Droplet Detection and Sorting System in Microfluidics: A Review
Abstract
1. Introduction
2. Detection Methods
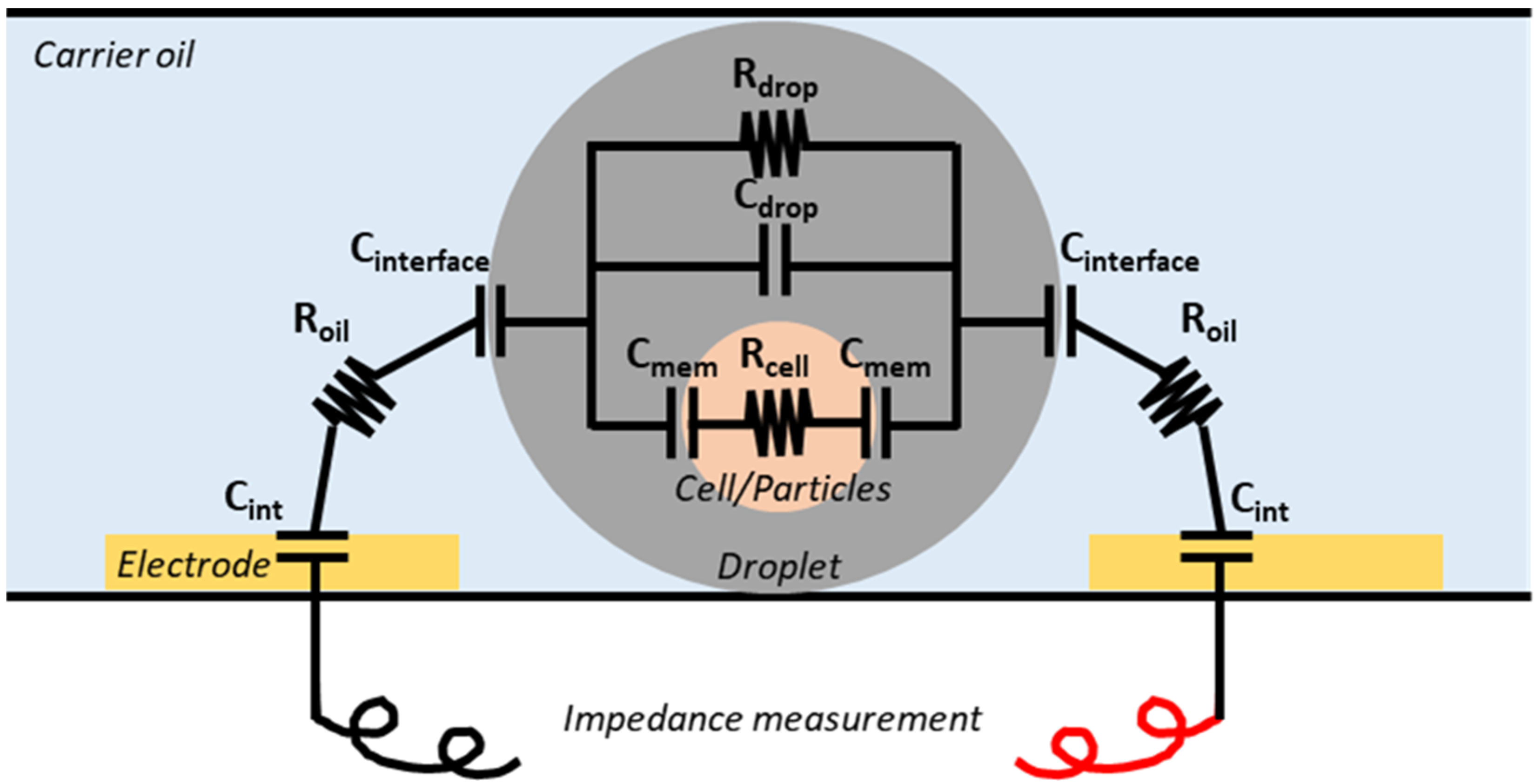
2.1. Impedance-Based Droplet Detection
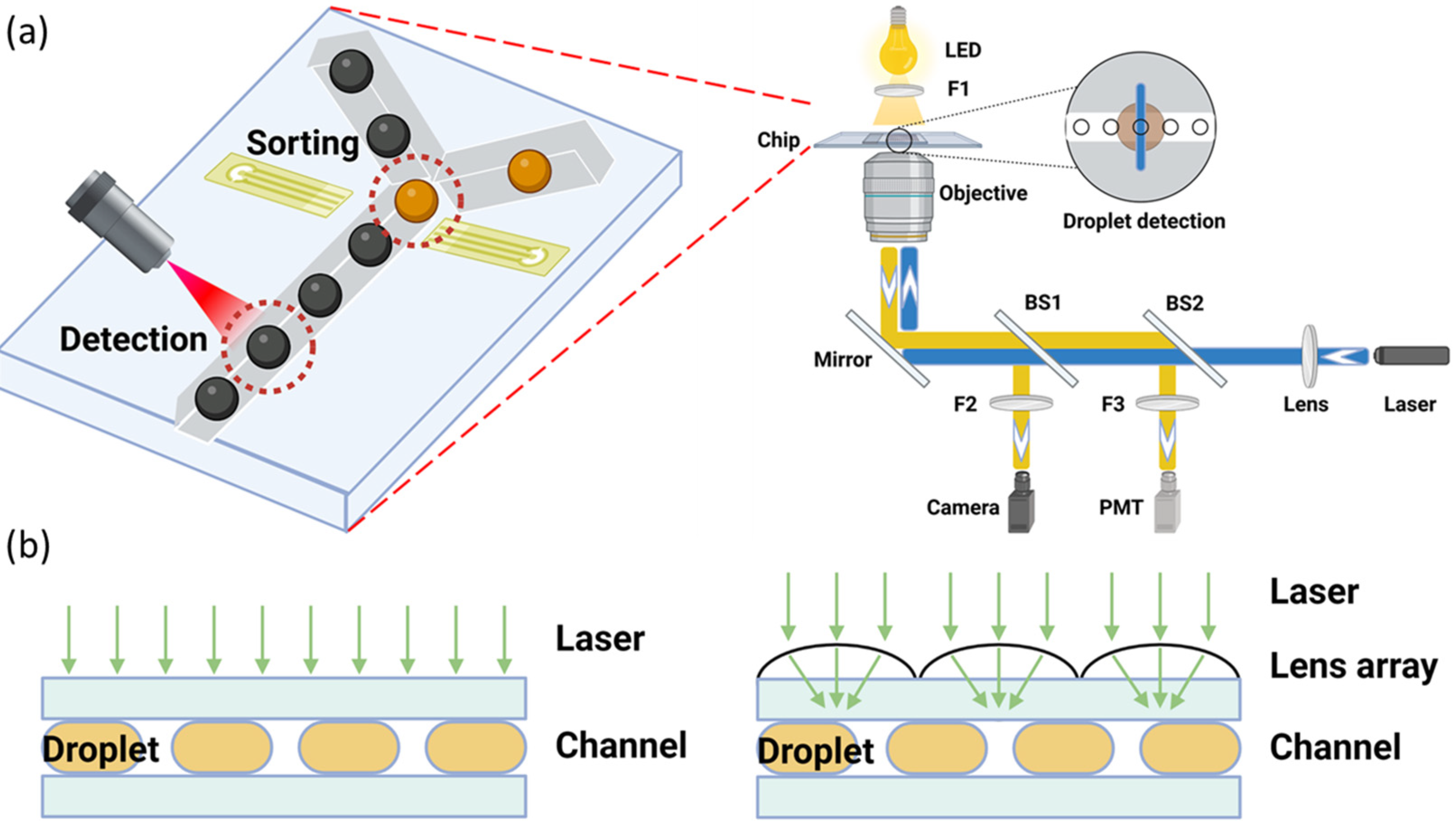
2.2. Fluorescent Emission-Based Droplet Detection
2.3. Other Visible Light-Based Droplet Detection
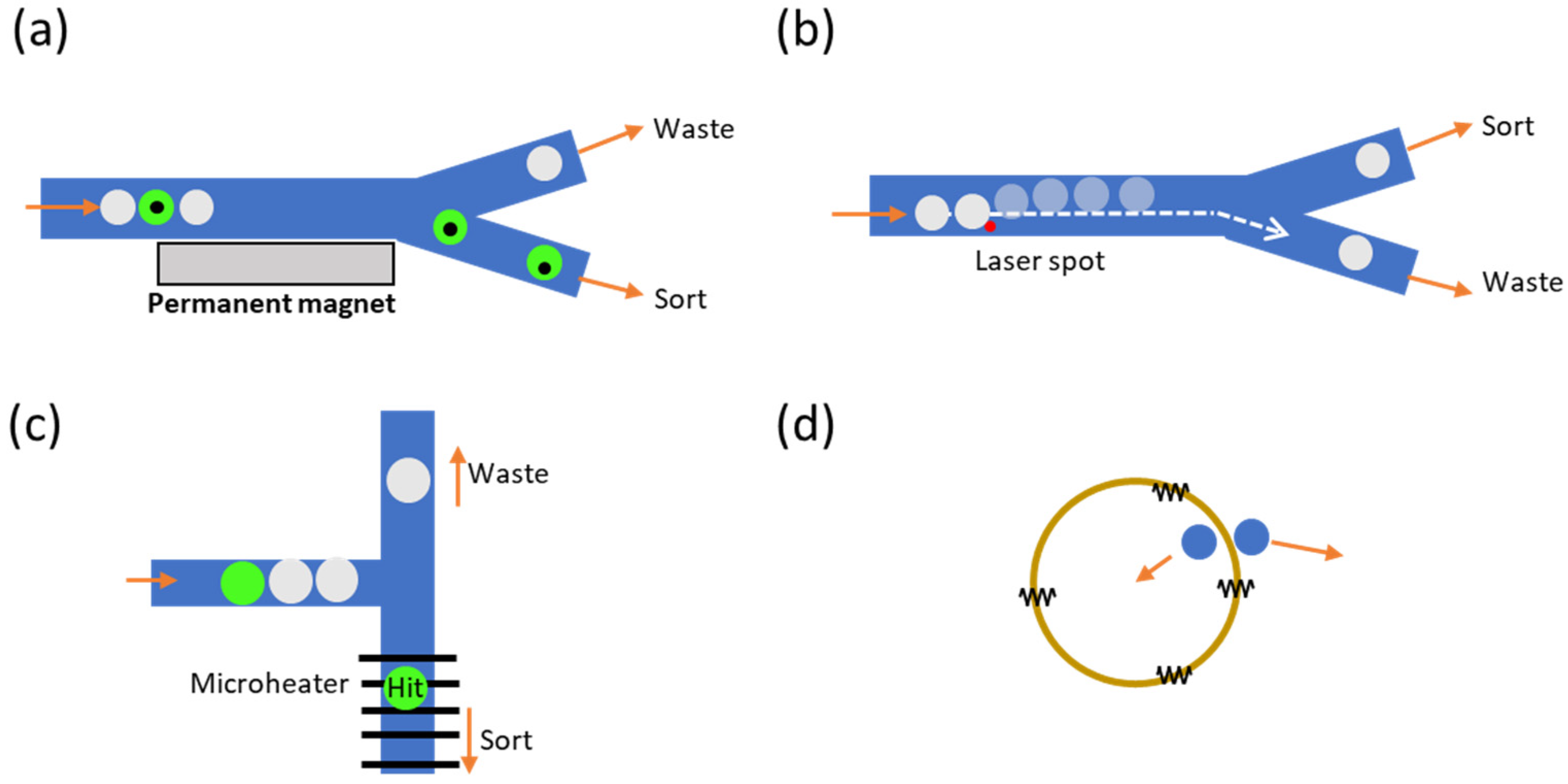
3. Droplet Sorting Methods
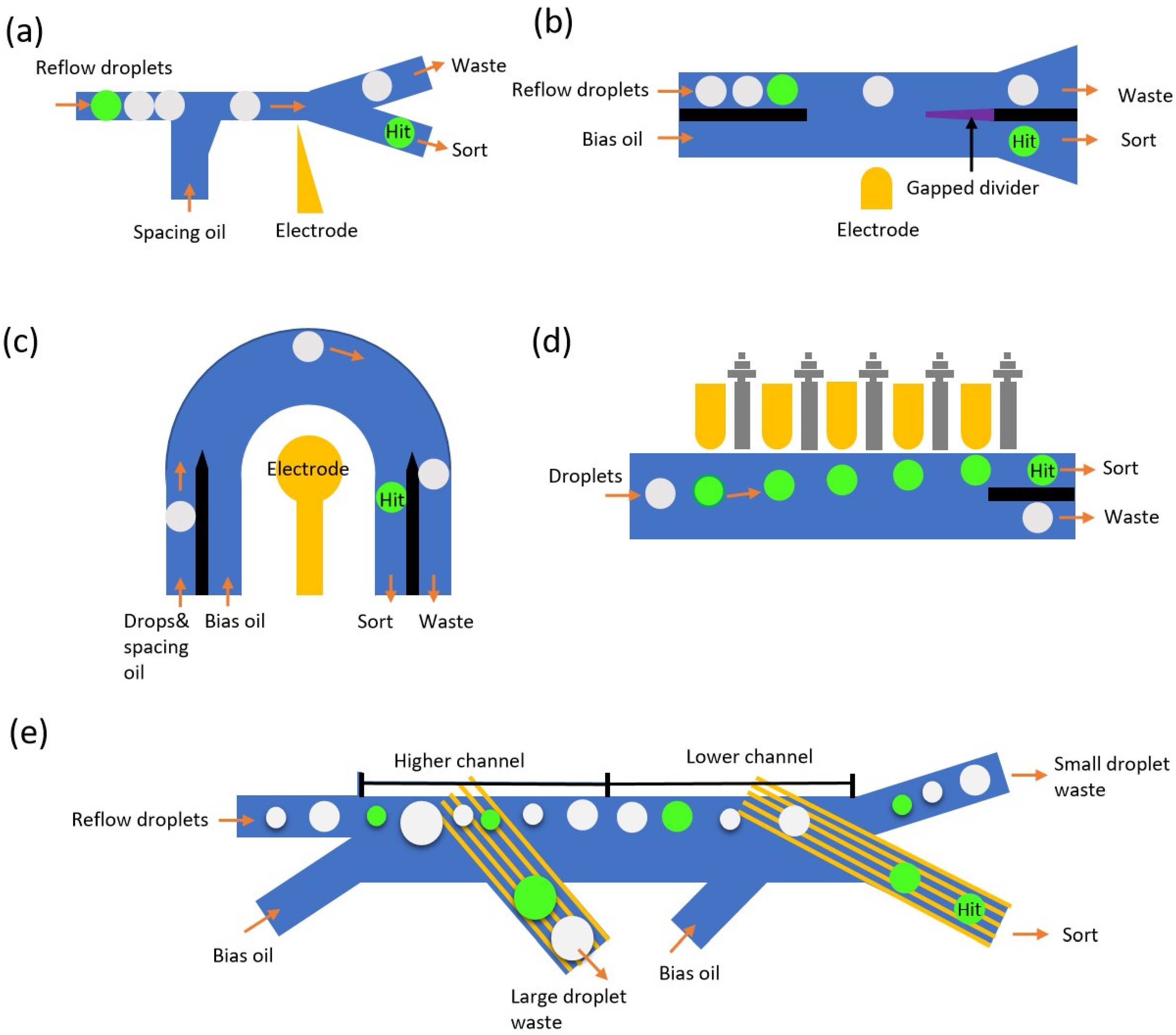
3.1. Pneumatic-Based Droplet Sorting
3.2. Dielectrophoretic-Based Droplet Sorting
3.2.1. Early Version of DEP Sorters
3.2.2. Improved DEP Sorters
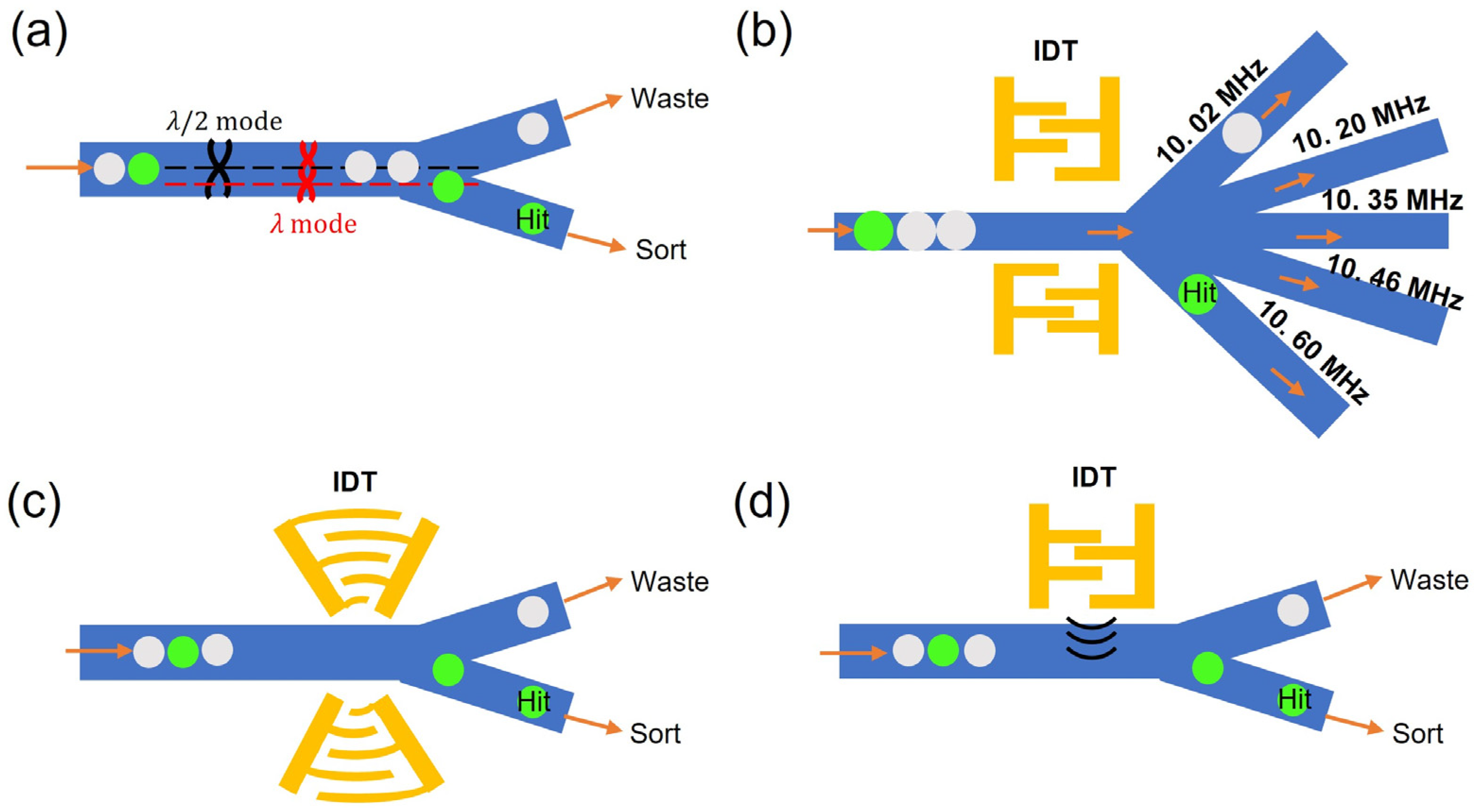
3.3. Acoustic-Based Droplet Sorting
3.4. Other Active Sorting Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Chen, Z.; Bian, F.; Shang, L.; Zhu, K.; Zhao, Y. Advances of droplet-based microfluidics in drug discovery. Expert Opin. Drug Discov. 2020, 15, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef]
- Schneider, G. Automating drug discovery. Nat. Rev. Drug Discov. 2018, 17, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yakar, A. High-content and high-throughput in vivo drug screening platforms using microfluidics. Assay Drug Dev. Technol. 2019, 17, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Elvira, K.S. Microfluidic technologies for drug discovery and development: Friend or foe? Trends Pharmacol. Sci. 2021, 42, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Torabinia, M.; Dakarapu, U.S.; Asgari, P.; Jeon, J.; Moon, H. Electrowetting-on-dielectric (EWOD) digital microfluidic device for in-line workup in organic reactions: A critical step in the drug discovery work cycle. Sens. Actuators B Chem. 2021, 330, 129252. [Google Scholar] [CrossRef]
- Chen, Z.; Kheiri, S.; Gevorkian, A.; Young, E.W.; Andre, V.; Deisenroth, T.; Kumacheva, E. Microfluidic arrays of dermal spheroids: A screening platform for active ingredients of skincare products. Lab Chip 2021, 21, 3952–3962. [Google Scholar] [CrossRef]
- Honrado, C.; Bisegna, P.; Swami, N.S.; Caselli, F. Single-cell microfluidic impedance cytometry: From raw signals to cell phenotypes using data analytics. Lab Chip 2021, 21, 22–54. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, T.; Zhang, J.; Wong, S.C.C.; Ritchie, M.; Hou, H.W.; Wang, Y. Single cell metabolite detection using inertial microfluidics-assisted ion mobility mass spectrometry. Anal. Chem. 2021, 93, 10462–10468. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Ohnuki, S.; Kondo, N.; Itto-Nakama, K.; Ghanegolmohammadi, F.; Isozaki, A.; Ohya, Y.; Goda, K. Are droplets really suitable for single-cell analysis? A case study on yeast in droplets. Lab Chip 2021, 21, 3793–3803. [Google Scholar] [CrossRef]
- Kim, Y.; Song, J.; Lee, Y.; Cho, S.; Kim, S.; Lee, S.-R.; Park, S.; Shin, Y.; Jeon, N.L. High-throughput injection molded microfluidic device for single-cell analysis of spatiotemporal dynamics. Lab Chip 2021, 21, 3150–3158. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.; Feng, Y.; Liang, F.; Wang, W. A microfluidic device enabling deterministic single cell trapping and release. Lab Chip 2021, 21, 2486–2494. [Google Scholar] [CrossRef]
- Terada, M.; Ide, S.; Naito, T.; Kimura, N.; Matsusaki, M.; Kaji, N. Label-Free Cancer Stem-like Cell Assay Conducted at a Single Cell Level Using Microfluidic Mechanotyping Devices. Anal. Chem. 2021, 93, 14409–14416. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Briggs, R.G.; Homburg, H.B.; Young, I.M.; Davis, E.J.; Lin, Y.-H.; Battiste, J.D.; Sughrue, M.E. Application of microfluidic devices for glioblastoma study: Current status and future directions. Biomed. Microdevices 2020, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, L.; Zhang, H.; Shen, X.; Zhu, Y.; Chen, H. Novel wax valves to improve distance-based analyte detection in paper microfluidics. Anal. Chem. 2019, 91, 5169–5175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Smith, E.; Zhang, W.; Zhou, A. Inkjet printed microfluidic paper-based analytical device (μPAD) for glucose colorimetric detection in artificial urine. Biomed. Microdevices 2019, 21, 48. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Dai, J.; Zhang, W.; Jiang, Y.; Zhou, A. A low-cost mobile platform for whole blood glucose monitoring using colorimetric method. Microchem. J. 2021, 162, 105814. [Google Scholar] [CrossRef]
- Ilkhani, H.; Zhang, H.; Zhou, A. A novel three-dimensional microTAS chip for ultra-selective single base mismatched Cryptosporidium DNA biosensor. Sens. Actuators B Chem. 2019, 282, 675–683. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, H.; Huang, C.; Chen, Z.; Han, A. A gel-based separation-free point-of-care device for whole blood glucose detection. Anal. Chem. 2020, 92, 16122–16129. [Google Scholar] [CrossRef]
- Zhang, H.; Anoop, K.; Huang, C.; Sadr, R.; Gupte, R.; Dai, J.; Han, A. A circular gradient-width crossflow microfluidic platform for high-efficiency blood plasma separation. Sens. Actuators B Chem. 2022, 354, 131180. [Google Scholar] [CrossRef]
- Battat, S.; Weitz, D.A.; Whitesides, G.M. An outlook on microfluidics: The promise and the challenge. Lab Chip 2022, 22, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Kamyabi, N.; Abbasgholizadeh, R.; Maitra, A.; Ardekani, A.; Biswal, S.L.; Grande-Allen, K.J. Isolation and mutational assessment of pancreatic cancer extracellular vesicles using a microfluidic platform. Biomed. Microdevices 2020, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-Z.; Tian, G.; Ko, A.C.-T.; Geissler, M.; Brassard, D.; Veres, T. Detection of renal biomarkers in chronic kidney disease using microfluidics: Progress, challenges and opportunities. Biomed. Microdevices 2020, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Takama, N.; Kinoshita, R.; Okitsu, T.; Kim, B. Flexible and porous microneedles of PDMS for continuous glucose monitoring. Biomed. Microdevices 2020, 22, 79. [Google Scholar] [CrossRef]
