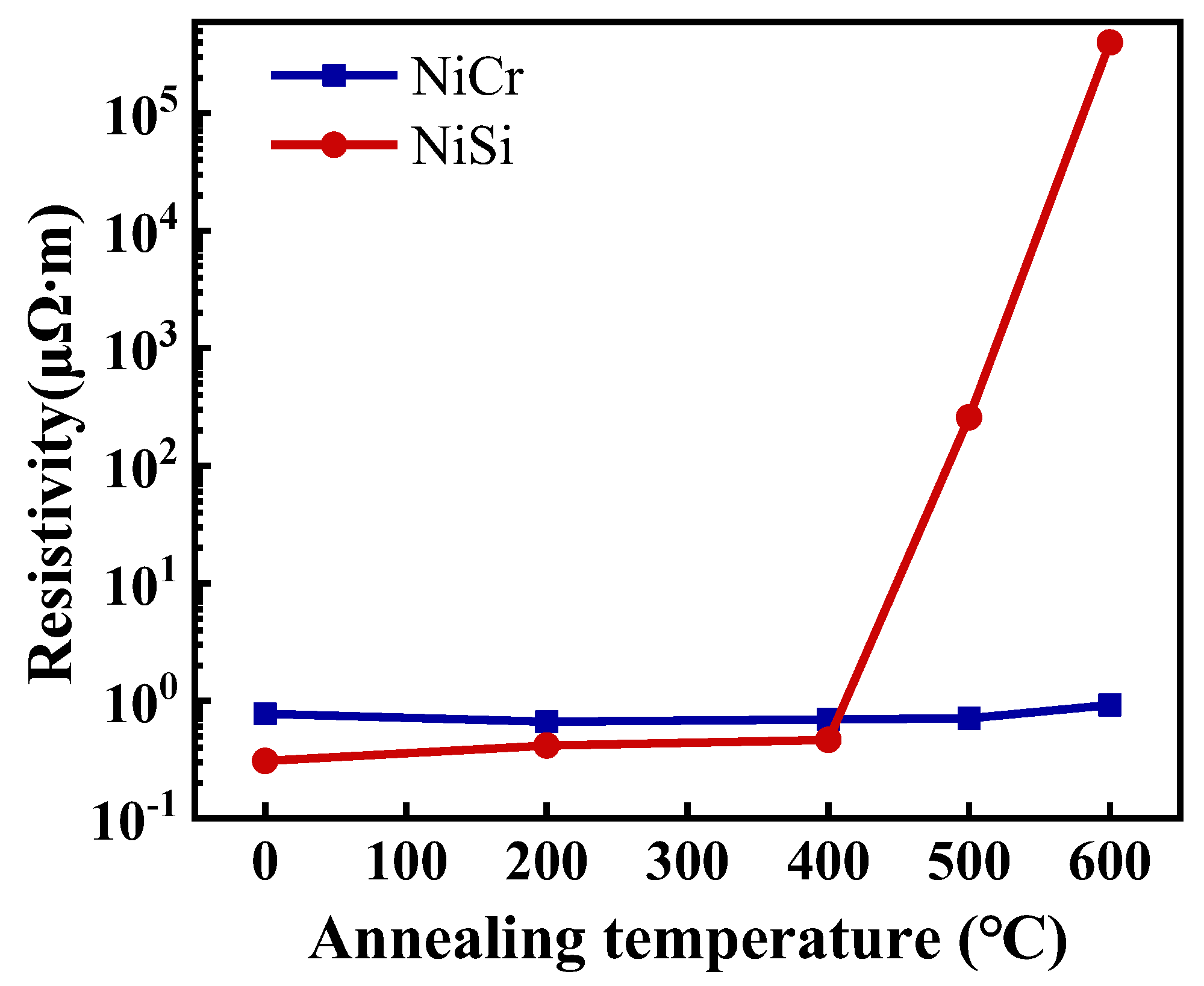
3.1. Characterization and Testing of the NiCr and NiSi Thin Films
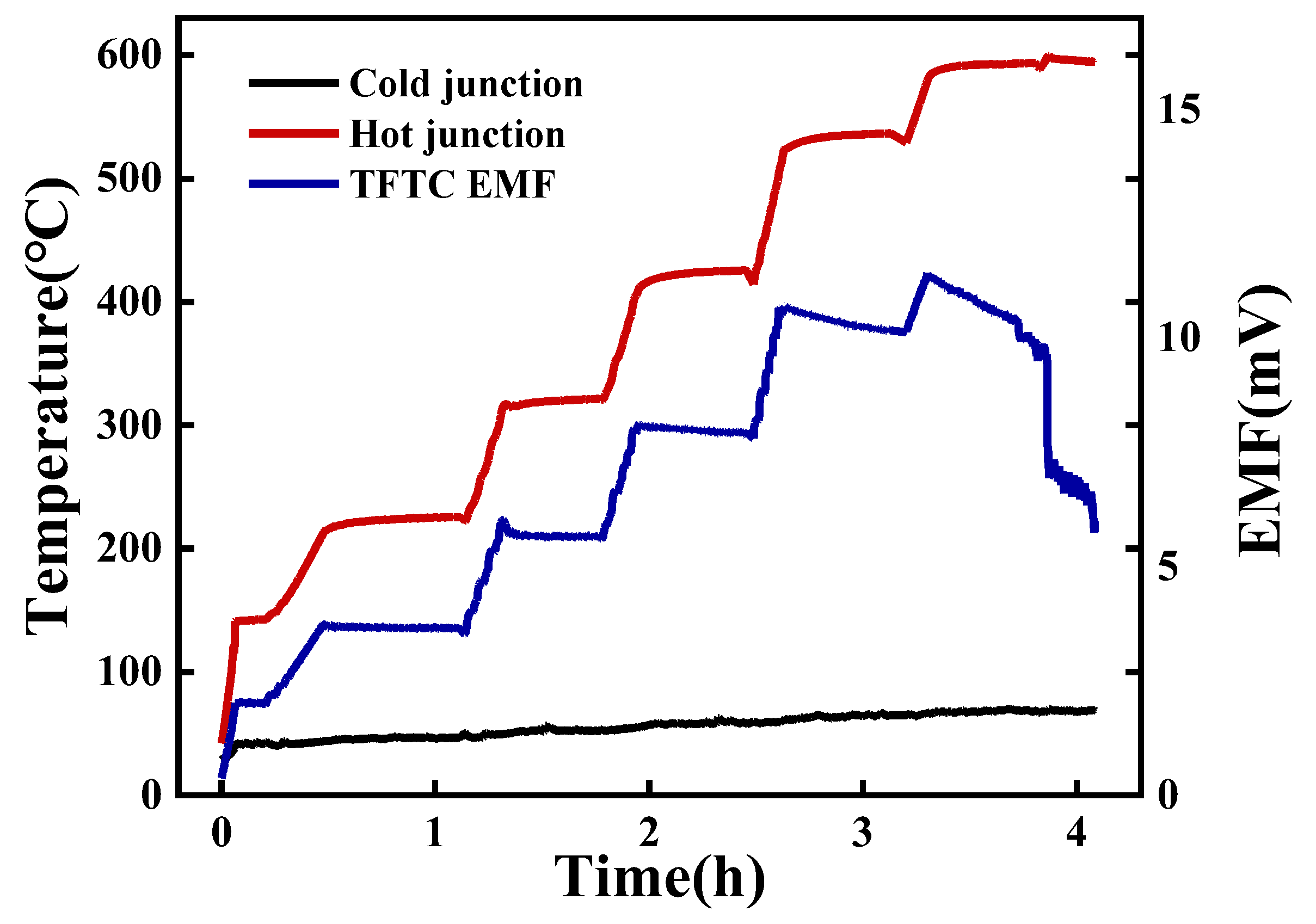
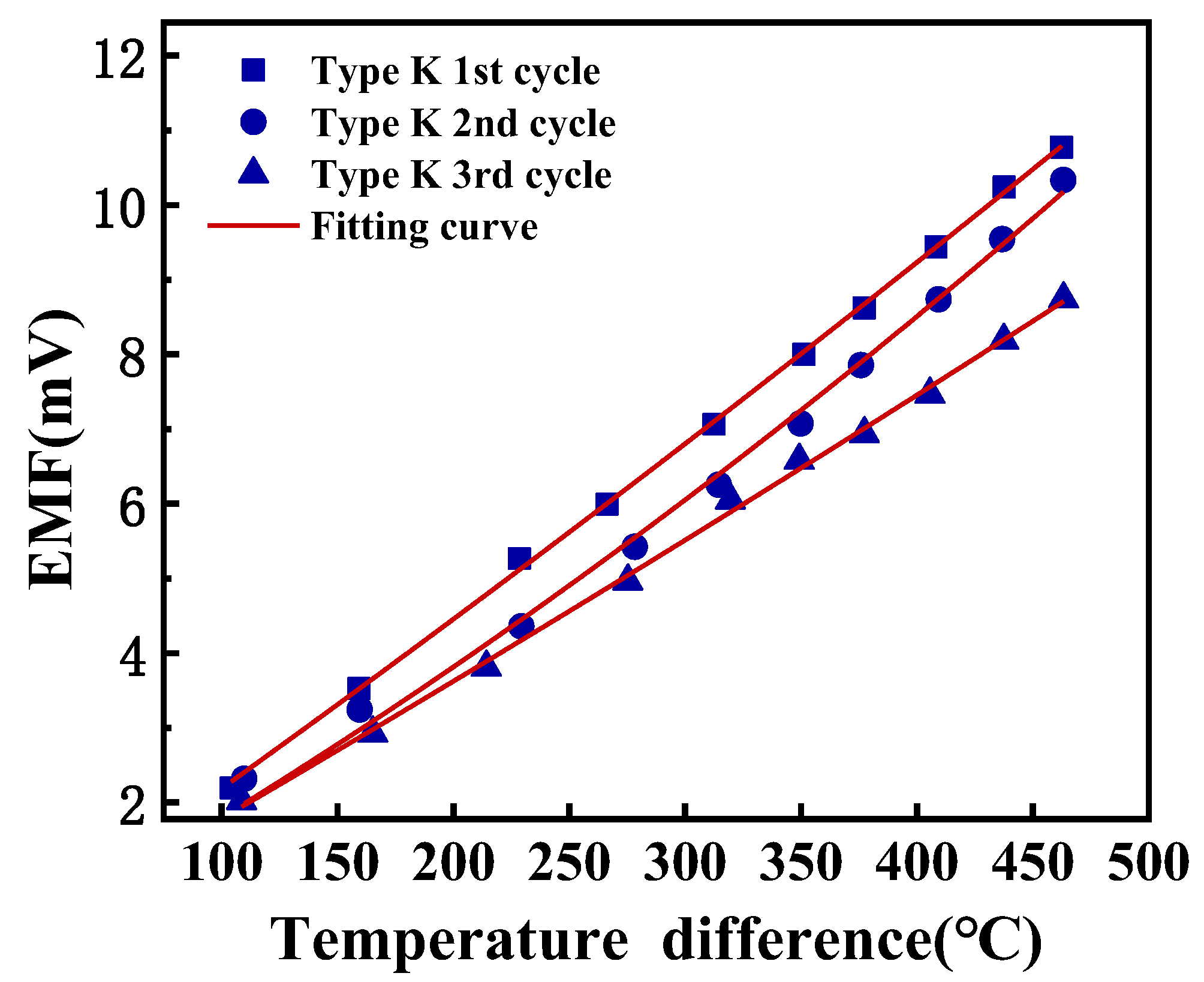
3.2. Thermoelectric Properties of Ni-Cr and Ni-Si Thin-Film Thermocouples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, Y. Dynamic Calibration of the Cutting Temperature Sensor of NiCr/NiSi Thin-Film Thermocouple. Chin. J. Mech. Eng. 2011, 24, 73–77. [Google Scholar] [CrossRef]
- Li, T.; Shi, T.; Tang, Z.; Liao, G.; Duan, J.; Han, J.; He, Z. Real-time tool wear monitoring using thin-film thermocouple. J. Mater. Process. Technol. 2021, 288, 116901. [Google Scholar] [CrossRef]
- Li, T.; Shi, T.; Tang, Z.; Liao, G.; Han, J.; Duan, J. Temperature monitoring of the tool-chip interface for PCBN tools using built-in thin-film thermocouples in turning of titanium alloy. J. Mater. Process. Technol. 2020, 275, 116376. [Google Scholar] [CrossRef]
- Zeng, Q.Y.; Sun, B.Y.; Xu, J.; Deng, X.L.; Xu, J.; Jia, Y. Development of NiCr/NiSi thin-film thermocouple sensor for workpiece temperature measurement in chemical explosive material machining. J. Manuf. Sci. Eng.-Trans. ASME 2006, 128, 175–179. [Google Scholar] [CrossRef]
