Biosynthesis of Copper Nanoparticles with Medicinal Plants Extracts: From Extraction Methods to Applications
Abstract
:1. Introduction
2. Copper NPs
3. Green Synthesis of Nanoparticles
4. Potential of Medicinal Plants
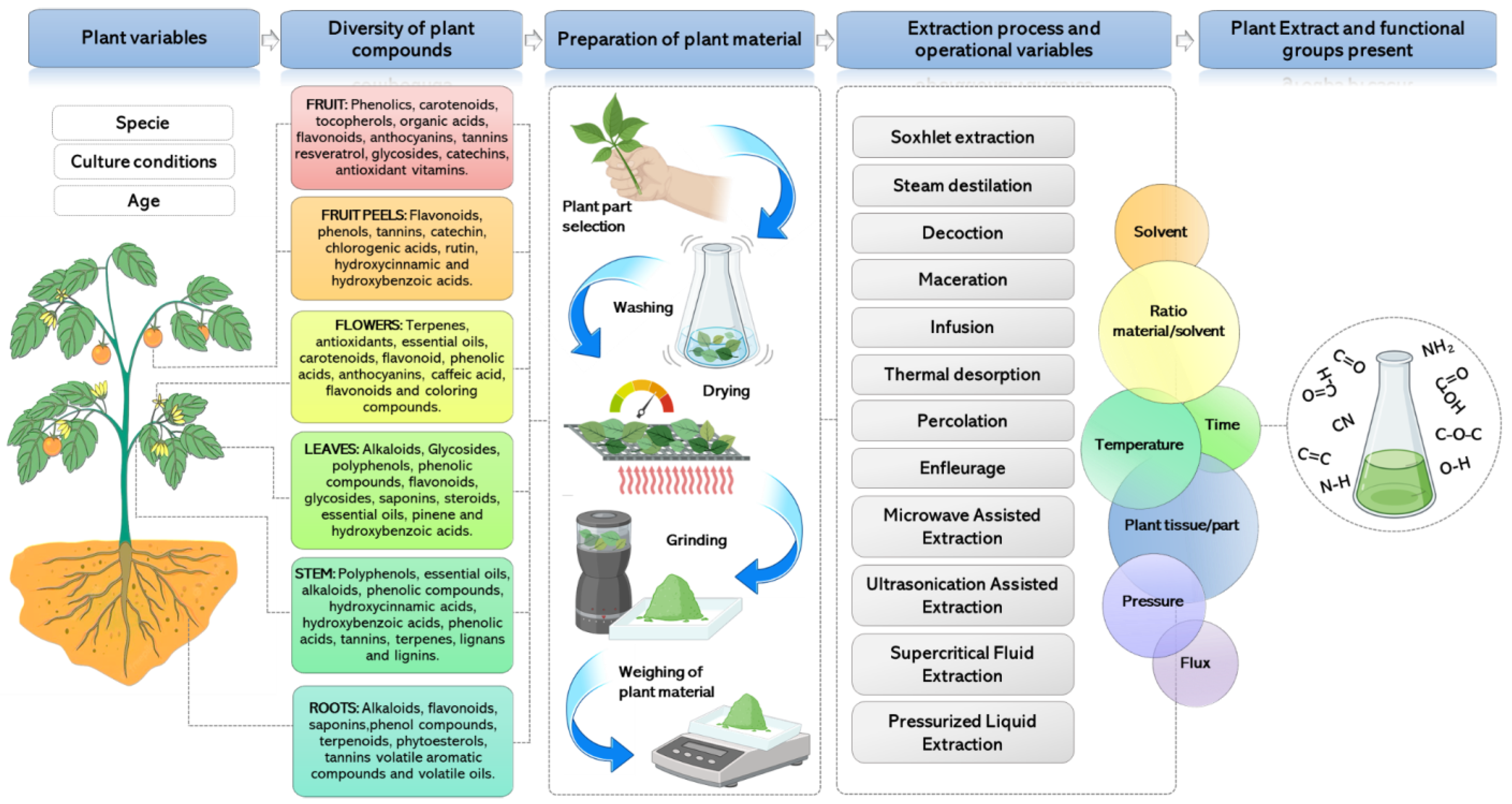
5. Characteristics of Medicinal Plant Extracts to Synthesize NPs
6. Extraction Methods to Obtain Plant-Derived Compounds
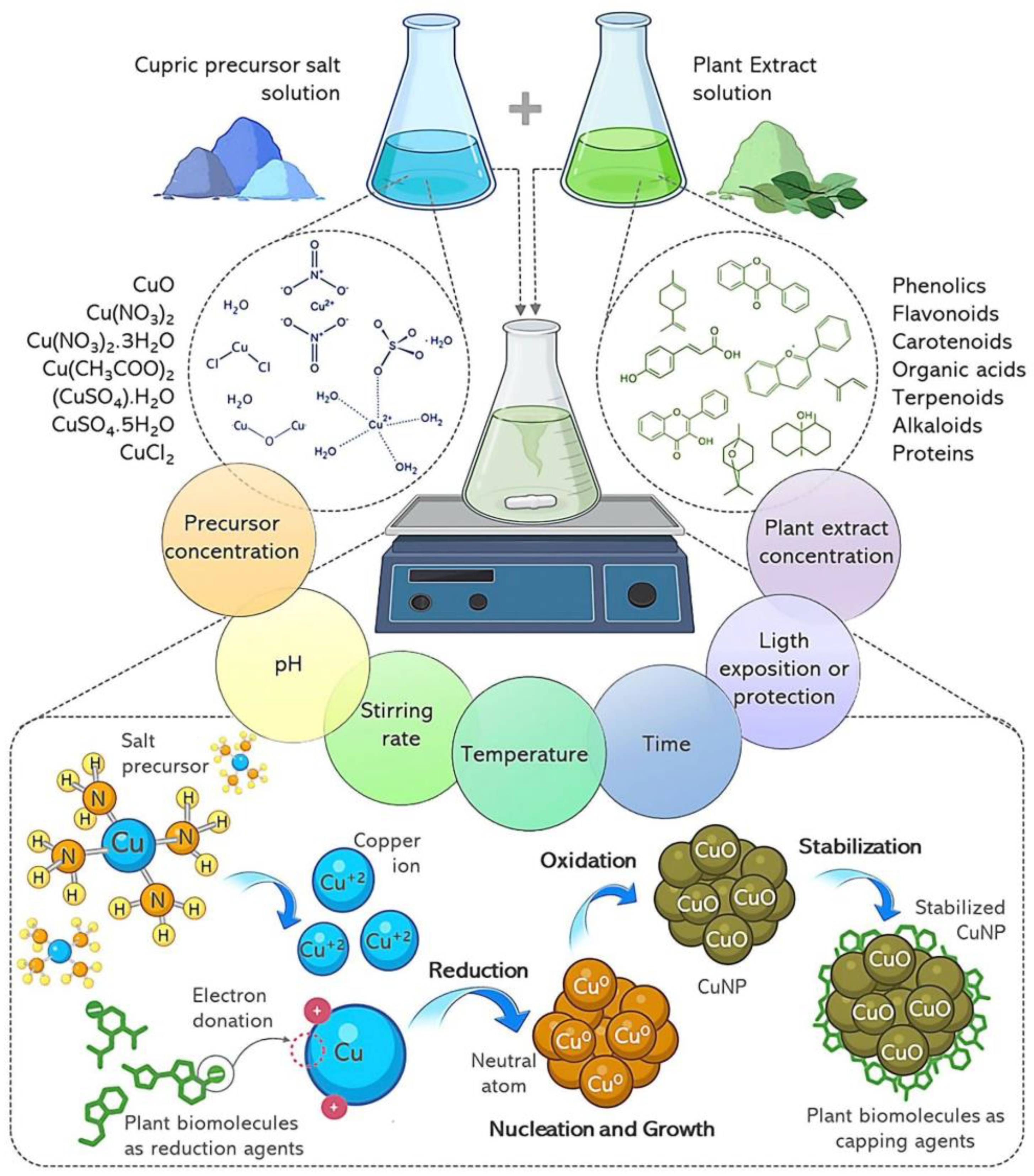
7. Mechanism of CuNP Biosynthesis with Plant Extracts
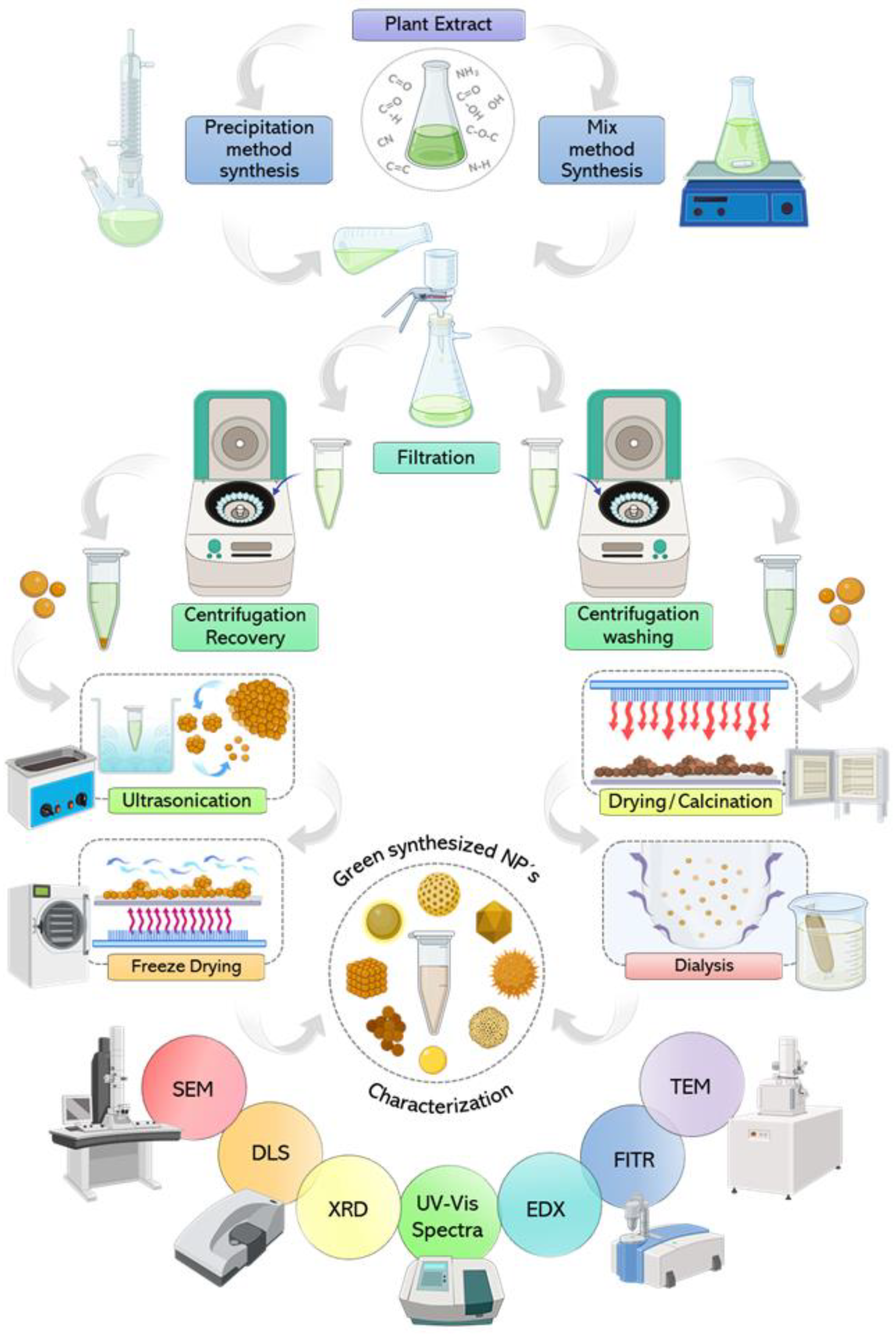
8. Downstream Process of NP Synthesis
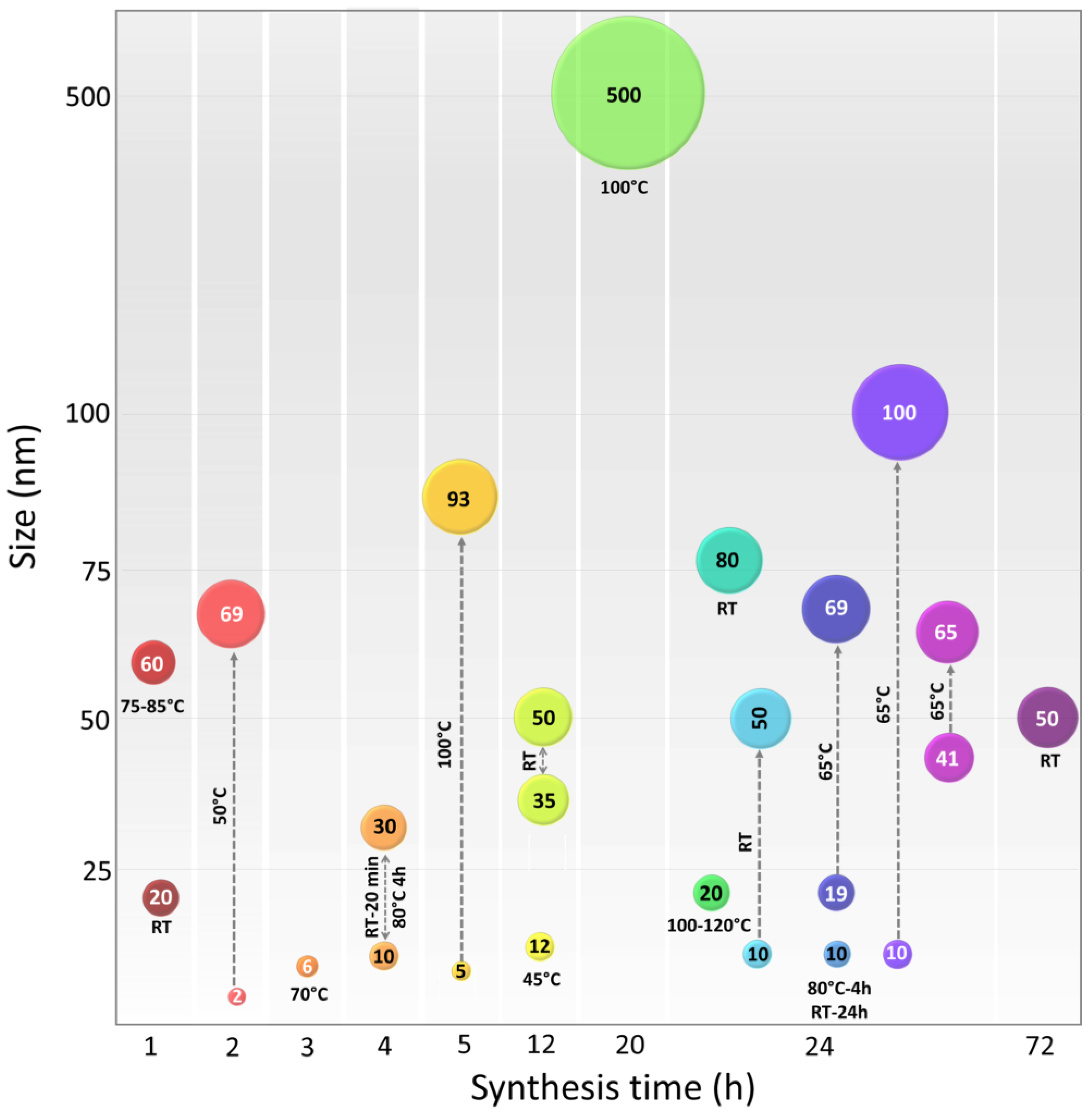
9. Relationship of the Synthesis Process Variables and CuNP Properties
| Plant | Extraction Procedure | Extract Phytochemical Compounds | Precursor | Synthesis Method | NPs Characteristics | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Azadirachta indica, Hibiscus rosa-sinensis, Murraya koenigii, Moringaoleifera, and Tamarindus indica | Powdered leaves were boiled with distilled water for 20 min at 60 °C. Cooled at RT and filtered. | Alkaloids, carbohydrates, flavonoids, glycosides, phenolic compounds, saponins, steroids, tannins, and volatile oils. | CuO | Mix of plant extract and precursor was boiled at 80 °C until the formation of a deep-green paste. The paste was heated at 400 °C for 2 h resulting in black colored powder. | Spherical with particle size range between 9.8 and 10.77 nm. | Antioxidant activity and cytotoxicity against four cancer cell lines such as human breast (MCF-7), S cervical (HeLa), epithelioma (Hep-2), and lung (A549). | [113] |
| Kigelia africana | Fruit extract was obtained by ethanol without thermal treatment for 48 h with light protection. | Alkaloids, anthraquinone, flavonoids, glycosides, phenols, quinones, saponins, steroids, tannins and terpenoids. | Cu(CH 3COO)2 | Mix of plant extract and precursor was stirred for 3 h and the absence of light for 24 h The mixture was centrifuged and the precipitate was washed and dried at 80 °C. | The study does not report the morphology or size of NPs, it focuses only on antimicrobial activity. | Antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi, Shigella sp., and Staphylococcus aureus. | [114] |
| Centella asiatica | Powdered leaves were infused in double distilled water at 80 °C for 30 min. Cooled at 4 °C and filtered. | Alkaloids, flavonoids, saponins, terpenoids, tannins, glycosides, carbohydrates, quinines, organic acids, centellose, phellandrene, and vitamin C. | CuCl2∙2H2O MnO2 | A mixture of MnO2 and plant extract was stirred for 20 min at room temperature. Following this, CuCl2 solution was added dropwise with vigorous stirring and heated for 4 h at 80 °C. | Cu/MnO2 nanocomposites, size range 10–30 nm. | High catalytic activity for the reduction of inorganic and organic dyes in aqueous media at ambient temperature. (Congo red, rhodamine B, and methylene blue). | [91] |
| Camelia sinensis | Powdered leaves were infused at 75–85 °C for 30 min and continuously stirred. The extract was centrifuged and the supernatant was separated for reaction. | Polyphenols, flavonoids, and alkaloids. | CuCl2 | Mix of plant extract and precursor salt was heated at 75–85 °C for 1 h and continuously stirred. The mixture was cooled at RT for 2 h and then centrifuged. | Agglomerated form with an average size of 60 ± 6 nm. | Efficient photocatalyst in dye degradation (using bromophenol blue). | [61] |
| Ageratum houstonianum | Fresh leaves were washed with water, then chopped and boiled at 60 °C for 20 min and filtered. | Alkaloids, flavonoids, tannins, triterpenes, diterpenes, steroids, and saponins. | CuCl2 | 3 mM solution of CuCl2 as precursor was stirred for 2 h; then mixed with leaf extract and stirred for another 24 h at room temperature. | Size around 80 nm, agglomerate, and not specific shape. NPs behave as a semiconductor. | Dye degradation against Congo red (azo dye). Antibacterial activity against E. coli (MTCC no. 40). | [94] |
| Ehretia acuminata | The fruit, leaves, and bark of E. acuminata were dried, ground, soaked and macerated with dichloromethane or methanol for 14 days. | Phenolic acids, steroids, terpenoids, polyphenolic compounds, tannins and flavonoids. | CuCl2·2H2O | L-ascorbic acid and precursor solution were mixed and heated at 100 °C continuously until the color changed (20 h). | NPs of 500 nm. The shape was not reported. Green NPs and phytochemicals were coated on a cotton textile surface. | Antiviral action was shown by the fabrics treated with CuNPs tested by coronavirus-infected Vero-E6 cultures. | [115] |
| Aloe vera | Powdered leaves were boiled for 5 min at 80 °C with deionized water. | Polysaccharides, flavonoids, and phenolic compounds. | Cu(NO3)2·3H2O | Mix of plant extract and precursor was stirred for 24 h at 100–120 °C. | Monoclinic phase with average particle size of 20 nm. | Bactericidal properties against three fish bacterial pathogens: Aeromonas hydrophila, Pseudomonas fluorescens and Flavobacterium branchiophilum. | [116] |
| Galeopsis herba | Powdered Galeopsidis herba was mixed with water and stirred for 50 min at 85 °C and filtered. | Iridoids, saponins, flavonoids, phenolic acids and tannins. | Cu(NO3)2·3H2O | The extract was mixed with Cu(NO3)2 in 90:10 (W:W) proportion and stirred 4 h at 80 °C then was stored for 24 h in dark place at 25 °C. | The size of NPs was 5–10 nm with spherical shape, dispersed and crystalline. | CuO- NPs showed high antioxidant activity against free radicals with a value of 4.12 µg/mL. NPs presented catalytic activity. | [90] |
| Hagenia abyssinica | Powdered leaves were boiled in deionized water at 50 °C for 1 h, with light protection. | Tannins, anthraquinone glycosides, cardiac glycosides, phenolic compounds. | Cu(NO3)2 ·3H2O | Mix of plant extract and precursor salt has been incubated at RTfor 24 h. The precipitate was washed and dried. | Spherical, hexagonal, triangular and cylindrical, and prismatic shapes. Size range of 10–50 nm. | Antibacterial activities against Escherichia coli Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus subtilis. Cu NPs presented concentric circular patterns with d-spacing of 0.24 nm. | [108] |
| Cinnamomum zelanicum | Powdered leaves of were macerated in boiling water for 6 h. The extract was filtered and evaporated to concentrate. | Cinnamic, coumaric, sinapic, ferulic and caffeic acids, camphor, linalool, benzyl benzoate, cinnamyl acetate, eugenol, and cinnamaldehyde. | Cu(NO3)2·3H2O | A mixture of plant extract and precursor salt was heated and stirred at 65 °C for 24 h. CuNPs were washed, centrifuged and dried at 55 °C. | Spherical morphology with size of 19.55 to 69.70 nm. | Antioxidant activity and anti-lung carcinoma properties against NCI-H2126, NCI-H1437, NCI-H1573, and NCI-H661 cell lines. | [117] |
| Berberis vulgaris | Leaves were macerated in water for 3 h at 90 °C. The extract was filtered and evaporated to concentrate. | Carbohydrates, fiber, several minerals, berberine, vitamin C, iron, zinc, copper and anthocyanins. | Cu(NO3)2·3H2O | Plant extract and precursor salt mixture was heated and stirred at 65 °C for 24 h. | Spherical with size of 10–100 nm. | Cardioprotective potential against isoproterenol-induced myocardial ischemia in mice. | [118] |