- Chen, H.; Cornwell, J.; Zhang, H.; Lim, T.; Resurreccion, R.; Port, T.; Rosengarten, G.; Nordon, R.E. Cardiac-like flow generator for long-term imaging of endothelial cell responses to circulatory pulsatile flow at microscale. Lab Chip 2013, 13, 2999–3007. [Google Scholar] [CrossRef] [PubMed]
- Guenat, O.T.; Berthiaume, F. Incorporating mechanical strain in organs-on-a-chip: Lung and skin. Biomicrofluidics 2018, 12, 042207. [Google Scholar] [CrossRef] [PubMed]
- Schneider, O.; Zeifang, L.; Fuchs, S.; Sailer, C.; Loskill, P. User-friendly and parallelized generation of human induced pluripotent stem cell-derived microtissues in a centrifugal heart-on-a-chip. Tissue Eng. Part A 2019, 25, 786–798. [Google Scholar] [CrossRef]
- Fois, C.A.; Schindeler, A.; Valtchev, P.; Dehghani, F. Dynamic flow and shear stress as key parameters for intestinal cells morphology and polarization in an organ-on-a-chip model. Biomed. Microdevices 2021, 23, 55. [Google Scholar] [CrossRef]
- Sugimura, R.; Ohta, R.; Mori, C.; Li, A.; Mano, T.; Sano, E.; Kosugi, K.; Nakahata, T.; Niwa, A.; Saito, M.K. Biomimetic aorta-gonad-mesonephros-on-a-chip to study human developmental hematopoiesis. Biomed. Microdevices 2020, 22, 34. [Google Scholar] [CrossRef]
- Arık, Y.B.; Buijsman, W.; Loessberg-Zahl, J.; Cuartas-Vélez, C.; Veenstra, C.; Logtenberg, S.; Grobbink, A.M.; Bergveld, P.; Gagliardi, G.; den Hollander, A.I. Microfluidic organ-on-a-chip model of the outer blood–retinal barrier with clinically relevant read-outs for tissue permeability and vascular structure. Lab Chip 2021, 21, 272–283. [Google Scholar] [CrossRef]
- Brooks, Z.; Kim, K.; Zhao, K.; Goswami, T.; Hussain, S.; Dixon, A.R. 3D printed transwell-integrated nose-on-chip model to evaluate effects of air flow-induced mechanical stresses on mucous secretion. Biomed. Microdevices 2022, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Al-Mofty, S.; Elsayed, M.; Ali, H.; Ahmed, O.; Altayyeb, A.; Wahby, A.; Abdelgawad, M.; Mousa, N. A microfluidic platform for dissociating clinical scale tissue samples into single cells. Biomed. Microdevices 2021, 23, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, S.; Chen, C.; Huang, H.; Tao, L.; Qian, Z.; Li, W. Microfluidic-enabled self-organized tumor model for in vitro cytotoxicity assessment of doxorubicin. Biomed. Microdevices 2020, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Sun, M.; Zhang, J.; Hu, R.; Fu, W.; Xuanyuan, T.; Liu, W. Single-cell droplet microfluidics for biomedical applications. Analyst 2022, 147, 2294–2316. [Google Scholar] [CrossRef]
- Lin, W.N.; Tay, M.Z.; Wong, J.X.E.; Lee, C.Y.; Fong, S.-W.; Wang, C.-I.; Ng, L.F.P.; Renia, L.; Chen, C.-H.; Cheow, L.F. Rapid microfluidic platform for screening and enrichment of cells secreting virus neutralizing antibodies. Lab Chip 2022, 22, 2578–2589. [Google Scholar] [CrossRef]
- Hsieh, K.; Mach, K.E.; Zhang, P.; Liao, J.C.; Wang, T.-H. Combating Antimicrobial Resistance via Single-Cell Diagnostic Technologies Powered by Droplet Microfluidics. Acc. Chem. Res. 2021, 55, 123–133. [Google Scholar] [CrossRef]
- Yu, Z.; Geisler, K.; Leontidou, T.; Young, R.E.; Vonlanthen, S.E.; Purton, S.; Abell, C.; Smith, A.G. Droplet-based microfluidic screening and sorting of microalgal populations for strain engineering applications. Algal Res. 2021, 56, 102293. [Google Scholar] [CrossRef]
- Zhang, H.; Guzman, A.R.; Wippold, J.A.; Li, Y.; Dai, J.; Huang, C.; Han, A. An ultra high-efficiency droplet microfluidics platform using automatically synchronized droplet pairing and merging. Lab Chip 2020, 20, 3948–3959. [Google Scholar] [CrossRef]
- Huang, C.; Wippold, J.A.; Stratis-Cullum, D.; Han, A. Eliminating air bubble in microfluidic systems utilizing integrated in-line sloped microstructures. Biomed. Microdevices 2020, 22, 76. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, H.; Han, S.-I.; Han, A. Cell washing and solution exchange in droplet microfluidic systems. Anal. Chem. 2021, 93, 8622–8630. [Google Scholar] [CrossRef]
- Han, A.; Guzman, A.; Zhang, H.; Wippold, J.; Dai, J. Ultra High Efficiency Microfluidic Platform. U.S. Patent US20220097066A1, 31 March 2022. [Google Scholar]
- Zhang, P.; Chang, K.-C.; Abate, A.R. Precision ejection of microfluidic droplets into air with a superhydrophobic outlet. Lab Chip 2021, 21, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Abate, A.R. High-Definition Single-Cell Printing: Cell-by-Cell Fabrication of Biological Structures. Adv. Mater. 2020, 32, 2005346. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Huang, C.; Zhang, H.; Samuel, R.; Li, Y.; Jayaraman, A.; de Figueiredo, P.; Han, A. Microfluidic Dielectrophoretic Method Enables On-Demand Spatial Arrangement of Bacteria-Encapsulated Agarose Gel Microparticles. Anal. Chem. 2022, 94, 13197–13204. [Google Scholar] [CrossRef] [PubMed]
- Gencturk, E.; Yurdakul, E.; Celik, A.Y.; Mutlu, S.; Ulgen, K.O. Cell trapping microfluidic chip made of Cyclo olefin polymer enabling two concurrent cell biology experiments with long term durability. Biomed. Microdevices 2020, 22, 20. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, Y.; Xu, Q.; Sun, X.; Meng, F. Recent advances on sorting methods of high-throughput droplet-based microfluidics in enzyme directed evolution. Front. Chem. 2021, 9, 666867. [Google Scholar] [CrossRef]
- Zhong, R.; Yang, S.; Ugolini, G.S.; Naquin, T.; Zhang, J.; Yang, K.; Xia, J.; Konry, T.; Huang, T.J. Acoustofluidic Droplet Sorter Based on Single Phase Focused Transducers. Small 2021, 17, 2103848. [Google Scholar] [CrossRef]
- Clark, I.C.; Thakur, R.; Abate, A.R. Concentric electrodes improve microfluidic droplet sorting. Lab Chip 2018, 18, 710–713. [Google Scholar] [CrossRef]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging Droplet Microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, Z.; Wu, M.; Lan, Y.; Jia, C.; Zhao, J. Single-cell sorting using integrated pneumatic valve droplet microfluidic chip. Talanta 2023, 253, 124044. [Google Scholar] [CrossRef]