- Ji, Z.; Duan, F.L.; Xie, Z. Transient Measurement of Temperature Distribution Using Thin Film Thermocouple Array on Turbine Blade Surface. IEEE Sens. J. 2021, 21, 207–212. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.; Zhao, X.; Deng, X.; Zhang, W. High temperature electrical insulation and adhesion of nanocrystalline YSZ/Al2O3 composite film for thin-film thermocouples on Ni-based superalloy substrates. Appl. Surf. Sci. 2022, 579, 152169. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.; Zhao, X.; Liu, B.; Jia, Z.; Deng, X.; Zhang, W. High temperature protection performance of sandwich structure Al2O3/Si3N4/YAlO multilayer films for Pt–Pt10%Rh thin film thermocouples. Ceram. Int. 2022, 48, 33943–33948. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, B.; Yu, Q.; Shi, P.; Lin, Q.; Zhao, N.; Jing, W.; Jiang, Z. Range Analysis of Thermal Stress and Optimal Design for Tungsten-Rhenium Thin Film Thermocouples Based on Ceramic Substrates. Sensors 2017, 17, 857. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, B.; Liu, Y.; Du, Z.; Lin, Q.; Jiang, Z. Thermoelectric Characteristics of Silicon Carbide and Tungsten-Rhenium-Based Thin-Film Thermocouples Sensor with Protective Coating Layer by RF Magnetron Sputtering. Materials 2019, 12, 1981. [Google Scholar] [CrossRef]
- Ruan, Y.; Xue, M.; Teng, J.; Wu, Y.; Shi, M. Horizontal Oxidation Diffusion Behavior of MEMS-Based Tungsten-Rhenium Thin Film Thermocouples. Materials 2022, 15, 5071. [Google Scholar] [CrossRef]