| Carum carvi | Leaves were macerated in double-distilled water using shaker incubator for 24 h at 45 °C, then cooled at RT and filtered. | Carvacrol, carvone, α-pinene, limonene, γ-terpinene, linalool, carvenone, and p-cymene, carveol, camphene and fenchen. | Cu(NO3)2·3H2O | Plant extract was added dropwise to precursor solution, with continuous stirring until color change after 12 h at 45 °C. | Regular and homogenous distribution spherical form with 12.4 nm size. | CuONPs had positive effect on the various physiological and biochemical characteristics of the Solanum lycopersicum seedlings (increased sugar content and pigment). | [105] |
| Syzygiumalternifolium | Dried fruits were boiled at a water bath at 80 °C for 30 min. The extract was filtered and stored at 4 °C. | Alkaloids, anthocyanins, anthraquinones, glycosides, emorins, flavonoids, and phenols. | CuSO4·5H2O | Plant extract was mixed with salt precursor at 50 °C for 2 h. The pH was adjusted to 8.2–9.0 by adding NaOH. | Spherical shape, size from 2 to 69 nm, non-agglomerated and polydisperse nature. | Antiviral ability against Newcastle disease virus. | [119] |
| Falcaria vulgaris | Powdered leaves were infused under magnetic stirring for 30 min at 50 °C. | Carvacrol, spathulenol, genistin, rutin, quercetin-3-O-glucoside, and quercetin. | CuSO4·5H2O | Mix of plant extract and precursor salt was rapidly stirred. Then NaOH was added to catalyzed and adjusted to pH 12. Stirring continued for 1 h. | Spherical shape with average diameter size of 20 nm. | Antioxidant activity. Antifungal activity against C. albicans, C. glabrata C. guilliermondii C. kruse. Antibacterial activity against E. coli and S. aureus. Cutaneous wound healing potential without any cytotoxicity. | [109] |
| Orobanche aegyptiaca | Powdered stems were treated by reflux extraction with distilled water, for 30 min. | Polyphenols, tannins, alkaloids and peptides. | CuSO4·5H2O | Mix of plant extract and precursor salt was stirred for 10–15 min at room temperature. The resultant mixture was kept in dark for 72 h. | Spherical shape with particle size less than 50 nm. | Nematicidal properties against Meloidogyne incognita. Antibacterial activity against Escherichia coli and Staphylococcus aureus. | [120] |
| Gnidia glauca Plumbago zeylanica. | Flower, leaves, and stem of G. glauca and leaves of P. zeylanica were boiled at 100 °C for 5 min. Extract was filtered, and stored at 4 °C. | G. glauca: Heterocyclic polyol components, flavonoids, and terpenoids. P. zeylanica: phenolics, flavonoids, reducing sugar, citric acid, and plumbagin. | CuSO4·5H2O | Mix of each plant extracts and precursor salt was stirred within 5 h at 100 °C. | Variable size according to plant extract used from 5–93 nm. Irregular brush border rods and spherical shape. | Antidiabetic activity evaluated by α-amylase inhibitory assay using the chromogenic 3,5-dinitrosalicylic acid (DNSA) method. | [74] |
| Eucalyptus camaldulensis, Azadirachta indica, Murraya koenigii, Rosa rubiginosa and Datura stramonium | Extract from leaves of each plant were obtained by soaking individually in aqueous ethanol (80% v/v) at RT for 3 h. | Alkaloids, flavonoids, terpenoids, polyphenols and proteins. | CuSO4·5H2O | Mix of plant extract and precursor salt was stirred at 80 °C for 10 min. After, mix was continuously stirred at 200 rpm for 24 h at RT and centrifuged. | Spherical shape with Variable size according to plant extract used from 41–65 nm. | Showed destruction of cell membrane and cell lysis of S. aureus, S. mutans, E. coli, K. pneumoniae and S. typhi and the multidrug-resistant P. aeruginosa. | [59] |
| Prunus nepalensis | Fruit extract was obtained by heated in deionized water in a water bath to 80 °C for 1 h. | Polyphenolic compounds, flavonoids, amino acids, alkaloids, saccharides, and tannins. | CuSO4·H2O | Mix of plant extract and precursor salt was stirred and incubated at RT overnight under dark conditions. Then the precipitate was centrifuged and washed. | Spherical with size ranging from 35 to 50 nm. | Anticancer activity on human breast cancer cell lines by increasing the gene expression of apoptotic genes in a dose-dependent manner. | [121] |
| Nigella sativa | Seeds were heated in water at 30 °C for 40 min. The extract was cooled, filtered and centrifuged. | Enzymes, phenols, flavonoids, terpenoids. | (CuSO4)·5H2O | A solution of precursor salt was heated up to 80 °C on a hot plate and seed extract was added dropwise with constant stirring at 150 rpm. | NPs with size of 98.23 nm with changes of size particle increase CuNPs concentration. Form was not reported. | Antiobesity activity tested by lipase and amylase inhibition assays. High antibacterial activity against Pseudomonas aeruginosa and E. coli. | [122] |
| Zingiber officinale | Ginger root powder was boiled at 50–60 °C for 10 min. Extract was filtered, and stored at 4 °C. | Polyphenols, such as 6-gingerol, 8-gingerol, and 10-gingerol. | CuSO4·5H2O | Mix of plant extract and precursor was stirred at RT until color changed. After the solution was centrifuged and the precipitate was dried and heated at 90 °C for 12 h. | Crystalline configuration with size of 60 nm. | Antibacterial activity against Staphylococcus aureus and Escherichia coli. | [123] |
| Haplophyllumtuberculatum | Complete dried plant was placed in a water bath at 70 °C with continuous stirring, for 3 h. After that, it was left at 4 °C and it was valid for use for a week. | Gallic acid, ferulic acid, catechin, quinol, syringic, caffeic, vanillic, ellagic and cinnamic acids, catechol and benzoic acid. | Cu(NO3)2·3H2O | Mix of plant extract was stirred and the precursor was added slowly at 500 rpm at RT. The solution was centrifuged and the precipitate was dried (18 h at 50 °C). | Amorphous particles with the average of about 85 nm. | Nematicide activity against Meloidogyne incognita. | [124] |
| Krameria sp. | Krameria roots were macerated and boiled. | Tannins (cate-chins and proanthocyanidins), rhataniatannic acid, and tannic acid. | (CuSO4)·5H2O | Mix of plant extract and precursor salt was stirred at 70 °C during 3 h. After that, the solution was centrifuged and the precipitate was rinsed and dried at 80 °C for 6 h. | Spherical NP’s and average size of 6.16 nm. | Antioxidant therapy. Antimicrobial agent against Escherichia coli, Staphylococcus aureus, Alternaria alternata, and Fusarium oxyporium. | [104] |
| Nigella sativa | Seeds were heated in water at 30 °C for 40 min. The extract was cooled, filtered and centrifuged. | Enzymes, phenols, flavonoids, terpenoids. | (CuSO4)·5H2O | A solution of precursor salt was heated up to 80 °C on a hot plate and seed extract was added dropwise with constant stirring at 150 rpm. | NPs with size of 98.23 nm with changes of size particle increase CuNPs concentration. Form was not reported. | Antiobesity activity tested by lipase and amylase inhibition assays. High antibacterial activity against Pseudomonas aeruginosa and E. coli. | [122] |
10. Application of Biosynthesized CuNPs
10.1. Therapeutics
10.2. Metabolic Disease Treatment
10.3. Antibacterial Activity
10.4. Antiviral Activity
10.5. Antioxidant Activity