- Banerjee, U.; Jain, S.; Sen, A. Particle encapsulation in aqueous ferrofluid drops and sorting of particle-encapsulating drops from empty drops using a magnetic field. Soft Matter 2021, 17, 6020–6028. [Google Scholar] [CrossRef]
- Yap, Y.-F.; Tan, S.-H.; Nguyen, N.-T.; Murshed, S.S.; Wong, T.-N.; Yobas, L. Thermally mediated control of liquid microdroplets at a bifurcation. J. Phys. D Appl. Phys. 2009, 42, 065503. [Google Scholar] [CrossRef]
- Gerlt, M.S.; Haidas, D.; Ratschat, A.; Suter, P.; Dittrich, P.S.; Dual, J. Manipulation of single cells inside nanoliter water droplets using acoustic forces. Biomicrofluidics 2020, 14, 064112. [Google Scholar] [CrossRef] [PubMed]
- Mernier, G.; Piacentini, N.; Tornay, R.; Buffi, N.; Renaud, P. Cell viability assessment by flow cytometry using yeast as cell model. Sens. Actuators B Chem. 2011, 154, 160–163. [Google Scholar] [CrossRef]
- Sun, T.; van Berkel, C.; Green, N.G.; Morgan, H. Digital signal processing methods for impedance microfluidic cytometry. Microfluid. Nanofluid. 2009, 6, 179–187. [Google Scholar] [CrossRef]
- Gawad, S.; Holmes, D.; Benazzi, G.; Renaud, P.; Morgan, H. Impedance spectroscopy and optical analysis of single biological cells and organisms in microsystems. Methods Mol. Biol. 2010, 583, 149–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y. A Digital Microfluidic Device Integrated with Electrochemical Impedance Spectroscopy for Cell-Based Immunoassay. Biosensors 2022, 12, 330. [Google Scholar] [CrossRef]
- Clausen, C.H.; Dimaki, M.; Bertelsen, C.V.; Skands, G.E.; Rodriguez-Trujillo, R.; Thomsen, J.D.; Svendsen, W.E. Bacteria Detection and Differentiation Using Impedance Flow Cytometry. Sensors 2018, 18, 3496. [Google Scholar] [CrossRef]
- Ishai, P.B.; Sobol, Z.; Nickels, J.D.; Agapov, A.L.; Sokolov, A.P. An assessment of comparative methods for approaching electrode polarization in dielectric permittivity measurements. Rev. Sci. Instrum. 2012, 83, 083118. [Google Scholar] [CrossRef]
- Chawla, K.; Bürgel, S.C.; Schmidt, G.W.; Kaltenbach, H.-M.; Rudolf, F.; Frey, O.; Hierlemann, A. Integrating impedance-based growth-rate monitoring into a microfluidic cell culture platform for live-cell microscopy. Microsyst. Nanoeng. 2018, 4, 8. [Google Scholar] [CrossRef]
- Wang, H.; Sobahi, N.; Han, A. Impedance spectroscopy-based cell/particle position detection in microfluidic systems. Lab Chip 2017, 17, 1264–1269. [Google Scholar] [CrossRef]
- Wang, N.; Liu, R.; Asmare, N.; Chu, C.-H.; Sarioglu, A.F. Processing code-multiplexed Coulter signals via deep convolutional neural networks. Lab Chip 2019, 19, 3292–3304. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, X.; Zhou, Z.; Han, Y.; Xiang, N.; Ni, Z. Microfluidic impedance cytometry for single-cell sensing: Review on electrode configurations. Talanta 2021, 233, 122571. [Google Scholar] [CrossRef] [PubMed]
- Salahi, A.; Rane, A.; Xiao, L.; Honrado, C.; Li, X.; Jin, L.; Swami, N.S. Single-cell assessment of the modulation of macrophage activation by ex vivo intervertebral discs using impedance cytometry. Biosens. Bioelectron. 2022, 210, 114346. [Google Scholar] [CrossRef] [PubMed]
- de Wagenaar, B.; Dekker, S.; de Boer, H.L.; Bomer, J.G.; Olthuis, W.; van den Berg, A.; Segerink, L.I. Towards microfluidic sperm refinement: Impedance-based analysis and sorting of sperm cells. Lab Chip 2016, 16, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Brian, P.C. Optimization of an impedance sensor for droplet-based microfluidic systems. In Proceedings of the SPIE Microtechnologies, Prague, Czech Republic, 18–20 April 2011; p. 80660F. [Google Scholar]
- Gu, S.; Lu, Y.; Ding, Y.; Li, L.; Song, H.; Wang, J.; Wu, Q. A droplet-based microfluidic electrochemical sensor using platinum-black microelectrode and its application in high sensitive glucose sensing. Biosens. Bioelectron. 2014, 55, 106–112. [Google Scholar] [CrossRef]
- Yakdi, N.E.; Huet, F.; Ngo, K. Detection and sizing of single droplets flowing in a lab-on-a-chip device by measuring impedance fluctuations. Sens. Actuators B Chem. 2016, 236, 794–804. [Google Scholar] [CrossRef]
- Kemna, E.W.M.; Segerink, L.I.; Wolbers, F.; Vermes, I.; van den Berg, A. Label-free, high-throughput, electrical detection of cells in droplets. Analyst 2013, 138, 4585–4592. [Google Scholar] [CrossRef]
- Marcali, M.; Elbuken, C. Impedimetric detection and lumped element modelling of a hemagglutination assay in microdroplets. Lab Chip 2016, 16, 2494–2503. [Google Scholar] [CrossRef]
- Fan, W.; Chen, X.; Ge, Y.; Jin, Y.; Jin, Q.; Zhao, J. Single-cell impedance analysis of osteogenic differentiation by droplet-based microfluidics. Biosens. Bioelectron. 2019, 145, 111730. [Google Scholar] [CrossRef]
- Cao, J.; Pliquett, U.; Yang, L.; Wiedemeier, S.; Cahill, B.; Michael Köhler, J. Contactless optical and impedimetric sensing for droplet-based dose-response investigations of microorganisms. Sens. Actuators B Chem. 2022, 372, 132688. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, H.S.; Frazier, A.B.; Chen, Z.G.; Shin, D.M.; Han, A. Whole-Cell Impedance Analysis for Highly and Poorly Metastatic Cancer Cells. J. Microelectromech. S 2009, 18, 808–817. [Google Scholar] [CrossRef]
- Wippold, J.A.; Huang, C.; Stratis-Cullum, D.; Han, A. Enhancing droplet transition capabilities using sloped microfluidic channel geometry for stable droplet operation. Biomed. Microdevices 2020, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Miller, O.J.; El Harrak, A.; Mangeat, T.; Baret, J.C.; Frenz, L.; El Debs, B.; Mayot, E.; Samuels, M.L.; Rooney, E.K.; Dieu, P.; et al. High-resolution dose-response screening using droplet-based microfluidics. Proc. Natl. Acad. Sci. USA 2012, 109, 378–383. [Google Scholar] [CrossRef]