- Zhao, X.-H.; Wang, Y.-R.; Chen, Y.-Z.; Jiang, H.-C.; Zhang, W.-L. Enhanced thermoelectric property and stability of NiCr–NiSi thin film thermocouple on superalloy substrate. Rare Met. 2016, 36, 512–516. [Google Scholar] [CrossRef]
- Luo, L.; Zou, L.; Schreiber, D.K.; Baer, D.R.; Bruemmer, S.M.; Zhou, G.; Wang, C.-M. In-situ transmission electron microscopy study of surface oxidation for Ni–10Cr and Ni–20Cr alloys. Scr. Mater. 2016, 114, 129–132. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Cui, Y.; Ding, W. The oxidation process of NiSix film at high temperature in air and the antioxidant effect of SiOxNy/NiSix film. J. Mater. Sci. Mater. Electron. 2015, 26, 3291–3298. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, X.; Jiang, S.; Zhang, W.; Jiang, H. Microstructure Evolution and Thermoelectric Property of Pt-PtRh Thin Film Thermocouples. Crystals 2017, 7, 96. [Google Scholar] [CrossRef]
- Kreider, K.G.; DiMeo, F. Platinum/palladium thin-film thermocouples for temperature measurements on silicon wafers. Sens. Actuator A-Phys. 1998, 69, 46–52. [Google Scholar] [CrossRef]
- Tian, B.; Zhang, Z.; Shi, P.; Zheng, C.; Yu, Q.; Jing, W.; Jiang, Z. Tungsten-rhenium thin film thermocouples for SiC-based ceramic matrix composites. Rev. Sci. Instrum. 2017, 88, 015007. [Google Scholar] [CrossRef] [PubMed]