10.6. Food Packaging
10.7. Wastewater Treatment
10.8. Vegetal Tissue Culture
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miu, B.A.; Dinischiotu, A. New Green Approaches in Nanoparticles Synthesis: An Overview. Molecules 2022, 27, 6472. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Alam, M.; Sarkar, A.; Haque, M.I.; Hasan, M.M.; Hoque, M. Application of Nanotechnology in Food: Processing, Preservation, Packaging and Safety Assessment. Heliyon 2022, 8, e11795. [Google Scholar] [CrossRef]
- Nongbet, A.; Mishra, A.K.; Mohanta, Y.K.; Mahanta, S.; Ray, M.K.; Khan, M.; Baek, K.H.; Chakrabartty, I. Nanofertilizers: A Smart and Sustainable Attribute to Modern Agriculture. Plants 2022, 11, 2587. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Sharmin, S.; Rahaman, M.M.; Sarkar, C.; Atolani, O.; Islam, M.T.; Adeyemi, O.S. Nanoparticles as Antimicrobial and Antiviral Agents: A Literature-Based Perspective Study. Heliyon 2021, 7, e06456. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Atapour, A.; Amani, A.M.; Savar Dashtaki, A.; Babapoor, A.; Arjmand, O. Green Synthesis of Silver Nanoparticles toward Bio and Medical Applications: Review Study. Artif. Cells Nanomed. Biotechnol. 2018, 46, S855–S872. [Google Scholar] [CrossRef]
- Ermini, M.L.; Summa, M.; Zamborlin, A.; Frusca, V.; Mapanao, A.K.; Mugnaioli, E.; Bertorelli, R.; Voliani, V. Copper Nano-Architecture Topical Cream for the Accelerated Recovery of Burnt Skin. Nanoscale Adv. 2023, 5, 1212–1219. [Google Scholar] [CrossRef]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The Emerging Role of Nanotechnology in Skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef]
- Guerrini, G.; Magrì, D.; Gioria, S.; Medaglini, D.; Calzolai, L. Characterization of Nanoparticles-Based Vaccines for COVID-19. Nat. Nanotechnol. 2022, 17, 570–576. [Google Scholar] [CrossRef]
- Colino, C.I.; Millán, C.G.; Lanao, J.M. Nanoparticles for Signaling in Biodiagnosis and Treatment of Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1627. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Yao, Q. Copper-Based Biomaterials for Bone and Cartilage Tissue Engineering. J. Orthop. Transl. 2021, 29, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal Nanoparticles Synthesis: An Overview on Methods of Preparation, Advantages and Disadvantages, and Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Thandapani, G.; Arthi, K.; Pazhanisamy, P.; John, J.J.; Vinothini, C.; Rekha, V.; Santhanalakshmi, K.; Sekar, V. Green Synthesis of Copper Oxide Nanoparticles Using Spinacia Oleracea Leaf Extract and Evaluation of Biological Applications: Antioxidant, Antibacterial, Larvicidal and Biosafety Assay. Mater. Today Commun. 2023, 34, 105248. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Copper and Copper Nanoparticles Toxicity and Their Impact on Basic Functions in the Body. Bratisl. Med. J. 2019, 120, 397–409. [Google Scholar] [CrossRef]
- Prohaska, J. Copper. In Present Knowledge in Nutrition; Erdman, J., Macdonald, I., Zeisel, S., Eds.; Wiley-Blackwell: Washington, DC, USA, 2012; pp. 540–553. [Google Scholar]
- Bellu, E.; Medici, S.; Coradduzza, D.; Cruciani, S.; Amler, E.; Maioli, M. Nanomaterials in Skin Regeneration and Rejuvenation. Int. J. Mol. Sci. 2021, 22, 7095. [Google Scholar] [CrossRef]
- Sandoval, C.; Ríos, G.; Sepúlveda, N.; Salvo, J.; Souza-Mello, V.; Farías, J. Effectiveness of Copper Nanoparticles in Wound Healing Process Using In Vivo and In Vitro Studies: A Systematic Review. Pharmaceutics 2022, 14, 1838. [Google Scholar] [CrossRef]
- Mitra, D.; Kang, E.T.; Neoh, K.G. Antimicrobial Copper-Based Materials and Coatings: Potential Multifaceted Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 21159–21182. [Google Scholar] [CrossRef]
- Malarkodi, C.; Rajeshkumar, S. In Vitro Bactericidal Activity of Biosynthesized CuS Nanoparticles against UTI-Causing Pathogens. Inorg. Nano-Metal. Chem. 2017, 47, 1290–1297. [Google Scholar] [CrossRef]
- Sánchez, S.V.; Navarro, N.; Catalán-Figueroa, J.; Morales, J.O. Nanoparticles as Potential Novel Therapies for Urinary Tract Infections. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute Toxicological Effects of Copper Nanoparticles in Vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Wang, Y.; Sun, Q.Q.; Xia, L.L.; Hu, J.J.; Cheng, K.; Wang, X.; Fu, X.X.; Gu, H. Copper Nanoparticles Show Obvious in Vitro and in Vivo Reproductive Toxicity via ERK Mediated Signaling Pathway in Female Mice. Int. J. Biol. Sci. 2018, 14, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, P.; Shafiee, G.; Ziamajidi, N.; Abbasalipourkabir, R. Copper Nanoparticles Induce Apoptosis and Oxidative Stress in SW480 Human Colon Cancer Cell Line. Biol. Trace Elem. Res. 2023, 201, 3746–3754. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green Synthesis of Nanoparticles Using Plant Extracts: A Review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of Plant Phytochemicals and Microbial Enzymes in Biosynthesis of Metallic Nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef]
- Debnath, S.; Swetha, D.; Niranjan Babu, M. Green Synthesis of Nanoparticles Using Herbal Extract. In Herbal Medicine in India: Indigenous Knowledge, Practice, Innovation and Its Value; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Iravani, S. Green Synthesis of Metal Nanoparticles Using Plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Chen, Z.S.; Chen, G. Biosynthesis of Nanoparticles by Microorganisms and Their Applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef]
- Pantidos, N. Biological Synthesis of Metallic Nanoparticles by Bacteria, Fungi and Plants. J. Nanomed. Nanotechnol. 2014, 5, 1. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Herbal Beverages: Bioactive Compounds and Their Role in Disease Risk Reduction–A Review. J. Tradit. Complement. Med. 2018, 8, 451–458. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Atarod, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. Plant-Mediated Green Synthesis of Nanostructures: Mechanisms, Characterization, and Applications. In Electrokinetics in Microfluidics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 199–322. [Google Scholar]
- Musa, H.H.; Musa, T.H.; Oderinde, O.; Musa, I.H.; Shonekan, O.O.; Akintunde, T.Y.; Onasanya, A.K. Traditional Herbal Medicine: Overview of Research Indexed in the Scopus Database. Adv. Tradit. Med. 2022. [CrossRef]
- Bauer Petrovska, B. Historical Review of Medicinal Plants’ Usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Chen, S.; Cheng, C.; Wang, J.; Xiao, H.; Qin, H. Developing New Drugs from Annals of Chinese Medicine. Acta Pharm. Sin. B 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zhang, Y.Q.; Liu, Z.M.; Chen, T.; Lv, C.Y.; Tang, S.H.; Zhang, X.B.; Zhang, W.; Li, Z.Y.; Zhou, R.R.; et al. ETCM: An Encyclopaedia of Traditional Chinese Medicine. Nucleic Acids Res. 2019, 47, D976–D982. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Sun, L.; Zhang, L. Biomedical Applications of Chinese Herb-Synthesized Silver Nanoparticles by Phytonanotechnology. Nanomaterials 2021, 11, 2757. [Google Scholar] [CrossRef]