- Seemann, R.; Brinkmann, M.; Pfohl, T.; Herminghaus, S. Droplet based microfluidics. Rep. Prog. Phys. 2012, 75, 016601. [Google Scholar] [CrossRef]
- Baret, J.C.; Miller, O.J.; Taly, V.; Ryckelynck, M.; El-Harrak, A.; Frenz, L.; Rick, C.; Samuels, M.L.; Hutchison, J.B.; Agresti, J.J.; et al. Fluorescence-activated droplet sorting (FADS): Efficient microfluidic cell sorting based on enzymatic activity. Lab Chip 2009, 9, 1850–1858. [Google Scholar] [CrossRef]
- Agresti, J.J.; Antipov, E.; Abate, A.R.; Ahn, K.; Rowat, A.C.; Baret, J.C.; Marquez, M.; Klibanov, A.M.; Griffiths, A.D.; Weitz, D.A. Ultrahigh-throughput screening in drop-based microfluidics for directed evolution. Proc. Natl. Acad. Sci. USA 2010, 107, 4004–4009. [Google Scholar] [CrossRef] [PubMed]
- Fallah-Araghi, A.; Baret, J.-C.; Ryckelynck, M.; Griffiths, A.D. A completely in vitro ultrahigh-throughput droplet-based microfluidic screening system for protein engineering and directed evolution. Lab Chip 2012, 12, 882–891. [Google Scholar] [CrossRef]
- Mazutis, L.; Gilbert, J.; Ung, W.L.; Weitz, D.A.; Griffiths, A.D.; Heyman, J.A. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 2013, 8, 870–891. [Google Scholar] [CrossRef]
- El Debs, B.; Utharala, R.; Balyasnikova, I.V.; Griffiths, A.D.; Merten, C.A. Functional single-cell hybridoma screening using droplet-based microfluidics. Proc. Natl. Acad. Sci. USA 2012, 109, 11570–11575. [Google Scholar] [CrossRef]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C.; et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 2011, 83, 8604–8610. [Google Scholar] [CrossRef]
- Li, Y.; Huang, C.; Han, S.-I.; Han, A. Measurement of dielectric properties of cells at single-cell resolution using electrorotation. Biomed. Microdevices 2022, 24, 23. [Google Scholar] [CrossRef] [PubMed]
- Kapuscinski, J. DAPI: A DNA-specific fluorescent probe. Biotech. Histochem. 1995, 70, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Bucevičius, J.; Lukinavičius, G.; Gerasimaitė, R. The Use of Hoechst Dyes for DNA Staining and beyond. Chemosensors 2018, 6, 18. [Google Scholar] [CrossRef]
- Hasan, S.; Geissler, D.; Wink, K.; Hagen, A.; Heiland, J.J.; Belder, D. Fluorescence lifetime-activated droplet sorting in microfluidic chip systems. Lab Chip 2019, 19, 403–409. [Google Scholar] [CrossRef]
- Royer, C.A. Fluorescence spectroscopy. Methods Mol. Biol. 1995, 40, 65–89. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006. [Google Scholar]
- Leonard, J.; Dumas, N.; Causse, J.P.; Maillot, S.; Giannakopoulou, N.; Barre, S.; Uhring, W. High-throughput time-correlated single photon counting. Lab Chip 2014, 14, 4338–4343. [Google Scholar] [CrossRef]
- Skilitsi, A.I.; Turko, T.; Cianfarani, D.; Barre, S.; Uhring, W.; Hassiepen, U.; Leonard, J. Towards sensitive, high-throughput, biomolecular assays based on fluorescence lifetime. Methods Appl. Fluoresc. 2017, 5, 034002. [Google Scholar] [CrossRef]
- Roulet, J.C.; Volkel, R.; Herzig, H.P.; Verpoorte, E.; de Rooij, N.F.; Dandliker, R. Performance of an integrated microoptical system for fluorescence detection in microfluidic systems. Anal. Chem. 2002, 74, 3400–3407. [Google Scholar] [CrossRef]
- Li, Q.; Chen, P.; Fan, Y.; Wang, X.; Xu, K.; Li, L.; Tang, B. Multicolor Fluorescence Detection-Based Microfluidic Device for Single-Cell Metabolomics: Simultaneous Quantitation of Multiple Small Molecules in Primary Liver Cells. Anal. Chem. 2016, 88, 8610–8616. [Google Scholar] [CrossRef]
- Michener, J.K.; Smolke, C.D. High-throughput enzyme evolution in Saccharomyces cerevisiae using a synthetic RNA switch. Metab. Eng. 2012, 14, 306–316. [Google Scholar] [CrossRef]
- Chen, C.H.; Miller, M.A.; Sarkar, A.; Beste, M.T.; Isaacson, K.B.; Lauffenburger, D.A.; Griffith, L.G.; Han, J. Multiplexed protease activity assay for low-volume clinical samples using droplet-based microfluidics and its application to endometriosis. J. Am. Chem. Soc. 2013, 135, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.H.; Gartner, Z.J.; Abate, A.R. Multicolor Fluorescence Detection for Droplet Microfluidics Using Optical Fibers. J. Vis. Exp. 2016, 111, e54010. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.H.; de Lange, N.; Gartner, Z.J.; Abate, A.R. Compact and modular multicolour fluorescence detector for droplet microfluidics. Lab Chip 2015, 15, 2754–2758. [Google Scholar] [CrossRef] [PubMed]
- Franch, N.; Alonso, O.; Canals, J.; Vilà, A.; Dieguez, A. A low cost fluorescence lifetime measurement system based on SPAD detectors and FPGA processing. J. Instrum. 2017, 12, C02070. [Google Scholar] [CrossRef]
- Lu, H.; Caen, O.; Vrignon, J.; Zonta, E.; El Harrak, Z.; Nizard, P.; Baret, J.C.; Taly, V. High throughput single cell counting in droplet-based microfluidics. Sci. Rep. 2017, 7, 1366. [Google Scholar] [CrossRef]
- Lim, J.; Gruner, P.; Konrad, M.; Baret, J.C. Micro-optical lens array for fluorescence detection in droplet-based microfluidics. Lab Chip 2013, 13, 1472–1475. [Google Scholar] [CrossRef]
- Schonbrun, E.; Abate, A.R.; Steinvurzel, P.E.; Weitz, D.A.; Crozier, K.B. High-throughput fluorescence detection using an integrated zone-plate array. Lab Chip 2010, 10, 852–856. [Google Scholar] [CrossRef]
- Eggeling, C.; Volkmer, A.; Seidel, C.A. Molecular photobleaching kinetics of rhodamine 6G by one-and two-photon induced confocal fluorescence microscopy. ChemPhysChem 2005, 6, 791–804. [Google Scholar] [CrossRef]
- Vaijayanthimala, V.; Tzeng, Y.-K.; Chang, H.-C.; Li, C.-L. The biocompatibility of fluorescent nanodiamonds and their mechanism of cellular uptake. Nanotechnology 2009, 20, 425103. [Google Scholar] [CrossRef]
- Sivaramakrishnan, M.; Kothandan, R.; Govindarajan, D.K.; Meganathan, Y.; Kandaswamy, K. Active microfluidic systems for cell sorting and separation. Curr. Opin. Biomed. Eng. 2020, 13, 60–68. [Google Scholar] [CrossRef]