- Kreider, K.G.; Gillen, G. High temperature materials for thin-film thermocouples on silicon wafers. Thin Solid Film. 2000, 376, 32–37. [Google Scholar] [CrossRef]
- Tillmann, W.; Kokalj, D.; Stangier, D.; Schöppner, V.; Benis, H.B.; Malatyali, H. Influence of Cr-Content on the thermoelectric and mechanical properties of NiCr thin film thermocouples synthesized on thermally sprayed Al2O3. Thin Solid Film. 2018, 663, 148–158. [Google Scholar] [CrossRef]
- Bruno, E.; Ginatempo, B.; Giuliano, E.S.; Ruban, A.V.; Vekilov, Y.K. Fermi surfaces and electronic topological transitions in metallic solid-solutions. Phys. Rep.-Rev. Sec. Phys. Lett. 1994, 249, 353–419. [Google Scholar] [CrossRef]
- Khachaturyan, A.G. Problem of symmetry in statistical thermodynamics of substitutional and interstitial ordered solid-solutions. Phys. Status Solidi B-Basic Solid State Phys. 1973, 60, 9–37. [Google Scholar] [CrossRef]
- Petley, V.; Sathishkumar, S.; Raman, K.H.T.; Rao, G.M.; Chandrasekhar, U. Microstructural and mechanical characteristics of Ni-Cr thin films. Mater. Res. Bull. 2015, 66, 59–64. [Google Scholar] [CrossRef]
- Vinayak, S.; Vyas, H.P.; Vankar, V. Microstructure and electrical characteristics of Ni-Cr thin films. Thin Solid Film. 2007, 515, 7109–7116. [Google Scholar] [CrossRef]