- Sun, K.; Wu, L.; Wang, S.; Deng, W. Antitumor Effects of Chinese Herbal Medicine Compounds and Their Nano-Formulations on Regulating the Immune System Microenvironment. Front. Oncol. 2022, 12, 949332. [Google Scholar] [CrossRef]
- Roberti di Sarsina, P.; Ottaviani, L.; Mella, J. Tibetan Medicine: A Unique Heritage of Person-Centered Medicine. EPMA J. 2011, 2, 385–389. [Google Scholar] [CrossRef]
- Reuter, K.P.; Weißhuhn, T.E.R.; Witt, C.M. Tibetan Medicine: A Systematic Review of the Clinical Research Available in the West. Evid. -Based Complement. Altern. Med. 2013, 2013, 213407. [Google Scholar] [CrossRef]
- Pan, L.; Gao, J.; Han, Y.; Shi, Y.; Tang, X.; Pu, L.; Lai, X.; Dongzhu, R.; Zhang, J.; Xiangmao, Q.; et al. The Treatment of Cholecystitis and Cholelithiasis by Tibetan Medicine. Evid. -Based Complement. Altern. Med. 2021, 2021, 9502609. [Google Scholar] [CrossRef]
- Fu, K.; Xu, M.; Zhou, Y.; Li, X.; Wang, Z.; Liu, X.; Meng, X.; Zeng, Y.; Zhang, H. The Status Quo and Way Forwards on the Development of Tibetan Medicine and the Pharmacological Research of Tibetan Materia Medica. Pharmacol. Res. 2020, 155, 104688. [Google Scholar]
- Yu, F.; Takahashi, T.; Moriya, J.; Kawaura, K.; Yamakawa, J.; Kusaka, K.; Itoh, T.; Morimoto, S.; Yamaguchi, N.; Kanda, T. Traditional Chinese Medicine and Kampo: A Review from the Distant Past for the Future. J. Int. Med. Res. 2006, 34, 231–239. [Google Scholar] [CrossRef]
- Kimata, Y.; Ogawa, K.; Okamoto, H.; Chino, A.; Namiki, T. Efficacy of Japanese Traditional (Kampo) Medicine for Treating Chemotherapy-Induced Peripheral Neuropathy: A Retrospective Case Series Study. World J. Clin. Cases 2016, 4, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Iwase, S.; Yamaguchi, T.; Miyaji, T.; Terawaki, K.; Inui, A.; Uezono, Y. The Clinical Use of Kampo Medicines (Traditional Japanese Herbal Treatments) for Controlling Cancer Patients’ Symptoms in Japan: A National Cross-Sectional Survey. BMC Complement. Altern. Med. 2012, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Veilleux, M.-P.; Moriyama, S.; Yoshioka, M.; Hinode, D.; Grenier, D. A Review of Evidence for a Therapeutic Application of Traditional Japanese Kampo Medicine for Oral Diseases/Disorders. Medicines 2018, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.C.; Makino, I.; Ikemoto, T.; Saisu, H.; Terajima, Y.; Owari, K. Kampo for the Treatment of Pain in Japan: A Review. Pain. Ther. 2020, 9, 161–170. [Google Scholar] [CrossRef]
- Komuro, A. Kampo Medicines for Infectious Diseases. In Japanese Kampo Medicines for the Treatment of Common Diseases: Focus on Inflammation; Somasundaram, A., Kenichi, W., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 127–142. [Google Scholar]
- Ahmad, S.; Zahiruddin, S.; Parveen, B.; Basist, P.; Parveen, A.; Gaurav; Parveen, R.; Ahmad, M. Indian Medicinal Plants and Formulations and Their Potential Against COVID-19–Preclinical and Clinical Research. Front. Pharmacol. 2021, 11, 578970. [Google Scholar]
- Varghese, K.J.; Anila, J.; Nagalekshmi, R.; Resiya, S.; Sonu, J. Dasapushpam: The Traditional Uses and the Therapeutic Potential of Ten Sacred Plants of Kerala State in India. Int. J. Pharm. Sci. Res. 2010, 1, 50–59. [Google Scholar]
- Sansores-España, D.; Pech-Aguilar, A.G.; Cua-Pech, K.G.; Medina-Vera, I.; Guevara-Cruz, M.; Gutiérrez-Solis, A.L.; Reyes-García, J.G.; Avila-Nava, A. Plants Used in Mexican Traditional Medicine for the Management of Urolithiasis: A Review of Preclinical Evidence, Bioactive Compounds, and Molecular Mechanisms. Molecules 2022, 27, 2008. [Google Scholar] [CrossRef]
- Valdivia-Correa, B.; Gómez-Gutiérrez, C.; Uribe, M.; Méndez-Sánchez, N. Herbal Medicine in Mexico: A Cause of Hepatotoxicity. A Critical Review. Int. J. Mol. Sci. 2016, 17, 235. [Google Scholar] [CrossRef]
- Lucía, C.P.A.; Jacqueline, B.R.; Alberto, B.R.L.; David, B.A.; Beatriz, R.A. Actualized Inventory of Medicinal Plants Used in Traditional Medicine in Oaxaca, Mexico. J. Ethnobiol. Ethnomed. 2021, 17, 7. [Google Scholar] [CrossRef]
- Lilian Pérez-Ochoa, M.; Luis Chávez-Servia, J.; Minerva Vera-Guzmán, A.; Nora Aquino-Bolaños, E.; Cruz Carrillo-Rodríguez, J. Medicinal Plants Used by Indigenous Communities of Oaxaca, Mexico, to Treat Gastrointestinal Disorders. In Pharmacognosy-Medicinal Plants; Intechopen: London, UK, 2019. [Google Scholar]
- Cabada-Aguirre, P.; López López, A.M.; Mendoza, K.C.O.; Garay Buenrostro, K.D.; Luna-Vital, D.A.; Mahady, G.B. Mexican Traditional Medicines for Women’s Reproductive Health. Sci. Rep. 2023, 13, 2807. [Google Scholar] [CrossRef]
- Silveira, D.; Boylan, F. Medicinal Plants: Advances in Phytochemistry and Ethnobotany. Plants 2023, 12, 1682. [Google Scholar] [CrossRef] [PubMed]
- Gebreslassie, Y.T.; Gebretnsae, H.G. Green and Cost-Effective Synthesis of Tin Oxide Nanoparticles: A Review on the Synthesis Methodologies, Mechanism of Formation, and Their Potential Applications. Nanoscale Res. Lett. 2021, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.I.M. Green Synthesis and Characterizations of Copper Nanoparticles. Mater. Chem. Phys. 2020, 240, 122283. [Google Scholar] [CrossRef]
- Asghar, M.A.; Asghar, M.A. Green Synthesized and Characterized Copper Nanoparticles Using Various New Plants Extracts Aggravate Microbial Cell Membrane Damage after Interaction with Lipopolysaccharide. Int. J. Biol. Macromol. 2020, 160, 1168–1176. [Google Scholar] [CrossRef]
- Rana, A.; Yadav, K.; Jagadevan, S. A Comprehensive Review on Green Synthesis of Nature-Inspired Metal Nanoparticles: Mechanism, Application and Toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Ahmed, A.; Usman, M.; Liu, Q.Y.; Shen, Y.Q.; Yu, B.; Cong, H.L. Plant Mediated Synthesis of Copper Nanoparticles by Using Camelia Sinensis Leaves Extract and Their Applications in Dye Degradation. Ferroelectrics 2019, 549, 61–69. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Chung, I.M.; Gomathi, T.; Ansari, M.A.; Gopiesh Khanna, V.; Babu, V.; Rajakumar, G. Synthesis, Characterization and Pharmacological Potential of Green Synthesized Copper Nanoparticles. Bioprocess. Biosyst. Eng. 2019, 42, 1769–1777. [Google Scholar] [CrossRef]
- Montes, O.; Vázquez-Hernández, A.; Fenton-Navarro, B. Active Compounds of Medicinal Plants, Mechanism for Antioxidant and Beneficial Effects. Phyton 2019, 88, 1–10. [Google Scholar] [CrossRef]
- Kumar, M.; Prakash, S.; Radha; Kumari, N.; Pundir, A.; Punia, S.; Saurabh, V.; Choudhary, P.; Changan, S.; Dhumal, S.; et al. Beneficial Role of Antioxidant Secondary Metabolites from Medicinal Plants in Maintaining Oral Health. Antioxidants 2021, 10, 1061. [Google Scholar] [CrossRef]
- Godlewska-żyłkiewicz, B.; Świsłocka, R.; Kalinowska, M.; Golonko, A.; Świderski, G.; Arciszewska, Ż.; Nalewajko-Sieliwoniuk, E.; Naumowicz, M.; Lewandowski, W. Biologically Active Compounds of Plants: Structure-Related Antioxidant, Microbiological and Cytotoxic Activity of Selected Carboxylic Acids. Materials 2020, 13, 4454. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The Role of Plant-Derived Natural Antioxidants in Reduction of Oxidative Stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef] [PubMed]