- Pereira, H.; Schulze, P.S.; Schüler, L.M.; Santos, T.; Barreira, L.; Varela, J. Fluorescence activated cell-sorting principles and applications in microalgal biotechnology. Algal Res. 2018, 30, 113–120. [Google Scholar] [CrossRef]
- Gielen, F.; Hours, R.; Emond, S.; Fischlechner, M.; Schell, U.; Hollfelder, F. Ultrahigh-throughput–directed enzyme evolution by absorbance-activated droplet sorting (AADS). Proc. Natl. Acad. Sci. USA 2016, 113, E7383–E7389. [Google Scholar] [CrossRef] [PubMed]
- Sesen, M.; Whyte, G. Image-based single cell sorting automation in droplet microfluidics. Sci. Rep. 2020, 10, 8736. [Google Scholar] [CrossRef] [PubMed]
- Adan, A.; Alizada, G.; Kiraz, Y.; Baran, Y.; Nalbant, A. Flow cytometry: Basic principles and applications. Crit. Rev. Biotechnol. 2017, 37, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.F.; Engh, G.v.d. Flow cytometry and cell sorting. Cell Sep. 2007, 19–39. [Google Scholar]
- Polli, D.; Kumar, V.; Valensise, C.M.; Marangoni, M.; Cerullo, G. Broadband coherent Raman scattering microscopy. Laser Photonics Rev. 2018, 12, 1800020. [Google Scholar] [CrossRef]
- Zhang, H.; Silva, A.C.; Zhang, W.; Rutigliano, H.; Zhou, A. Raman Spectroscopy characterization extracellular vesicles from bovine placenta and peripheral blood mononuclear cells. PLoS ONE 2020, 15, e0235214. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, L.; Li, Q.; Qi, X.; Zhou, A. Microfluidic chip for non-invasive analysis of tumor cells interaction with anti-cancer drug doxorubicin by AFM and Raman spectroscopy. Biomicrofluidics 2018, 12, 024119. [Google Scholar] [CrossRef]
- Zhou, A.; Zhang, H.; Li, Q.; Xiao, L. AFM and Raman Spectroscopy, Applications in Cellular Imaging and Assays; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Geng, J.; Zhang, W.; Chen, C.; Zhang, H.; Zhou, A.; Huang, Y. Tracking the differentiation status of human neural stem cells through label-free raman spectroscopy and machine learning-based analysis. Anal. Chem. 2021, 93, 10453–10461. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Q.; Tang, M.; Zhang, H.; Sun, X.; Zou, S.; Jensen, J.L.; Liou, T.G.; Zhou, A. A multi-scale approach to study biochemical and biophysical aspects of resveratrol on diesel exhaust particle-human primary lung cell interaction. Sci. Rep. 2019, 9, 18178. [Google Scholar] [CrossRef]
- Cialla, D.; März, A.; Böhme, R.; Theil, F.; Weber, K.; Schmitt, M.; Popp, J. Surface-enhanced Raman spectroscopy (SERS): Progress and trends. Anal. Bioanal. Chem. 2012, 403, 27–54. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, F.; Liu, Y.; Zhang, H.; Gilbertson, T.A.; Zhou, A. Spatiotemporal dynamic monitoring of fatty acid–receptor interaction on single living cells by multiplexed Raman imaging. Proc. Natl. Acad. Sci. USA 2020, 117, 3518–3527. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Fang, J.; Xu, Z. Advances in droplet microfluidics for SERS and Raman analysis. Biosens. Bioelectron. 2021, 198, 113822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, W.; Xiao, L.; Liu, Y.; Gilbertson, T.A.; Zhou, A. Use of surface-enhanced Raman scattering (SERS) probes to detect fatty acid receptor activity in a microfluidic device. Sensors 2019, 19, 1663. [Google Scholar] [CrossRef]
- Sun, D.; Cao, F.; Tian, Y.; Li, A.; Xu, W.; Chen, Q.; Shi, W.; Xu, S. Label-free detection of multiplexed metabolites at single-cell level via a SERS-microfluidic droplet platform. Anal. Chem. 2019, 91, 15484–15490. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jeon, C.S.; Choi, N.; Moon, J.-I.; Lee, K.M.; Pyun, S.H.; Kang, T.; Choo, J. Sensitive and reproducible detection of SARS-CoV-2 using SERS-based microdroplet sensor. Chem. Eng. J. 2022, 446, 137085. [Google Scholar] [CrossRef] [PubMed]
- Hengoju, S.; Shvydkiv, O.; Tovar, M.; Roth, M.; Rosenbaum, M.A. Advantages of optical fibers for facile and enhanced detection in droplet microfluidics. Biosens. Bioelectron. 2021, 200, 113910. [Google Scholar] [CrossRef]
- Yang, T.; Stavrakis, S.; deMello, A. A high-sensitivity, integrated absorbance and fluorescence detection scheme for probing picoliter-volume droplets in segmented flows. Anal. Chem. 2017, 89, 12880–12887. [Google Scholar] [CrossRef]
- Duncombe, T.A.; Ponti, A.; Seebeck, F.P.; Dittrich, P.S. UV–Vis spectra-activated droplet sorting for label-free chemical identification and collection of droplets. Anal. Chem. 2021, 93, 13008–13013. [Google Scholar] [CrossRef]
- Colozza-Gama, G.A.; Callegari, F.; Bešič, N.; Paniza, A.C.d.J.; Cerutti, J.M. Machine learning algorithm improved automated droplet classification of ddPCR for detection of BRAF V600E in paraffin-embedded samples. Sci. Rep. 2021, 11, 12648. [Google Scholar] [CrossRef]
- Anagnostidis, V.; Sherlock, B.; Metz, J.; Mair, P.; Hollfelder, F.; Gielen, F. Deep learning guided image-based droplet sorting for on-demand selection and analysis of single cells and 3D cell cultures. Lab Chip 2020, 20, 889–900. [Google Scholar] [CrossRef] [PubMed]
- LaBelle, C.A.; Massaro, A.; Cortés-Llanos, B.; Sims, C.E.; Allbritton, N.L. Image-based live cell sorting. Trends Biotechnol. 2021, 39, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Girault, M.; Kim, H.; Arakawa, H.; Matsuura, K.; Odaka, M.; Hattori, A.; Terazono, H.; Yasuda, K. An on-chip imaging droplet-sorting system: A real-time shape recognition method to screen target cells in droplets with single cell resolution. Sci. Rep. 2017, 7, 40072. [Google Scholar] [CrossRef] [PubMed]
- Joensson, H.N.; Uhlén, M.; Svahn, H.A. Droplet size based separation by deterministic lateral displacement—Separating droplets by cell-induced shrinking. Lab Chip 2011, 11, 1305–1310. [Google Scholar] [CrossRef]
- Tummons, E.N.; Tarabara, V.V.; Chew, J.W.; Fane, A.G. Behavior of oil droplets at the membrane surface during crossflow microfiltration of oil–water emulsions. J. Membr. Sci. 2016, 500, 211–224. [Google Scholar] [CrossRef]
