Abstract
A trace element copper (Cu2+) ion is the third most plentiful metal ion that necessary for all living organisms and playing a critical role in several processes. Nonetheless, according to cellular needs, deficient or excess Cu2+ ion cause various diseases. For all these reasons, optical sensors have been focused rapid Cu2+ ion detection in real-time with high selectivity and sensitivity. Optical sensors can measure fluorescence in the refractive index—adsorption from the relationships between light and matter. They have gained great attention in recent years due to the excellent advantages of simple and naked eye recognition, real-time detection, low cost, high specificity against analytes, a quick response, and the need for less complex equipment in analysis. This review aims to show the significance of Cu2+ ion detection and electively current trends in optical sensors. The integration of optical sensors with different systems, such as microfluidic systems, is mentioned, and their latest studies in medical and environmental applications also are depicted. Conclusions and future perspectives on these advances is added at the end of the review.
1. Introduction
The copper (Cu2+) ion is one of the most important metal ion and has a crucial part in many applications with very important functions in nature and the human body [1,2]. It plays an important role in the human body as a catalytic cofactor of redox-regulating enzymes such as for a diversity of metalloenzymes, including tyrosinase, lysyl oxidase, cytochrome c oxidase, and superoxide dismutase [3,4]. It is an indispensable catalyst in the absorption of iron and the synthesis of ferroheme [5]. Cu2+ ion does not have a toxic effect on the human body under normal conditions; however, excess and deficiency can cause various harms. Although trace amounts of Cu2+ ion are sufficient for the normal physiological process, intake above the required daily dose has negative consequences for human health [6,7].
In addition to the iron metabolism regulated by copper ions, many diseases occur with the increase of concentration due to a lack of this element or long-term exposure. Excess Cu2+ ion intake can cause complaints such as diarrhea, vomiting, dizziness, and stomach ache [7]. Free Cu2+ ion that has accumulated in the body as a result of long-term exposure produce reactive oxygen species due to redox activity and damage lipids, DNA, and proteins. Free oxygen species are potentially toxic to cells [8,9]. Many diseases may develop in the body depending on the defect in hemostasis. Increasing the concentration of Cu2+ ion causes many adverse health problems such as Wilson’s, Parkinson’s, Menkes, neurodegenerative and Alzheimer’s diseases [10,11,12], and also Huntington’s, and acute hepatic kidney failure [13,14,15]. According to the Environmental Protection Agency (EPA), the upper limit for Cu2+ ion in drinking water is 1.3 ppm [16,17]. The amount of copper concentration that should be in the blood serum of healthy individuals is 100–150 μg/dL [18,19]. Moreover, Cu2+ ion is released into the environment through domestic, industrial, and agricultural processes [20]. Cu2+-combined pesticides are commonly utilized to supplement agricultural growth and prohibit diseases. Since 1991, the EPA has restricted the level of Cu2+ ion in tap water to 20 μM [21], and the World Health Organization (WHO) has regulated drinking water at 30 μM [22]. Freshwater, agricultural products, soil, drinking water, and sediment can be contaminated because of the excess use of these pesticides.
Various methods are used to detect trace metals. In Cu2+ ion detection, atomic absorption spectrometry [23,24,25], anodic stripping voltammetry [26,27], high-performance liquid chromatography, inductively coupled plasma atomic emission spectrometry [28,29,30], and inductively coupled plasma mass spectrometry [31] are widely used. However, these methods have advantages, such as being reliable, accurate, and fast, as well as disadvantages, such as being expensive and complex processes, which must be overcome [32,33,34]. It is essential and immediately to improve sensitive, easy, rapid, reproducible, low-cost, and analytical methods for Cu2+ ion detection in environmental and medical samples [35]. In this regard, sensors are analytical devices that convert physical, chemical, and biological changes in the environment into electrical signals [36,37]. Generally, these sensors consist of three main parts: transducers, receptors, and electronic parts [38,39]. A typical biosensor is shown in Figure 1. The sensing principle is based on the specific interaction between the analyte and receptor. Depending on the interaction, properties such as changing pH, electron, mass transfer, temperature, a change of optical properties, and potential differences are detected by the transducers. This system converts the receptor’s response into an electronic signal that is directly related to the existence of the analyte or commensurate to the analyte concentration [40]. The increase and interest in sensor studies in recent years is due to their low cost, easy miniaturization, and production of semi-quantitative information in a short time. For all these reasons, sensors have become very significant tools for clinical environmental and food monitoring and detection of chemical and biological compounds [41,42,43]. The analyte used in sensor applications is defined as the substances and structures to be analyzed. Receptors are elements that make-up compounds or mixtures. The most common receptors are enzymes and antibodies, but in general, polymers, dyes or chelating agents are also used for sensor surface modification [44]. Transducers are part of sensors that convert the sensed energy from one form to another [45,46]. The selectivity of the sensors is a very important parameter, as the sensors must respond to analytes in complex matrices of real samples [47]. In sensors, transducers such as electrochemical, optical, and piezoelectric are used during the measurement and transmission of the signal formed as a result of the interaction between samples and ligands [48]. Thus, sensor technology is a widely used range of platforms such as biomedical, health technologies, pharmacological and environmental analysis [49,50,51].