- Anantharaju, P.G.; Reddy, D.B.; Padukudru, M.A.; Chitturi, C.M.K.; Vimalambike, M.G.; Madhunapantula, S.R.V. Induction of Colon and Cervical Cancer Cell Death by Cinnamic Acid Derivatives Is Mediated through the Inhibition of Histone Deacetylases (HDAC). PLoS ONE 2017, 12, e0186208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Jiang, Q.; Zhang, L.; Song, W. Zinc Binding Groups for Histone Deacetylase Inhibitors. J. Enzyme Inhib. Med. Chem. 2018, 33, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Sabeena, G.; Rajaduraipandian, S.; Pushpalakshmi, E.; Alhadlaq, H.A.; Mohan, R.; Annadurai, G.; Ahamed, M. Green and Chemical Synthesis of CuO Nanoparticles: A Comparative Study for Several in Vitro Bioactivities and in Vivo Toxicity in Zebrafish Embryos. J. King Saud. Univ. Sci. 2022, 34, 102092. [Google Scholar] [CrossRef]
- Bashir, R.; Tabassum, S.; Rashid, A.; Rehman, S.; Adnan, A.; Ghaffar, R. Bioactive Components of Root Vegetables. In Advances in Root Vegetables Research; Intechopen: London, UK, 2023. [Google Scholar]
- Dos Santos, M.S.N.; Wancura, J.H.C.; Oro, C.E.D.; Dallago, R.M.; Tres, M.V. Opportunities and Challenges of Plant Bioactive Compounds for Food and Agricultural-Related Areas. Phyton-Int. J. Exp. Bot. 2022, 91, 1105–1127. [Google Scholar] [CrossRef]
- Rodríguez De Luna, S.L.; Ramírez-Garza, R.E.; Serna Saldívar, S.O. Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties. Sci. World J. 2020, 2020, 6792069. [Google Scholar] [CrossRef] [PubMed]
- Jamdade, D.A.; Rajpali, D.; Joshi, K.A.; Kitture, R.; Kulkarni, A.S.; Shinde, V.S.; Bellare, J.; Babiya, K.R.; Ghosh, S. Gnidia Glauca-And Plumbago Zeylanica -Mediated Synthesis of Novel Copper Nanoparticles as Promising Antidiabetic Agents. Adv. Pharmacol. Sci. 2019, 2019, 9080279. [Google Scholar] [CrossRef]
- Ingle, K.P.; Deshmukh, A.G.; Padole, D.A.; Dudhare, M.S.; Moharil, M.P.; Khelurkar, V.C. Phytochemicals: Extraction Methods, Identification and Detection of Bioactive Compounds from Plant Extracts. J. Pharmacogn. Phytochem. 2017, 6, 32–36. [Google Scholar]
- Jha, A.K.; Sit, N. Extraction of Bioactive Compounds from Plant Materials Using Combination of Various Novel Methods: A Review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Horablaga, N.M.; Cozma, A.; Alexa, E.; Obistioiu, D.; Cocan, I.; Poiana, M.A.; Lalescu, D.; Pop, G.; Imbrea, I.M.; Buzna, C. Influence of Sample Preparation/Extraction Method on the Phytochemical Profile and Antimicrobial Activities of 12 Commonly Consumed Medicinal Plants in Romania. Appl. Sci. 2023, 13, 2530. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Naraniwal, M.; Kothari, V. Modern Extraction Methods for Preparation of Bioactive Plant Extracts. Int. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A Review of Modern and Conventional Extraction Techniques and Their Applications for Extracting Phytochemicals from Plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Nurdalilah, O.; Teoh, Y.P.; Ooi, Z.X.; Sam, S.T. Comparative Study on the Extraction of Bioactive Secondary Metabolites from Pomelo and Pineapple Peels Extract. IOP Conf. Series Mater. Sci. Eng. 2018, 429, 012040. [Google Scholar] [CrossRef]
- Ahmadi Tehrani, A.; Omranpoor, M.M.; Vatanara, A.; Seyedabadi, M.; Ramezani, V. Formation of Nanosuspensions in Bottom-up Approach: Theories and Optimization. J. Pharm. Sci. 2019, 27, 451–473. [Google Scholar] [CrossRef]
- Törnquist, M.; Michaels, T.C.T.; Sanagavarapu, K.; Yang, X.; Meisl, G.; Cohen, S.I.A.; Knowles, T.P.J.; Linse, S. Secondary Nucleation in Amyloid Formation. Chem. Commun. 2018, 54, 8667–8684. [Google Scholar] [CrossRef]
- Luque-Jacobo, C.M.; Cespedes-Loayza, A.L.; Echegaray-Ugarte, T.S.; Cruz-Loayza, J.L.; Cruz, I.; de Carvalho, J.C.; Goyzueta-Mamani, L.D. Biogenic Synthesis of Copper Nanoparticles: A Systematic Review of Their Features and Main Applications. Molecules 2023, 28, 4838. [Google Scholar] [CrossRef]
- Letchumanan, D.; Sok, S.P.M.; Ibrahim, S.; Nagoor, N.H.; Arshad, N.M. Plant-Based Biosynthesis of Copper/Copper Oxide Nanoparticles: An Update on Their Applications in Biomedicine, Mechanisms, and Toxicity. Biomolecules 2021, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Vishveshvar, K.; Aravind Krishnan, M.V.; Haribabu, K.; Vishnuprasad, S. Green Synthesis of Copper Oxide Nanoparticles Using Ixiro Coccinea Plant Leaves and Its Characterization. Bionanoscience 2018, 8, 554–558. [Google Scholar] [CrossRef]
- Wu, S.; Rajeshkumar, S.; Madasamy, M.; Mahendran, V. Green Synthesis of Copper Nanoparticles Using Cissus Vitiginea and Its Antioxidant and Antibacterial Activity against Urinary Tract Infection Pathogens. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1153–1158. [Google Scholar] [CrossRef]
- Dobrucka, R. Antioxidant and Catalytic Activity of Biosynthesized CuO Nanoparticles Using Extract of Galeopsidis Herba. J. Inorg. Organomet. Polym. Mater. 2018, 28, 812–819. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Mohammad Sajadi, S. Biosynthesis of Copper Nanoparticles Supported on Manganese Dioxide Nanoparticles Using Centella Asiatica L. Leaf Extract for the Efficient Catalytic Reduction of Organic Dyes and Nitroarenes. Cuihua Xuebao/Chin. J. Catal. 2018, 39, 109–117. [Google Scholar] [CrossRef]
- Iliger, K.S.; Sofi, T.A.; Bhat, N.A.; Ahanger, F.A.; Sekhar, J.C.; Elhendi, A.Z.; Al-Huqail, A.A.; Khan, F. Copper Nanoparticles: Green Synthesis and Managing Fruit Rot Disease of Chilli Caused by Colletotrichum Capsici. Saudi J. Biol. Sci. 2021, 28, 1477–1486. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” Nanotechnologies: Synthesis of Metal Nanoparticles Using Plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Chandraker, S.K.; Lal, M.; Ghosh, M.K.; Tiwari, V.; Ghorai, T.K.; Shukla, R. Green Synthesis of Copper Nanoparticles Using Leaf Extract of Ageratum Houstonianum Mill. and Study of Their Photocatalytic and Antibacterial Activities. Nano Express 2020, 1, 010033. [Google Scholar] [CrossRef]
- Malassis, L.; Dreyfus, R.; Murphy, R.J.; Hough, L.A.; Donnio, B.; Murray, C.B. One-Step Green Synthesis of Gold and Silver Nanoparticles with Ascorbic Acid and Their Versatile Surface Post-Functionalization. RSC Adv. 2016, 6, 33092–33100. [Google Scholar] [CrossRef]
- Liu, Q.M.; Yasunami, T.; Kuruda, K.; Okido, M. Preparation of Cu Nanoparticles with Ascorbic Acid by Aqueous Solution Reduction Method. Trans. Nonferrous Met. Soc. China 2012, 22, 2198–2203. [Google Scholar] [CrossRef]
- Zain, N.M.; Stapley, A.G.F.; Shama, G. Green Synthesis of Silver and Copper Nanoparticles Using Ascorbic Acid and Chitosan for Antimicrobial Applications. Carbohydr. Polym. 2014, 112, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Dalwadi, G.; Benson, H.A.E.; Chen, Y. Comparison of Diafiltration and Tangential Flow Filtration for Purification of Nanoparticle Suspensions. Pharm. Res. 2005, 22, 2152–2162. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, B.; Lagzi, I.; Grzybowski, B.A. Nanoseparations: Strategies for Size and/or Shape-Selective Purification of Nanoparticles. Curr. Opin. Colloid Interface Sci. 2011, 16, 135–148. [Google Scholar] [CrossRef]