- Li, M.; van Zee, M.; Goda, K.; Di Carlo, D. Size-based sorting of hydrogel droplets using inertial microfluidics. Lab Chip 2018, 18, 2575–2582. [Google Scholar] [CrossRef]
- Boitard, L.; Cottinet, D.; Kleinschmitt, C.; Bremond, N.; Baudry, J.; Yvert, G.; Bibette, J. Monitoring single-cell bioenergetics via the coarsening of emulsion droplets. Proc. Natl. Acad. Sci. USA 2012, 109, 7181–7186. [Google Scholar] [CrossRef]
- Geersens, É.; Vuilleumier, S.; Ryckelynck, M. Growth-Associated Droplet Shrinkage for Bacterial Quantification, Growth Monitoring, and Separation by Ultrahigh-Throughput Microfluidics. ACS Omega 2022, 7, 12039–12047. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Lu, Y.-T.; Chang, C.-M.; Liu, C.-H. Finger-powered cell-sorting microsystem chip for cancer-study applications. Sens. Actuators B Chem. 2022, 370, 132430. [Google Scholar] [CrossRef]
- Gilet, T.; van Loo, S. Multiple interactions between microfluidic droplets and on-chip pneumatic valves. Microfluid. Nanofluid. 2022, 26, 20. [Google Scholar] [CrossRef]
- Ferraro, D.; Serra, M.; Ferrante, I.; Viovy, J.L.; Descroix, S. Microfluidic valve with zero dead volume and negligible back-flow for droplets handling. Sens. Actuators B Chem. 2018, 258, 1051–1059. [Google Scholar] [CrossRef]
- Abate, A.R.; Weitz, D.A. Single-layer membrane valves for elastomeric microfluidic devices. Appl. Phys. Lett. 2008, 92, 243509. [Google Scholar] [CrossRef]
- Abate, A.R.; Agresti, J.J.; Weitz, D.A. Microfluidic sorting with high-speed single-layer membrane valves. Appl. Phys. Lett. 2010, 96, 203509. [Google Scholar] [CrossRef]
- Jin, S.H.; Lee, B.; Kim, J.S.; Lee, C.-S. Improvement strategy of a microfluidic sorter using a pneumatic bilayer valve. Chem. Eng. Sci. 2021, 245, 116834. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, C.; Tian, L.; Wang, R.; Hong, J.; Gao, M.; Gui, L. Dynamic pneumatic rails enabled microdroplet manipulation. Lab Chip 2021, 21, 105–112. [Google Scholar] [CrossRef]
- Yoon, D.H.; Ito, J.; Sekiguchi, T.; Shoji, S. Active and Precise Control of Microdroplet Division Using Horizontal Pneumatic Valves in Bifurcating Microchannel. Micromachines 2013, 4, 197–205. [Google Scholar] [CrossRef]
- Yoon, D.H.; Wakui, D.; Nakahara, A.; Sekiguchi, T.; Shoji, S. Selective droplet sampling using a minimum number of horizontal pneumatic actuators in a high aspect ratio and highly flexible PDMS device. RSC Adv. 2015, 5, 2070–2074. [Google Scholar] [CrossRef]
- Sciambi, A.; Abate, A.R. Accurate microfluidic sorting of droplets at 30 kHz. Lab Chip 2015, 15, 47–51. [Google Scholar] [CrossRef]
- Leman, M.; Abouakil, F.; Griffiths, A.D.; Tabeling, P. Droplet-based microfluidics at the femtolitre scale. Lab Chip 2015, 15, 753–765. [Google Scholar] [CrossRef]
- Beneyton, T.; Wijaya, I.P.M.; Postros, P.; Najah, M.; Leblond, P.; Couvent, A.; Mayot, E.; Griffiths, A.D.; Drevelle, A. High-throughput screening of filamentous fungi using nanoliter-range droplet-based microfluidics. Sci. Rep. 2016, 6, 27223. [Google Scholar] [CrossRef]
- Xi, H.-D.; Zheng, H.; Guo, W.; Gañán-Calvo, A.M.; Ai, Y.; Tsao, C.-W.; Zhou, J.; Li, W.; Huang, Y.; Nguyen, N.-T.; et al. Active droplet sorting in microfluidics: A review. Lab Chip 2017, 17, 751–771. [Google Scholar] [CrossRef] [PubMed]
- Sciambi, A.; Abate, A.R. Generating electric fields in PDMS microfluidic devices with salt water electrodes. Lab Chip 2014, 14, 2605–2609. [Google Scholar] [CrossRef]
- Ahn, K.; Kerbage, C.; Hunt, T.P.; Westervelt, R.M.; Link, D.R.; Weitz, D.A. Dielectrophoretic manipulation of drops for high-speed microfluidic sorting devices. Appl. Phys. Lett. 2006, 88, 024104. [Google Scholar] [CrossRef]
- Link, D.R.; Grasland-Mongrain, E.; Duri, A.; Sarrazin, F.; Cheng, Z.; Cristobal, G.; Marquez, M.; Weitz, D.A. Electric control of droplets in microfluidic devices. Angew. Chem. Int. Ed. Engl. 2006, 45, 2556–2560. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Whitesides, G.M. SOFT LITHOGRAPHY. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Tan, S.H.; Nguyen, N.T.; Chua, Y.C.; Kang, T.G. Oxygen plasma treatment for reducing hydrophobicity of a sealed polydimethylsiloxane microchannel. Biomicrofluidics 2010, 4, 32204. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, M.; Peng, S.; Wen, W.; Sheng, P. Real-time detection, control, and sorting of microfluidic droplets. Biomicrofluidics 2007, 1, 44101. [Google Scholar] [CrossRef]
- Tan, S.H.; Maes, F.; Semin, B.; Vrignon, J.; Baret, J.-C. The Microfluidic Jukebox. Sci. Rep. 2014, 4, 4787. [Google Scholar] [CrossRef]
- Tan, S.H.; Semin, B.; Baret, J.-C. Microfluidic flow-focusing in ac electric fields. Lab Chip 2014, 14, 1099–1106. [Google Scholar] [CrossRef]
- Castro-Hernández, E.; García-Sánchez, P.; Tan, S.H.; Gañán-Calvo, A.M.; Baret, J.-C.; Ramos, A. Breakup length of AC electrified jets in a microfluidic flow-focusing junction. Microfluid. Nanofluid. 2015, 19, 787–794. [Google Scholar] [CrossRef]
- Castro-Hernández, E.; García-Sánchez, P.; Alzaga-Gimeno, J.; Tan, S.H.; Baret, J.-C.; Ramos, A. AC electrified jets in a flow-focusing device: Jet length scaling. Biomicrofluidics 2016, 10, 043504. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, J.L.; Schoepp, N.G.; Xu, L.; Powell, C.S.; Delley, C.L.; Siltanen, C.A.; Danao, J.; Srinivasan, M.; Cole, R.H.; Abate, A.R. Characterizing cell interactions at scale with made-to-order droplet ensembles (MODEs). Proc. Natl. Acad. Sci. USA 2022, 119, e2110867119. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Cai, B.; Yu, X.-L.; Guo, S.-S.; Liu, W.; Zhao, X.-Z. One-step fabrication of 3D silver paste electrodes into microfluidic devices for enhanced droplet-based cell sorting. AIP Adv. 2015, 5, 057134. [Google Scholar] [CrossRef]