Figure 1.
Typical detection principle of a biosensor.
2. Optical Sensors
Optical sensors are highly sensitive and selective to the analyte to be analyzed. They have gained great attention in recent years due to the excellent advantages of simple and naked eye recognition, real-time detection, low cost, high specificity against analytes, their quick response, and less complex equipment in analyses [52,53,54]. Optical sensors have countlessly subdivided according to the change in the signal resulting from the interaction between the recognition element and target molecule. The subdivisions are refractive index, fluorescence, chemiluminescence, infrared spectrum, colorimetric, and Raman spectra [55,56,57,58,59,60]. Nowadays, optical sensors have proceeded to develop in many applications including food safety [61], virus detection [62], cancer diagnosis [63,64], cardiac biomarkers detection [65], environmental monitoring [66], DNA sensing [67], and blood glucose monitoring [68].
There are several kinds of optical sensors in the literature with different platforms. For instance, the technology of processes with small amounts of fluids (up to 10−18 L) utilizing channels called microfluidics [69,70]. The microfluidic platforms have the potential to alter subject areas from analysis to information technology. The first applications of microfluidics aim to have many helpful superiorities including the capability to employ low amounts of samples and to carry out detections with high resolution, short time, and low cost [71,72]. Microfluidics works through its most obvious properties such as its small size and less visible properties of fluids in microchannels. It proposes fundamentally novel abilities in the control of concentrations of biomolecules [73].
2.1. Colorimetric Sensors
Colorimetric sensors are used to detect the instantaneous modification in color that occurs as a result of the interaction between the target analyte and reacting sensing element [74,75]; this color change is seen by the naked eyes. The main purpose of colorimetric sensors is how stimuli such as pH values, temperature change, and stress cause a visible change in the analyte [76,77,78]. Colorimetric sensors have many advantages, such as naked eye detection, low capital cost, utmost simplicity, good selectivity and specificity, short-time analysis, reversibility, and lacking the need for the requirement of complex instruments [79,80,81]. There are several colorimetric sensors for Cu2+ ion detection.
For instance, Gangapuram et al. improved gold nanoparticles (AuNPs)-modified colorimetric sensor for the selective and sensitive Cu2+ ion detection. Synthesized AuNPs were characterized and they showed excellent stability in different conditions. Carboxymethyl gum karaya capped with AuNPs demonstrated a selective colorimetric response with a visible change from red to blue. These results were approved by TEM and DLS analysis. This assay showed a well linear correlation in the range of 10–1000 nM of Cu2+. The limit of detection was calculated as 10 nM. Tap water, human plasma, and human urine samples were used to evaluate the detection capability of this sensor [82].
For the detection of Cu2+ ion, Park et al. also prepared a receptor 1. The chromogenic perception capability of receptor 1 was examined in the asset of 18 different cations. Figure 2(Ai) shows the absorption spectra changed only when Cu2+ induced, and Figure 2(Aii) depicts the solution color of receptor 1 changing from yellow to purple with the addition of Cu2+ ion [83]. Deng and colleagues developed a colorimetric sensor to detect Cu2+ with gold nanoparticles. The existence of Cu2+ ion causes the color to alter from red to purple–blue. The limit of detection was determined as 0.04 μM with the UV-Vis spectrometry and 2 μM with the naked eye [84]. A colorimetric hydrazone-based ligand was developed for Cu2+ ion detection by Abdulazeez et al. This developed ligand did not show the selectivity for Cu2+ ion against different metals and a color change was only observed in the prepared Cu2+ ion solution. In other metals, the color change was insignificant or not at all. Ligand showed good selectivity and sensitivity. The detection limit value was reported as 0.34 μg/L [85].
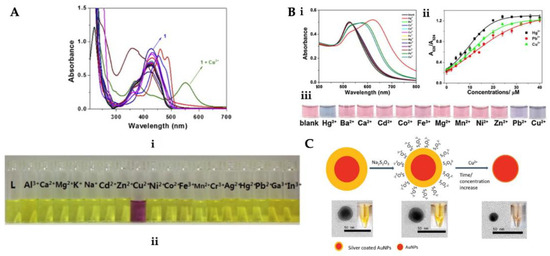
Figure 2.