- Zhang, W.; Brongersma, S.H.; Richard, O.; Brijs, B.; Palmans, R.; Froyen, L.; Maex, K. Influence of the electron mean free path on the resistivity of thin metal films. Microelectron. Eng. 2004, 76, 146–152. [Google Scholar] [CrossRef]
- Davidson, J.L.; Gibson, J.D.; Harris, S.A.; Rossiter, T.J. Fusing mechanism of nichrome thin films. In Proceedings of the 14th International Reliability Physics Symposium, Las Vegas, NV, USA, 20–22 April 1976; pp. 173–181. [Google Scholar] [CrossRef]
- Peck, M.A.; Langell, M.A. Comparison of Nanoscaled and Bulk NiO Structural and Environmental Characteristics by XRD, XAFS, and XPS. Chem. Mater. 2012, 24, 4483–4490. [Google Scholar] [CrossRef]
- Mu, S.L.; Li, N.; Li, D.Y.; Xu, L.Y. Corrosion behavior and composition analysis of chromate passive film on electroless Ni-P coating. Appl. Surf. Sci. 2010, 256, 4089–4094. [Google Scholar] [CrossRef]
- Scarioni, L.; Castro, E.M. Thermoelectric power in thin film Fe–CuNi alloy (type-J) couples. J. Appl. Phys. 2000, 87, 4337–4339. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.; Guo, S.; Wang, H.; Jiang, W.; Liu, S.; Liu, C.; Wang, N.; Cui, Y.; Ding, W. The preliminary exploration on change mechanism of Seebeck coefficient for NiCr/NiSi thin film thermocouple with different thickness. J. Alloys Compd. 