- Guzman, A.R.; Kim, H.S.; de Figueiredo, P.; Han, A. A three-dimensional electrode for highly efficient electrocoalescence-based droplet merging. Biomed. Microdevices 2015, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Isozaki, A.; Nakagawa, Y.; Loo, M.H.; Shibata, Y.; Tanaka, N.; Setyaningrum, D.L.; Park, J.W.; Shirasaki, Y.; Mikami, H.; Huang, D.; et al. Sequentially addressable dielectrophoretic array for high-throughput sorting of large-volume biological compartments. Sci. Adv. 2020, 6, eaba6712. [Google Scholar] [CrossRef]
- Bowman, T.; Frechette, J.; Drazer, G. Force driven separation of drops by deterministic lateral displacement. Lab Chip 2012, 12, 2903–2908. [Google Scholar] [CrossRef]
- Ding, R.; Ung, W.L.; Heyman, J.A.; Weitz, D.A. Sensitive and predictable separation of microfluidic droplets by size using in-line passive filter. Biomicrofluidics 2017, 11, 014114. [Google Scholar]
- HyunáYoon, D. Hydrodynamic on-rail droplet pass filter for fully passive sorting of droplet-phase samples. RSC Adv. 2014, 4, 37721–37725. [Google Scholar]
- Rehman, A.U.; Coskun, U.C.; Rashid, Z.; Morova, B.; Jonas, A.; Erten, A.; Kiraz, A. Size-based sorting of emulsion droplets in microfluidic channels patterned with laser-ablated guiding tracks. Anal. Chem. 2020, 92, 2597–2604. [Google Scholar] [CrossRef]
- Maenaka, H.; Yamada, M.; Yasuda, M.; Seki, M. Continuous and size-dependent sorting of emulsion droplets using hydrodynamics in pinched microchannels. Langmuir 2008, 24, 4405–4410. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-C.; Lee, A.P. Microfluidic separation of satellite droplets as the basis of a monodispersed micron and submicron emulsification system. Lab Chip 2005, 5, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Isozaki, A.; Huang, D.; Nakagawa, Y.; Goda, K. Dual sequentially addressable dielectrophoretic array for high-throughput, scalable, multiplexed droplet sorting. Microfluid. Nanofluid. 2021, 25, 32. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, C.; Li, Y.; Gupte, R.; Samuel, R.; Dai, J.; Guzman, A.; Sabnis, R.; de Figueiredo, P.; Han, A. FIDELITY: A quality control system for droplet microfluidics. Sci. Adv. 2022, 8, eabc9108. [Google Scholar] [CrossRef] [PubMed]
- Yosioka, K.; Kawasima, Y. Acoustic radiation pressure on a compressible sphere. Acta Acust. United Acust. 1955, 5, 167–173. [Google Scholar]
- Leibacher, I.; Reichert, P.; Dual, J. Microfluidic droplet handling by bulk acoustic wave (BAW) acoustophoresis. Lab Chip 2015, 15, 2896–2905. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, C.; Su, X.; Mai, J.; Gu, Y.; Tian, Z.; Zhu, H.; Zhong, Z.; Fu, H.; Yang, S.; et al. Acoustic streaming vortices enable contactless, digital control of droplets. Sci. Adv. 2020, 6, eaba0606. [Google Scholar] [CrossRef]
- Guo, F.; Lapsley, M.I.; Nawaz, A.A.; Zhao, Y.; Lin, S.-C.S.; Chen, Y.; Yang, S.; Zhao, X.-Z.; Huang, T.J. A Droplet-Based, Optofluidic Device for High-Throughput, Quantitative Bioanalysis. Anal. Chem. 2012, 84, 10745–10749. [Google Scholar] [CrossRef][Green Version]
- Tang, S.-Y.; Ayan, B.; Nama, N.; Bian, Y.; Lata, J.P.; Guo, X.; Huang, T.J. On-Chip Production of Size-Controllable Liquid Metal Microdroplets Using Acoustic Waves. Small 2016, 12, 3861–3869. [Google Scholar] [CrossRef]
- Weis, R.S.; Gaylord, T.K. Lithium niobate: Summary of physical properties and crystal structure. Appl. Phys. A 1985, 37, 191–203. [Google Scholar] [CrossRef]
- Shi, J.; Huang, H.; Stratton, Z.; Huang, Y.; Huang, T.J. Continuous particle separation in a microfluidic channelvia standing surface acoustic waves (SSAW). Lab Chip 2009, 9, 3354–3359. [Google Scholar] [CrossRef] [PubMed]
- Lighthill, S.J. Acoustic streaming. J. Sound Vib. 1978, 61, 391–418. [Google Scholar] [CrossRef]
- Dentry, M.B.; Yeo, L.Y.; Friend, J.R. Frequency effects on the scale and behavior of acoustic streaming. Phys. Rev. E 2014, 89, 013203. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Shen, W.; Li, Y.; Zhao, H.; Li, X.; Wang, C.; He, F. Continuous separation of particles with different densities based on standing surface acoustic waves. Sens. Actuators A Phys. 2022, 341, 113589. [Google Scholar] [CrossRef]
- Li, S.; Ding, X.; Guo, F.; Chen, Y.; Lapsley, M.I.; Lin, S.-C.S.; Wang, L.; McCoy, J.P.; Cameron, C.E.; Huang, T.J. An On-Chip, Multichannel Droplet Sorter Using Standing Surface Acoustic Waves. Anal. Chem. 2013, 85, 5468–5474. [Google Scholar] [CrossRef]
- Doinikov, A.A. Acoustic radiation forces: Classical theory and recent advances. Recent Res. Dev. Acoust. 2003, 1, 39–67. [Google Scholar]
- Franke, T.; Abate, A.R.; Weitz, D.A.; Wixforth, A. Surface acoustic wave (SAW) directed droplet flow in microfluidics for PDMS devices. Lab Chip 2009, 9, 2625–2627. [Google Scholar] [CrossRef]
- Mutafopulos, K.; Lu, P.J.; Garry, R.; Spink, P.; Weitz, D.A. Selective cell encapsulation, lysis, pico-injection and size-controlled droplet generation using traveling surface acoustic waves in a microfluidic device. Lab Chip 2020, 20, 3914–3921. [Google Scholar] [CrossRef]
- Liu, G.; He, F.; Li, Y.; Zhao, H.; Li, X.; Tang, H.; Li, Z.; Yang, Z.; Zhang, Y. Effects of two surface acoustic wave sorting chips on particles multi-level sorting. Biomed. Microdevices 2019, 21, 59. [Google Scholar] [CrossRef]
- Chen, A.; Byvank, T.; Chang, W.-J.; Bharde, A.; Vieira, G.; Miller, B.L.; Chalmers, J.J.; Bashir, R.; Sooryakumar, R. On-chip magnetic separation and encapsulation of cells in droplets. Lab Chip 2013, 13, 1172–1181. [Google Scholar] [CrossRef]
- Kühnemund, M.; Witters, D.; Nilsson, M.; Lammertyn, J. Circle-to-circle amplification on a digital microfluidic chip for amplified single molecule detection. Lab Chip 2014, 14, 2983–2992. [Google Scholar] [CrossRef] [PubMed]
- Surenjav, E.; Priest, C.; Herminghaus, S.; Seemann, R. Manipulation of gel emulsions by variable microchannel geometry. Lab Chip 2009, 9, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Baroud, C.N.; Delville, J.-P.; Gallaire, F.; Wunenburger, R. Thermocapillary valve for droplet production and sorting. Phys. Rev. E 2007, 75, 046302. [Google Scholar] [CrossRef] [PubMed]