(i) Absorption and (ii) color changes upon the addition of different metal ions (A), (i) UV-vis absorption spectra with distinct metal ions, (ii) P-AuNPs against several concentrations of Cu2+, Hg2+, and Pb2+ ions (iii) solution with different metal ions (B) and scheme of the Ag/AuNPs for Cu2+ ion detection (C). Republished with permission from [83,86,87].
Guo et al. presented an easy colorimetric sensor to determine Cu2+, Hg2+, and Pb2+ ions (Figure 2B). Cu2+, Hg2+, and Pb2+ ions were dropped into papain-coated gold nanoparticles (P-AuNPs) solution prepared for colorimetric detection. The P-AuNPs showed different responses to Cu2+, Hg2+, and Pb2+ ions in an aqueous solution. They examined the concentration, pH, and size impact on the sensitivity and stability of the sensor. To analyze the selectivity of the sensor, the colorimetric reaction was explored in the existence of diverse metals. The color intensity of the adsorbent enhanced with an increase in the Cu2+ concentration leading to the red-shift of the peak at 375 nm and also the emergence of a novel peak at about 490 nm by UV-vis-NIR spectroscopy [86]. Lou et al. synthesized silver-coated gold nanoparticles (Ag/AuNPs) for Cu2+ ion detection in water samples (Figure 2C). The leaching of Ag/AuNPs would cause a fair decrease in the surface plasmon resonance absorption as the dimensions of Ag/AuNPs decreased. This colorimetric sensor is dependent on the dimensions-based nanoparticles’ highly sensitive and selective sensing toward Cu2+ ion. They reported that this sensor ensured an easy and fast platform for heavy metal detection [87].
Xei et al. prepared a microfluidic system-integrated colorimetric sensor for Cu2+ ion detection. They observed that in the presence of Cu2+ ion, the channel color values decreased with increasing the Cu2+ concentration, and also, the intensity was linear in the range of Cu2+ ion concentration (0–30 mg/L) with a limit of the detection value of 0.096 mg/L. They also performed tap water analysis with this microfluidic system-integrated colorimetric sensor and observed high selectivity and recoveries and also, satisfying reproducibility. Additionally, by changing a hole punch with different shapes and numbers, it is very easy to produce sensors with different designs at a low cost [88].
2.2. Fluorescence Sensors
Fluorescence sensors are widely used for sensing different molecules. They do not need high excitation power [89]. The fluorescence has quite high sensitivity and selectivity. The fluorescence sensors observe the change of frequency of electromagnetic radiation emission induced by preceding radiation absorption and excited state generation that only rises for a very limited time [90]. Fluorescence sensors have advantages such as less response time with high selectivity and sensitivity and have applications in environmental monitoring, microscope-based analysis, in clinical diagnostics and food safety; they are broadly employed for the detection of heavy metal ions [91,92]. These advantages have proven to be the most appropriate approximation for metal ions detection [93]. Fluorescence detection of Cu2+ ion is more difficult than many other metals. The reason for this difficulty is due to the paramagnetic nature of Cu2+ ion, which has inherent quenching properties of Cu2+ ion [94,95].
For example, Liu et al. fabricated a fluorescence sensor for extremely sensitive Cu2+ ion detection. The surface of silica-coated CdSe quantum dots was conjugated to the Cu2+ ion nanoclusters and they designed the fluorescence sensor. In the asset of various quantities of Cu2+ ion, a color shift from yellow–green to red was observed in the sensor. The detection limit for Cu2+ ion was calculated to be 8.9 nM [96]. Xie and co-workers reported the quantum dots acted as a fluorescence sensor to distinguish and determine Cu2+ and Ag2+ ions. They observed bright yellow fluorescence emission under a 365 nm UV lamp and absorption at 525 nm. With this method, detection of Cu2+ and Ag2+ ions takes only a few minutes. The method can carry out the differentiation of Cu2+ and Ag2+ ions by principal components analysis plots; the detection limit was 35 nM, and this sensor also has good trustworthiness and correctness in real samples [97]. A switchable fluorescence sensor was improved for the determination of Cu2+ ion by Niu et al. (Figure 3A). This method showed good linearity for Cu2+ ion under optimum conditions. The fluorescence sensor in the search of Cu2+ ion showed a peak (emission) at 660 nm upon peak (excitation) at 495 nm. As a result of the experiments on living samples, the fluorescent sensor’s rapid, high stability, and sensitivity were promising for diagnosing Alzheimer’s [98]. Wang et al. prepared a pyrene-based fluorescence sensor for Cu2+ ion determination. In the asset of Cu2+ ion, the sensor ensured an important fluorescence increase. Maximum fluorescence increase was observed with the binding of Cu2+ ion to the sensor at the range of pH 2.0–8.5. Selectivity analyzes of the prepared fluorescence sensor were performed using Ag+, Ca2+, Cd2+, Co2+, Fe2+, Fe3+, Hg2+, K+, Mg2+, Mn2+, Ni2+, Pb2+, and Zn2+ metal ions. As depicted in Figure 3B, Cu2+ ion caused a visible color change in the sensor from light yellow or colorless and had a blue emission [99].