- Shah, N.K.; Ivone, R.; Shen, J.; Meenach, S.A. A Comparison of Centrifugation and Tangential Flow Filtration for Nanoparticle Purification: A Case Study on Acetalated Dextran Nanoparticles. Particuology 2020, 50, 189–196. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green Synthesis of Metallic Nanoparticles as Effective Alternatives to Treat Antibiotics Resistant Bacterial Infections: A Review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-Drying of Nanoparticles: Formulation, Process and Storage Considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef]
- Matsakas, L.; Gerber, M.; Yu, L.; Rova, U.; Christakopoulos, P. Preparation of Low Carbon Impact Lignin Nanoparticles with Controllable Size by Using Different Strategies for Particles Recovery. Ind. Crops Prod. 2020, 147, 112243. [Google Scholar] [CrossRef]
- Alshammari, S.O.; Mahmoud, S.Y.; Farrag, E.S. Synthesis of Green Copper Nanoparticles Using Medicinal Plant Krameria Sp. Root Extract and Its Applications. Molecules 2023, 28, 4629. [Google Scholar] [CrossRef]
- Oraibi, A.G.; Rashad, A.A.; Ahmed, M.H. Carum Carvi Mediated Green Synthesis of Copper Nanoparticles and Its Effect on Solanum Lycopersicum Seedlings. J. Arid. Agric. 2023, 9. [Google Scholar] [CrossRef]
- Alavi, M.; Kamarasu, P.; McClements, D.J.; Moore, M.D. Metal and Metal Oxide-Based Antiviral Nanoparticles: Properties, Mechanisms of Action, and Applications. Adv. Colloid Interface Sci. 2022, 306, 102726. [Google Scholar] [CrossRef]
- Majumdar, T.D.; Singh, M.; Thapa, M.; Dutta, M.; Mukherjee, A.; Ghosh, C.K. Size-Dependent Antibacterial Activity of Copper Nanoparticles against Xanthomonas Oryzae Pv. Oryzae–A Synthetic and Mechanistic Approach. Colloid Interface Sci. Commun. 2019, 32, 100190. [Google Scholar] [CrossRef]
- Murthy, H.C.A.; Desalegn, T.; Kassa, M.; Abebe, B.; Assefa, T. Copper Paper. J. Nanomater. 2020, 2020, 3924081. [Google Scholar]
- Zangeneh, M.M.; Ghaneialvar, H.; Akbaribazm, M.; Ghanimatdan, M.; Abbasi, N.; Goorani, S.; Pirabbasi, E.; Zangeneh, A. Novel Synthesis of Falcaria Vulgaris Leaf Extract Conjugated Copper Nanoparticles with Potent Cytotoxicity, Antioxidant, Antifungal, Antibacterial, and Cutaneous Wound Healing Activities under in Vitro and in Vivo Condition. J. Photochem. Photobiol. B 2019, 197, 111556. [Google Scholar] [CrossRef] [PubMed]
- Badeggi, U.M.; Lawal, B.A.; Akinfenwa, A.O.; Ayipo, Y.O.; Azeh, Y.; Dagaci, M.Z. Physicochemical Properties and in Vitro Stability Studies of Green Synthesized Gold Nanoparticles Using Pelargonium Sidoides. Niger. J. Technol. 2020, 39, 785–791. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, L.; Deng, H.; Zhang, Z. Structural Characterization and Stability Study of Green Synthesized Starch Stabilized Silver Nanoparticles Loaded with Isoorientin. Food Chem. 2021, 338, 127807. [Google Scholar] [CrossRef]
- Habibullah, G.; Viktorova, J.; Ulbrich, P.; Ruml, T. Effect of the Physicochemical Changes in the Antimicrobial Durability of Green Synthesized Silver Nanoparticles during Their Long-Term Storage. RSC Adv. 2022, 12, 30386–30403. [Google Scholar] [CrossRef] [PubMed]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Evaluation of Antioxidant and Anticancer Activity of Copper Oxide Nanoparticles Synthesized Using Medicinally Important Plant Extracts. Biomed. Pharmacother. 2017, 89, 1067–1077. [Google Scholar] [CrossRef]
- Alao, I.I.; Oyekunle, I.P.; Iwuozor, K.O.; Emenike, E.C. Green Synthesis of Copper Nanoparticles and Investigation of Its Antimicrobial Properties. Adv. J. Chem. -Sect. B Nat. Prod. Med. Chem. 2022, 4, 39–52. [Google Scholar]
- Tahir, M.F.; Khan, M.Z.; Attacha, S.; Asim, N.; Tayyab, M.; Ali, A.; Militky, J.; Tomková, B. The Comparative Performance of Phytochemicals, Green Synthesised Silver Nanoparticles, and Green Synthesised Copper Nanoparticles-Loaded Textiles to Avoid Nosocomial Infections. Nanomaterials 2022, 12, 3629. [Google Scholar] [CrossRef]
- Kumar, P.P.N.V.; Shameem, U.; Kollu, P.; Kalyani, R.L.; Pammi, S.V.N. Green Synthesis of Copper Oxide Nanoparticles Using Aloe Vera Leaf Extract and Its Antibacterial Activity Against Fish Bacterial Pathogens. Bionanoscience 2015, 5, 135–139. [Google Scholar] [CrossRef]
- Liu, H.; Wang, G.; Liu, J.; Nan, K.; Zhang, J.; Guo, L.; Liu, Y. Green Synthesis of Copper Nanoparticles Using Cinnamomum Zelanicum Extract and Its Applications as a Highly Efficient Antioxidant and Anti-Human Lung Carcinoma. J. Exp. Nanosci. 2021, 16, 410–423. [Google Scholar] [CrossRef]
- Tu, S.; Shen, C.; Bai, X.; Zhang, H.; Amirpour Amraii, S.; Dai, D. Copper Nanoparticles Green-Formulated by a Medicinal Plant: Preparation, Characterization and Investigation of Its Cardioprotective Effects. Inorg. Chem. Commun. 2023, 155, 111104. [Google Scholar] [CrossRef]
- Yugandhar, P.; Vasavi, T.; Jayavardhana Rao, Y.; Uma Maheswari Devi, P.; Narasimha, G.; Savithramma, N. Cost Effective, Green Synthesis of Copper Oxide Nanoparticles Using Fruit Extract of Syzygium alternifolium (Wt.) Walp., Characterization and Evaluation of Antiviral Activity. J. Clust. Sci. 2018, 29, 743–755. [Google Scholar] [CrossRef]
- Akhter, G.; Khan, A.; Ali, S.G.; Khan, T.A.; Siddiqi, K.S.; Khan, H.M. Antibacterial and Nematicidal Properties of Biosynthesized Cu Nanoparticles Using Extract of Holoparasitic Plant. SN Appl. Sci. 2020, 2, 1–6. [Google Scholar] [CrossRef]
- Biresaw, S.S.; Taneja, P. Copper Nanoparticles Green Synthesis and Characterization as Anticancer Potential in Breast Cancer Cells (MCF7) Derived from Prunus Nepalensis Phytochemicals. Mater. Today Proc. 2021, 49, 3501–3509. [Google Scholar] [CrossRef]
- Kumar, M.; Kaushik, D.; Kumar, A.; Gupta, P.; Proestos, C.; Oz, E.; Orhan, E.; Kaur, J.; Khan, M.R.; Elobeid, T.; et al. Green Synthesis of Copper Nanoparticles from Nigella sativa Seed Extract and Evaluation of Their Antibacterial and Antiobesity Activity. Int. J. Food Sci. Technol. 2023, 58, 2883–2892. [Google Scholar] [CrossRef]
- Abbas, A.H.; Fairouz, N.Y. Characterization, Biosynthesis of Copper Nanoparticles Using Ginger Roots Extract and Investigation of Its Antibacterial Activity. Mater. Today Proc. 2022, 61, 908–913. [Google Scholar] [CrossRef]
- Soliman, A.M.M.; Abdallah, E.A.M.; Hafez, E.E.; Kadous, E.A.; Kassem, F.A. Nematicidal Activity of Chemical and Green Biosynthesis of Copper Nanoparticles Against Root-Knot Nematode, Meloidogyne Incognita. Alex. Sci. Exch. J. 2022, 43, 583–591. [Google Scholar] [CrossRef]
- Najaflu, M.; Shahgolzari, M.; Bani, F.; Khosroushahi, A.Y. Green Synthesis of Near-Infrared Copper-Doped Carbon Dots from Alcea for Cancer Photothermal Therapy. ACS Omega 2022, 7, 34573–34582. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, K.; Di, L.; Wang, P.; Liu, Z.; Zhang, J.; Yue, P.; Song, W.; Zhang, J.; Chen, T.; et al. Traditional Herbal Medicine and Nanomedicine: Converging Disciplines to Improve Therapeutic Efficacy and Human Health. Adv. Drug Deliv. Rev. 2021, 178, 113964. [Google Scholar] [CrossRef]
- Amjad, S.; Mahdi, A.A. Application of Phyto-Nanomedicine for the Treatment of Different Disease Conditions Such as Diabetes, Cardiovascular Diseases, and Neurodegenerative Disorders. In Nanotechnology in Herbal Medicine; Elsevier: Amsterdam, The Netherlands, 2023; pp. 293–312. [Google Scholar]
- Bahloul, B.; Castillo-Henríquez, L.; Jenhani, L.; Aroua, N.; Ftouh, M.; Kalboussi, N.; Vega-Baudrit, J.; Mignet, N. Nanomedicine-Based Potential Phyto-Drug Delivery Systems for Diabetes. J. Drug Deliv. Sci. Technol. 2023, 82, 104377. [Google Scholar] [CrossRef]