2023, 931, 167573. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Jiang, H.C.; Zhang, W.L.; Liu, X.Z.; Jiang, S.W. Film thickness influences on the thermoelectric properties of NiCr/NiSi thin film thermocouples. Mod. Phys. Lett. B 2013, 27, 1350103. [Google Scholar] [CrossRef]
- Cattani, M.; Salvadori, M.C.; Vaz, A.R.; Teixeira, F.S.; Brown, I.G. Thermoelectric power in very thin film thermocouples: Quantum size effects. J. Appl. Phys. 2006, 100, 114905. [Google Scholar] [CrossRef]
- Cassinelli, M.; Muller, S.; Voss, K.O.; Trautmann, C.; Volklein, F.; Gooth, J.; Nielsch, K.; Toimil-Molares, M.E. Influence of surface states and size effects on the Seebeck coefficient and electrical resistance of Bi1-xSbx nanowire arrays. Nanoscale 2017, 9, 3169–3179. [Google Scholar] [CrossRef]
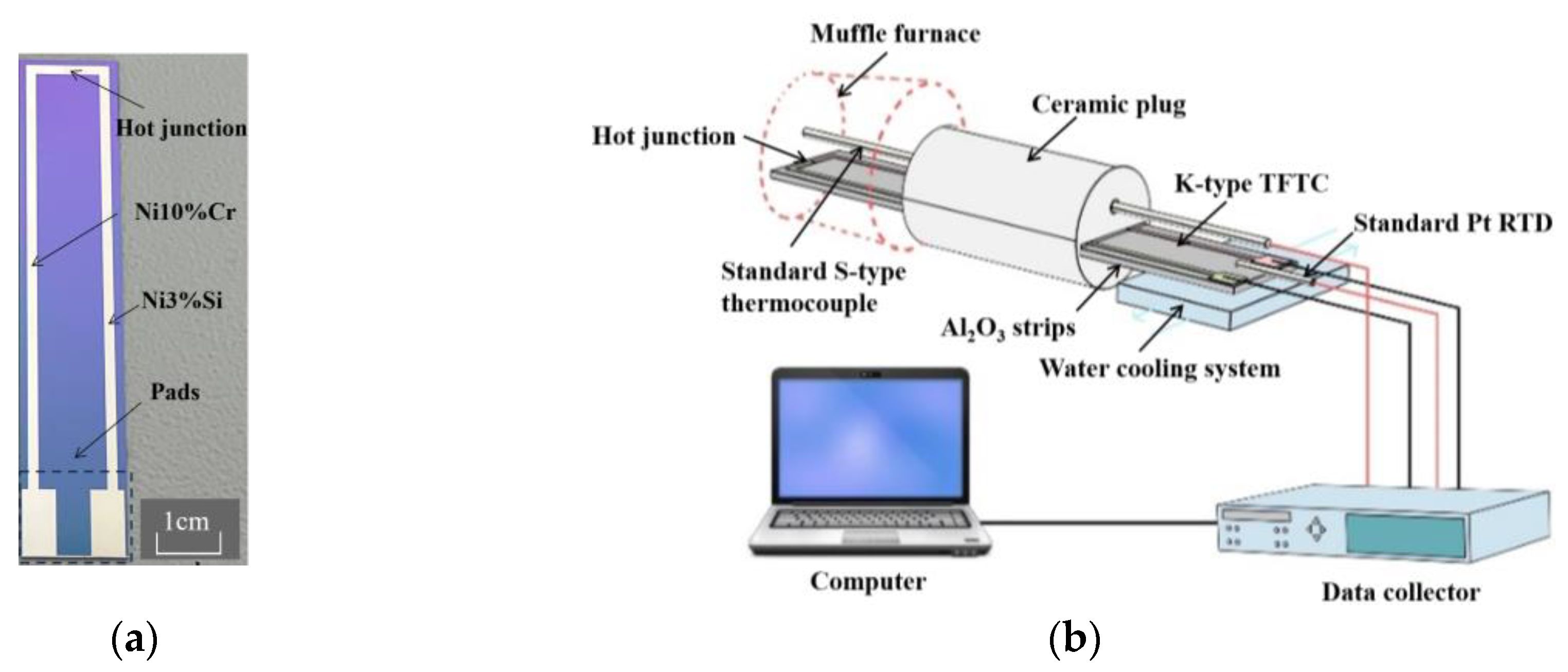
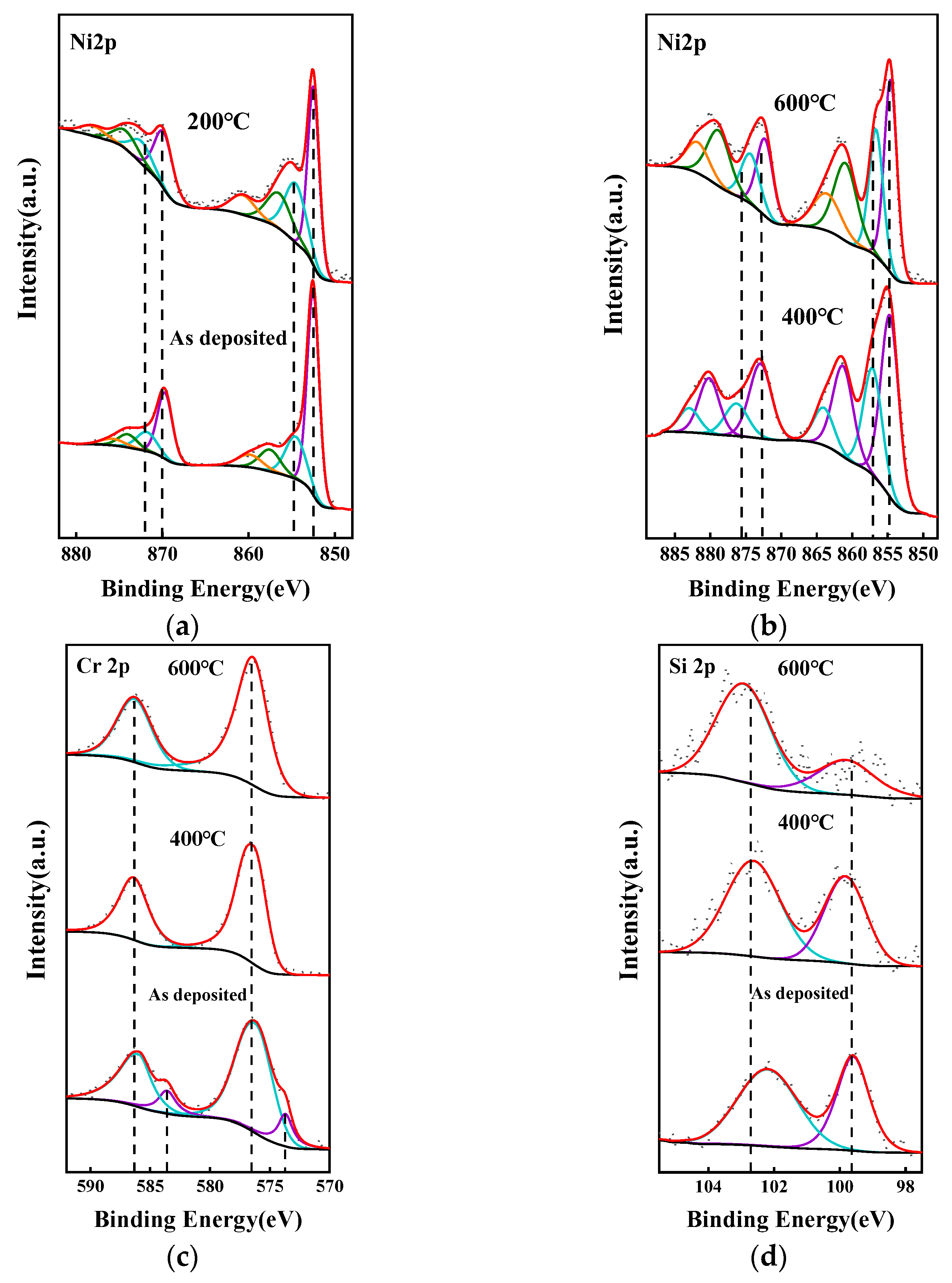
| Material | Target-Base Distance (mm) | Background Pressure (Torr) | Working Pressing (Torr) | Sputtering Power (W) | Ar2 Flow Rate (sccm) |
|---|---|---|---|---|---|
| NiCr | 65 | 5 × 10−6 | 6.1 × 10−3 | 180 | 25 |
| NiSi | 65 | 5 × 10−6 | 6.1 × 10−3 | 180 | 25 |
| Samples | Atomic % | ||
|---|---|---|---|
| Ni | Cr | O | |
| Ni-10%Cr annealed at 600 °C | 60.15 | 7.36 | 32.48 |