- Robert de Saint Vincent, M.; Wunenburger, R.; Delville, J.-P. Laser switching and sorting for high speed digital microfluidics. Appl. Phys. Lett. 2008, 92, 154105. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, J.; Huang, T.J.; Zou, J. Ring-shaped photoacoustic tweezers for single particle manipulation. Opt. Lett. 2022, 47, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Yesiloz, G.; Boybay, M.S.; Ren, C.L. Label-free high-throughput detection and content sensing of individual droplets in microfluidic systems. Lab Chip 2015, 15, 4008–4019. [Google Scholar] [CrossRef] [PubMed]
- Wen, N.; Zhao, Z.; Fan, B.; Chen, D.; Men, D.; Wang, J.; Chen, J. Development of Droplet Microfluidics Enabling High-Throughput Single-Cell Analysis. Molecules 2016, 21, 881. [Google Scholar] [CrossRef]
- Li, Y.; Dai, J.; Shim, W.B.; Han, A. Microfluidic droplet-based high-throughput screening of filamentous fungi. IEEE Sens. 2022. [Google Scholar] [CrossRef]
- Kim, H.S.; Hsu, S.C.; Han, S.I.; Thapa, H.R.; Guzman, A.R.; Browne, D.R.; Tatli, M.; Devarenne, T.P.; Stern, D.B.; Han, A. High-throughput droplet microfluidics screening platform for selecting fast-growing and high lipid-producing microalgae from a mutant library. Plant Direct 2017, 1, e00011. [Google Scholar] [CrossRef]
- Rauf, S.; Tashkandi, N.; de Oliveira Filho, J.I.; Oviedo-Osornio, C.I.; Danish, M.S.; Hong, P.Y.; Salama, K.N. Digital E. coli Counter: A Microfluidics and Computer Vision-Based DNAzyme Method for the Isolation and Specific Detection of E. coli from Water Samples. Biosensors 2022, 12, 34. [Google Scholar] [CrossRef]
- Wang, X.; Ren, L.; Su, Y.; Ji, Y.; Liu, Y.; Li, C.; Li, X.; Zhang, Y.; Wang, W.; Hu, Q. Raman-activated droplet sorting (RADS) for label-free high-throughput screening of microalgal single-cells. Anal. Chem. 2017, 89, 12569–12577. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.E.; Cygan, Z.T.; Yates, J.K.; Beers, K.L.; Amis, E.J. Raman spectroscopic monitoring of droplet polymerization in a microfluidic device. Analyst 2006, 131, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Waqued, S.C.; Nodurft, D.T.; Devarenne, T.P.; Yakovlev, V.V.; Han, A. Raman spectroscopy compatible PDMS droplet microfluidic culture and analysis platform towards on-chip lipidomics. Analyst 2017, 142, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Holstein, J.M.; Gylstorff, C.; Hollfelder, F. Cell-free Directed Evolution of a Protease in Microdroplets at Ultrahigh Throughput. ACS Synth. Biol. 2021, 10, 252–257. [Google Scholar] [CrossRef]
- Nuti, N.; Rottmann, P.; Stucki, A.; Koch, P.; Panke, S.; Dittrich, P.S. A Multiplexed Cell-Free Assay to Screen for Antimicrobial Peptides in Double Emulsion Droplets. Angew. Chem. Int. Ed. 2022, 61, e202114632. [Google Scholar] [CrossRef]
- Ahn, B.; Lee, K.; Panchapakesan, R.; Oh, K.W. On-demand electrostatic droplet charging and sorting. Biomicrofluidics 2011, 5, 024113. [Google Scholar] [CrossRef]
- Guo, F.; Ji, X.-H.; Liu, K.; He, R.-X.; Zhao, L.-B.; Guo, Z.-X.; Liu, W.; Guo, S.-S.; Zhao, X.-Z. Droplet electric separator microfluidic device for cell sorting. Appl. Phys. Lett. 2010, 96, 193701. [Google Scholar] [CrossRef]
- Rao, L.; Cai, B.; Wang, J.; Meng, Q.; Ma, C.; He, Z.; Xu, J.; Huang, Q.; Li, S.; Cen, Y.; et al. A microfluidic electrostatic separator based on pre-charged droplets. Sens. Actuators B Chem. 2015, 210, 328–335. [Google Scholar] [CrossRef]


| Detection Methods | Throughput | System Cost | Specificity | Applications | Ref | |
|---|---|---|---|---|---|---|
| Impedance | 0.1~3.3 kHz | Low | Medium | Empty droplet, viable/non-viable cells | [69,190] | |
| Fluorescent | 10~30 kHz | Medium | High | Yeast, filamentous fungi, microalgae, bacteria, direct evolution (cell-free) and particle detection (cell-free) | [79,170,191,192,193,194] | |
| Absorbance | 1~2.1 kHz | Lowest | Low | Chemical identification | [122,123] | |
| Scattering | Raman | 10~42 Hz | High | High | Microalgal cells, chemical polymerization and lipid production | [195,196,197] |
| Visible | 0.25~3 kHz | High | Low | Droplet PCR, single-cell screening | [124,125,126,127] | |
| Sorting Methods | Types | Throughput | Accuracy | Applications | Ref |
|---|---|---|---|---|---|
| Pneumatic | Single Layer | 1~250 Hz | >99% | Single cell droplet, protein analysis | [136,137] |
| Multi-Layer | 1~50 Hz | >98% | [138,139] | ||
| Dielectrophoretic | AC | Up to 30 kHz | >99% | Single-cell assays, direct evolution (cell-free) | [79,142,198,199] |
| DC | 100 Hz~1.1 kHz | 92 ± 5.4% | [152,200,201,202] | ||
| Acoustophoretic | BAW | 1~5 Hz | - | Empty droplet, drug toxicity test, particle sorting (cell-free), on-demand droplet generation | [169,170] |
| SSAW | 222~1 kHz | 99.2% | [47,179] | ||
| TSAW | 1~1 kHz | 90~100% | [118,181,182,183] | ||
| Magnetophoretic | Perm Mag | 100 Hz | 95% | Empty droplet, cell viability assay, Single-cell analysis | [51,184] |
| Electro Mag | 0.5~50 Hz | 58~90% | [185] | ||
| Thermal | Laser Focus | <1 Hz | 100% | Empty droplet | [52,187,188] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Jiang, Y.; Li, Y.; Zhang, H. Droplet Detection and Sorting System in Microfluidics: A Review. Micromachines 2023, 14, 103. https://doi.org/10.3390/mi14010103
Huang C, Jiang Y, Li Y, Zhang H. Droplet Detection and Sorting System in Microfluidics: A Review. Micromachines. 2023; 14(1):103. https://doi.org/10.3390/mi14010103
Chicago/Turabian StyleHuang, Can, Yuqian Jiang, Yuwen Li, and Han Zhang. 2023. "Droplet Detection and Sorting System in Microfluidics: A Review" Micromachines 14, no. 1: 103. https://doi.org/10.3390/mi14010103
APA StyleHuang, C., Jiang, Y., Li, Y., & Zhang, H. (2023). Droplet Detection and Sorting System in Microfluidics: A Review. Micromachines, 14(1), 103. https://doi.org/10.3390/mi14010103