- Yi, M.H.; Simu, S.Y.; Ahn, S.; Aceituno, V.C.; Wang, C.; Mathiyalagan, R.; Hurh, J.; Batjikh, I.; Ali, H.; Kim, Y.-J.; et al. Anti-Obesity Effect of Gold Nanoparticles from Dendropanax Morbifera Léveille by Suppression of Triglyceride Synthesis and Downregulation of PPARγ and CEBPα Signaling Pathways in 3T3-L1 Mature Adipocytes and HepG2 Cells. Curr. Nanosci. 2020, 16, 196–203. [Google Scholar] [CrossRef]
- Rajivgandhi, G.; Maruthupandy, M.; Muneeswaran, T.; Ramachandran, G.; Manoharan, N.; Quero, F.; Anand, M.; Song, J.M. Biologically Synthesized Copper Oxide Nanoparticles Enhanced Intracellular Damage in Ciprofloxacin Resistant ESBL Producing Bacteria. Microb. Pathog. 2019, 127, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Zuily, L.; Lahrach, N.; Fassler, R.; Genest, O.; Faller, P.; Sénèque, O.; Denis, Y.; Castanié-Cornet, M.P.; Genevaux, P.; Jakob, U.; et al. Copper Induces Protein Aggregation, a Toxic Process Compensated by Molecular Chaperones. Mbio 2022, 13, e0325121. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, M.; Choudhury, B.; Yadav, R.N.S. Comparative Study of Antibiofilm Activity of Copper Oxide and Iron Oxide Nanoparticles Against Multidrug Resistant Biofilm Forming Uropathogens. Indian J. Microbiol. 2014, 54, 365–368. [Google Scholar] [CrossRef]
- Naz, S.; Gul, A.; Zia, M. Toxicity of Copper Oxide Nanoparticles: A Review Study. IET Nanobiotechnol. 2020, 14, 1–13. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, S.; Xu, X.; Du, Q. Copper-Containing Nanoparticles: Mechanism of Antimicrobial Effect and Application in Dentistry-a Narrative Review. Front. Surg. 2022, 9, 905892. [Google Scholar] [CrossRef]
- Truong, H.T.; Nguyen, L.C.T.; Quang Le, L. Synthesis and Antifungal Activity of Copper Nanoparticles against Fusarium Oxysporum Pathogen of Plants. Mater. Res. Express 2023, 10, 065001. [Google Scholar] [CrossRef]
- Everts, K.L.; Himmelstein, J.C. Fusarium Wilt of Watermelon: Towards Sustainable Management of a Re-Emerging Plant Disease. Crop Prot. 2015, 73, 93–99. [Google Scholar] [CrossRef]
- Rubina, M.S.; Vasil’kov, A.Y.; Naumkin, A.V.; Shtykova, E.V.; Abramchuk, S.S.; Alghuthaymi, M.A.; Abd-Elsalam, K.A. Synthesis and Characterization of Chitosan–Copper Nanocomposites and Their Fungicidal Activity against Two Sclerotia-Forming Plant Pathogenic Fungi. J. Nanostruct. Chem. 2017, 7, 249–258. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A Review of the Antibacterial Effects of Silver Nanomaterials and Potential Implications for Human Health and the Environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar]
- Kashyap, P.; Shirkot, P.; Das, R.; Pandey, H.; Singh, D. Biosynthesis and Characterization of Copper Nanoparticles from Stenotrophomonas Maltophilia and Its Effect on Plant Pathogens and Pesticide Degradation. J. Agric. Food Res. 2023, 13, 100654. [Google Scholar] [CrossRef]
- Kaningini, A.G.; Motlhalamme, T.; More, G.K.; Mohale, K.C.; Maaza, M. Antimicrobial, Antioxidant, and Cytotoxic Properties of Biosynthesized Copper Oxide Nanoparticles (CuO-NPs) Using Athrixia Phylicoides DC. Heliyon 2023, 9, e15265. [Google Scholar] [CrossRef] [PubMed]
- Yazdanian, M.; Rostamzadeh, P.; Rahbar, M.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Yazdanian, A. The Potential Application of Green-Synthesized Metal Nanoparticles in Dentistry: A Comprehensive Review. Bioinorg. Chem. Appl. 2022, 2022, 2311910. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Abd–Elsalam, K.A. Nanoantimicrobials for Plant Pathogens Control: Potential Applications and Mechanistic Aspects. In Nanotechnology in the Life Sciences; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of Antibacterial Activity of Copper Nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Young, G.M.; Yun, S. Il In Vitro Embryotoxicity and Mode of Antibacterial Mechanistic Study of Gold and Copper Nanoparticles Synthesized from Angelica keiskei (Miq.) Koidz. Leaves Extract. Saudi J. Biol. Sci. 2022, 29, 2552–2563. [Google Scholar] [CrossRef]
- Abad, M.J.; Guerra, J.A.; Bermejo, P.; Irurzun, A.; Carrasco, L. Search for Antiviral Activity in Higher Plant Extracts. Phytother. Res. 2000, 14, 604–607. [Google Scholar] [CrossRef]
- Alagarasu, K.; Patil, P.; Kaushik, M.; Chowdhury, D.; Joshi, R.K.; Hegde, H.V.; Kakade, M.B.; Hoti, S.L.; Cherian, S.; Parashar, D. In Vitro Antiviral Activity of Potential Medicinal Plant Extracts Against Dengue and Chikungunya Viruses. Front. Cell. Infect. Microbiol. 2022, 12, 866452. [Google Scholar] [CrossRef]
- Hegazy, A.; Mostafa, I.; Elshaier, Y.A.M.M.; Mahmoud, S.H.; Abo Shama, N.M.; Shehata, M.; Yahya, G.; Nasr, N.F.; El-Halawany, A.M.; Ali, M.A.; et al. Robust Antiviral Activity of Santonica Flower Extract (Artemisia cina) against Avian and Human Influenza A Viruses: In Vitro and Chemoinformatic Studies. ACS Omega 2022, 7, 41212–41223. [Google Scholar] [CrossRef]
- Dell’Annunziata, F.; Sellitto, C.; Franci, G.; Marcotullio, M.C.; Piovan, A.; Della Marca, R.; Folliero, V.; Galdiero, M.; Filippelli, A.; Conti, V.; et al. Antiviral Activity of Ficus Rubiginosa Leaf Extracts against HSV-1, HCoV-229E and PV-1. Viruses 2022, 14, 2257. [Google Scholar] [CrossRef]
- Karthik, C.; Punnaivalavan, K.A.; Prabha, S.P.; Caroline, D.G. Multifarious Global Flora Fabricated Phytosynthesis of Silver Nanoparticles: A Green Nanoweapon for Antiviral Approach Including SARS-CoV-2. Int. Nano Lett. 2022, 12, 313–344. [Google Scholar] [PubMed]
- Kumar, S.D.; Singaravelu, G.; Ajithkumar, S.; Murugan, K.; Nicoletti, M.; Benelli, G. Mangrove-Mediated Green Synthesis of Silver Nanoparticles with High HIV-1 Reverse Transcriptase Inhibitory Potential. J. Clust. Sci. 2017, 28, 359–367. [Google Scholar] [CrossRef]
- Sharma, V.; Kaushik, S.; Pandit, P.; Dhull, D.; Yadav, J.P.; Kaushik, S. Green Synthesis of Silver Nanoparticles from Medicinal Plants and Evaluation of Their Antiviral Potential against Chikungunya Virus. Appl. Microbiol. Biotechnol. 2019, 103, 881–891. [Google Scholar] [CrossRef]
- Haggag, E.G.; Elshamy, A.M.; Rabeh, M.A.; Gabr, N.M.; Salem, M.; Youssif, K.A.; Samir, A.; Bin Muhsinah, A.; Alsayari, A.; Abdelmohsen, U.R. Antiviral Potential of Green Synthesized Silver Nanoparticles of Lampranthus Coccineus and Malephora Lutea. Int. J. Nanomed. 2019, 14, 6217–6229. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, G.A.; Mustaqeem, M.; Hassan, I.U.; Awan, T.; Arshad, F.; Salim, H.; Qurashi, A. Bioinspired and Green Synthesis of Nanoparticles from Plant Extracts with Antiviral and Antimicrobial Properties: A Critical Review. J. Saudi Chem. Soc. 2021, 25, 101304. [Google Scholar] [CrossRef]
- Ahmadi, M.; Elikaei, A.; Ghadam, P. Antiviral Activity of Biosynthesized Copper Nanoparticle by Juglans Regia Green Husk Aqueous Extract and Iron Nanoparticle: Molecular Docking and in-Vitro Studies. Iran. J. Microbiol. 2023, 15, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Ermini, M.L.; Voliani, V. Antimicrobial Nano-Agents: The Copper Age. ACS Nano 2021, 15, 6008–6029. [Google Scholar] [CrossRef]