| Ni-10%Cr annealed at 400 °C | 72.47 | 9.37 | 18.17 |
| Ni-10%Cr as deposited | 80.22 | 9.95 | 9.83 |
| Coefficients of Polynomial V(T) = A(∆T)2 + B∆T + C | ||||
|---|---|---|---|---|
| Thermocouple | A (mV/°C2) | B (mV/°C) | C (mV) | Average Seebeck Coefficient (µV/°C) |
| Type K 1st cycle | 4.03 × 10−6 | 2.15 × 10−2 | 0 | 23.00 |
| Type K 2nd cycle | 1.09 × 10−5 | 1.69 × 10−2 | 0 | 21.04 |
| Type K 3rd cycle | 2.59 × 10−6 | 1.76 × 10−2 | 0 | 18.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, Y.; Li, J.; Xiao, Q.; Wu, Y.; Shi, M. High-Temperature Failure Evolution Analysis of K-Type Film Thermocouples. Micromachines 2023, 14, 2070. https://doi.org/10.3390/mi14112070
Ruan Y, Li J, Xiao Q, Wu Y, Shi M. High-Temperature Failure Evolution Analysis of K-Type Film Thermocouples. Micromachines. 2023; 14(11):2070. https://doi.org/10.3390/mi14112070
Chicago/Turabian StyleRuan, Yong, Jiaheng Li, Qian Xiao, Yu Wu, and Meng Shi. 2023. "High-Temperature Failure Evolution Analysis of K-Type Film Thermocouples" Micromachines 14, no. 11: 2070. https://doi.org/10.3390/mi14112070
APA StyleRuan, Y., Li, J., Xiao, Q., Wu, Y., & Shi, M. (2023). High-Temperature Failure Evolution Analysis of K-Type Film Thermocouples. Micromachines, 14(11), 2070. https://doi.org/10.3390/mi14112070