- Hang, X.; Peng, H.; Song, H.; Qi, Z.; Miao, X.; Xu, W. Antiviral Activity of Cuprous Oxide Nanoparticles against Hepatitis C Virus in Vitro. J. Virol. Methods 2015, 222, 150–157. [Google Scholar] [CrossRef]
- Broglie, J.J.; Alston, B.; Yang, C.; Ma, L.; Adcock, A.F.; Chen, W.; Yang, L. Antiviral Activity of Gold/Copper Sulfide Core/Shell Nanoparticles against Human Norovirus Virus-like Particles. PLoS ONE 2015, 10, e0141050. [Google Scholar] [CrossRef]
- Ishida, T. Antiviral Activities of Cu2+ Ions in Viral Prevention, Replication, RNA Degradation, and for Antiviral Efficacies of Lytic Virus, ROS-Mediated Virus, Copper Chelation. World Sci. News 2018, 99, 148–168. [Google Scholar]
- Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A Novel Anti-Influenza Copper Oxide Containing Respiratory Face Mask. PLoS ONE 2010, 5, e11295. [Google Scholar] [CrossRef] [PubMed]
- Sucipto, T.H.; Churrotin, S.; Setyawati, H.; Kotaki, T.; Martak, F.; Soegijanto, S. Antiviral Activity of Copper(Ii)Chloride Dihydrate against Dengue Virus Type-2 in Vero Cell. Indones. J. Trop. Infect. Dis. 2017, 6, 84. [Google Scholar] [CrossRef]
- Escoffery, C.C.; Dunn, I.; Patel, H.; Yan, S.; Shukla, S. A Novel Approach to Antiviral COVID-19 Masks; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Ghidan, A.Y.; Al-Antary, T.M.; Awwad, A.M. Green Synthesis of Copper Oxide Nanoparticles Using Punica Granatum Peels Extract: Effect on Green Peach Aphid. Environ. Nanotechnol. Monit. Manag. 2016, 6, 95–98. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Rinitha, G. Nanostructural Characterization of Antimicrobial and Antioxidant Copper Nanoparticles Synthesized Using Novel Persea Americana Seeds. OpenNano 2018, 3, 18–27. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Menon, S.; Venkat Kumar, S.; Tambuwala, M.M.; Bakshi, H.A.; Mehta, M.; Satija, S.; Gupta, G.; Chellappan, D.K.; Thangavelu, L.; et al. Antibacterial and Antioxidant Potential of Biosynthesized Copper Nanoparticles Mediated through Cissus Arnotiana Plant Extract. J. Photochem. Photobiol. B 2019, 197, 111531. [Google Scholar] [CrossRef]
- Peddi, P.; Ptsrk, P.R.; Rani, N.U.; Tulasi, S.L. Green Synthesis, Characterization, Antioxidant, Antibacterial, and Photocatalytic Activity of Suaeda maritima (L.) Dumort Aqueous Extract-Mediated Copper Oxide Nanoparticles. J. Genet. Eng. Biotechnol. 2021, 19, 131. [Google Scholar] [CrossRef]
- Shanmugapriya, J.; Reshma, C.A.; Srinidhi, V.; Harithpriya, K.; Ramkumar, K.M.; Umpathy, D.; Gunasekaran, K.; Subashini, R. Green Synthesis of Copper Nanoparticles Using Withania Somnifera and Its Antioxidant and Antibacterial Activity. J. Nanomater. 2022, 2022, 7967294. [Google Scholar] [CrossRef]
- Djamila, B.; Eddine, L.S.; Abderrhmane, B.; Nassiba, A.; Barhoum, A. In Vitro Antioxidant Activities of Copper Mixed Oxide (CuO/Cu2O) Nanoparticles Produced from the Leaves of Phoenix Dactylifera L. Biomass Convers. Biorefin. 2022. [CrossRef]
- Dash, K.K.; Deka, P.; Bangar, S.P.; Chaudhary, V.; Trif, M.; Rusu, A. Applications of Inorganic Nanoparticles in Food Packaging: A Comprehensive Review. Polymers 2022, 14, 521. [Google Scholar] [CrossRef]
- Zhang, W.; Roy, S.; Rhim, J.W. Copper-Based Nanoparticles for Biopolymer-Based Functional Films in Food Packaging Applications. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1933–1952. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.F.; Rhim, J.W. Preparation and Properties of Carbohydrate-Based Composite Films Incorporated with CuO Nanoparticles. Carbohydr. Polym. 2017, 169, 264–271. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Xiaowen, H.; Wang, M.H. Physical and Bioactivities of Biopolymeric Films Incorporated with Cellulose, Sodium Alginate and Copper Oxide Nanoparticles for Food Packaging Application. Int. J. Biol. Macromol. 2020, 153, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Störmer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical Review of the Migration Potential of Nanoparticles in Food Contact Plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar] [CrossRef]
- Shukla, A.K.; Iravani, S. Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Atri, A.; Echabaane, M.; Bouzidi, A.; Harabi, I.; Soucase, B.M.; Ben Chaâbane, R. Green Synthesis of Copper Oxide Nanoparticles Using Ephedra Alata Plant Extract and a Study of Their Antifungal, Antibacterial Activity and Photocatalytic Performance under Sunlight. Heliyon 2023, 9, e13484. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; Fahmy, A.H.; Ahmed, S.S. Copper Nanoparticles Elevate Regeneration Capacity of (Ocimum basilicum L.) Plant via Somatic Embryogenesis. Plant Cell Tissue Organ Cult. 2019, 136, 41–50. [Google Scholar] [CrossRef]
- Malik, W.A.; Mahmood, I.; Razzaq, A.; Afzal, M.; Shah, G.A.; Iqbal, A.; Zain, M.; Ditta, A.; Asad, S.A.; Ahmad, I.; et al. Exploring Potential of Copper and Silver Nano Particles to Establish Efficient Callogenesis and Regeneration System for Wheat (Triticum aestivum L.). GM Crops Food 2021, 12, 564–585. [Google Scholar] [CrossRef]
- Anwaar, S.; Maqbool, Q.; Jabeen, N.; Nazar, M.; Abbas, F.; Nawaz, B.; Hussain, T.; Hussain, S.Z. Addendum: The Effect of Green Synthesized CuO Nanoparticles on Callogenesis and Regeneration of Oryza sativa L. Front. Plant Sci. 2020, 11, 540. [Google Scholar] [CrossRef]
- Genady, E.A.; Qaid, E.A.; Fahmy, A.H. Copper Sulfate Nanoparticales In Vitro Applications on Verbena Bipinnatifida Nutt. Stimulating Growth and Total Phenolic Content Increasments. Int. J. Pharm. Res. Allied Sci. 2016, 5, 196–202. [Google Scholar]
- Grodetskaya, T.A.; Evlakov, P.M.; Fedorova, O.A.; Mikhin, V.I.; Zakharova, O.V.; Kolesnikov, E.A.; Evtushenko, N.A.; Gusev, A.A. Influence of Copper Oxide Nanoparticles on Gene Expression of Birch Clones In Vitro under Stress Caused by Phytopathogens. Nanomaterials 2022, 12, 864. [Google Scholar] [CrossRef]
- Chung, I.M.; Rajakumar, G.; Subramanian, U.; Venkidasamy, B.; Thiruvengadam, M. Impact of Copper Oxide Nanoparticles on Enhancement of Bioactive Compounds Using Cell Suspension Cultures of Gymnema sylvestre (Retz.) R. Br. Appl. Sci. 2019, 9, 2165. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonio-Pérez, A.; Durán-Armenta, L.F.; Pérez-Loredo, M.G.; Torres-Huerta, A.L. Biosynthesis of Copper Nanoparticles with Medicinal Plants Extracts: From Extraction Methods to Applications. Micromachines 2023, 14, 1882. https://doi.org/10.3390/mi14101882
Antonio-Pérez A, Durán-Armenta LF, Pérez-Loredo MG, Torres-Huerta AL. Biosynthesis of Copper Nanoparticles with Medicinal Plants Extracts: From Extraction Methods to Applications. Micromachines. 2023; 14(10):1882. https://doi.org/10.3390/mi14101882
Chicago/Turabian StyleAntonio-Pérez, Aurora, Luis Fernando Durán-Armenta, María Guadalupe Pérez-Loredo, and Ana Laura Torres-Huerta. 2023. "Biosynthesis of Copper Nanoparticles with Medicinal Plants Extracts: From Extraction Methods to Applications" Micromachines 14, no. 10: 1882. https://doi.org/10.3390/mi14101882
APA StyleAntonio-Pérez, A., Durán-Armenta, L. F., Pérez-Loredo, M. G., & Torres-Huerta, A. L. (2023). Biosynthesis of Copper Nanoparticles with Medicinal Plants Extracts: From Extraction Methods to Applications. Micromachines, 14(10), 1882. https://doi.org/10.3390/mi14101882