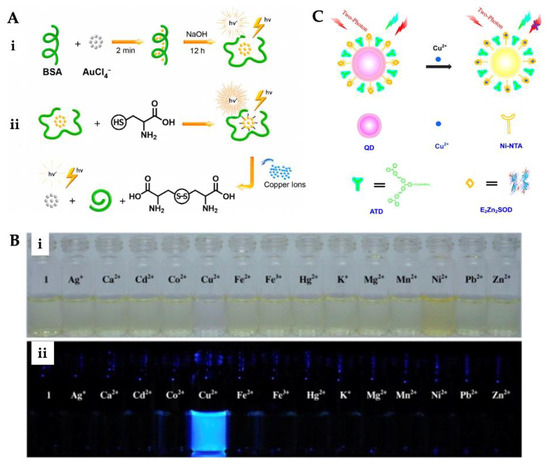
Figure 3.
Preparation steps of fluorescence sensor for Cu2+ ion detection (A), color (i) and fluorescence (ii) changes of sensor after addition of various metal ions (B). Scheme of the working principle of two-photon ratiometric imaging and sensing of Cu2+ ion (C). Republished with permission from [98,99,100].
Fu et al. designed a two-photon fluorescence sensor for imaging and sensing Cu2+ ion (Figure 3C). The selectivity of the fluorescence sensor was examined employing Na+, K+, Ca2+, Mg2+, Mn2+, Fe2+, Co2+, Ni2+, Zn2+, and Cu+ metal ions. The detection was achieved down to around 10 nM in a wide range (10−3–10−7 M) [100]. Peng et al. developed a fluorescence sensor containing two DNA sequences. The prepared sensor showed high selectivity for Cu2+ ion over various other metals. River water samples were analyzed by the standard addition method. The detection limit of the hybridized double-strand fluorescence sensor was calculated as 3.4 nM [101]. Furthermore, Du et al. designed a metal–organic framework-based fluorescence sensor for Cu2+ ion detection in aqueous conditions. The assess the ability of the sensor to determine Cu2+ ion, fluorescence quenching studies were made with distinct Cu2+ ion concentrations. The ability of selectivity and sensitivity experiments were also conducted. The results displayed that the designed fluorescence sensor could be a promising platform for real-time monitoring and bifunctional intelligent adsorbent Cu2+ ion detection with 1.91 × 10−7 M of detection limit [102]. Xie et al. prepared a fluorescence sensor for the determination of Cu2+ ions in tea infusions. The fluorescence sensor was prepared for homogeneous precipitation of CdS nanocrystals onto the SiO2 core surfaces. According to the selectivity analysis, the fluorescence sensor was more sensitive to Cu2+ ion than other metal ions. The reason for this is the strong fluorescence quenching effect of Cu2+ ion. The sensor determined the Cu2+ ion in extensive linear ranges from 0.01 to 2 μM and the detection limit was calculated as 6.3 nM [103]. Tan et al. reported a fluorescence sensor for Cu2+ ion detection in serum samples. Carbon dots and gold nanoclusters were embedded into ZIF-8 and the potential guideline of the sensor for detecting Cu2+ ion was exemplified. The synthesized sensor’s characterization by TEM and detection performance was optimized [104].
Chatterjee et al. designed an anthracene excimer fluorescence sensor on mesoporous silica, which has important advantages such as chemical stability and a large surface area. The structural characterization of cubic mesoporous silica was performed with several methods, and the reusability of materials was analyzed. This sensor material was analyzed in orange and grape juice samples for Cu2+ ion detection. In line with the tremendous results obtained, the sensor presents a very uncommon sample of an excimer-based heterogeneous sensor [105]. Cheng et al. also presented a fluorescence sensor in which Cu2+ ion was detected in an aqueous solution and in living cells. For rapid detection of Cu2+ ion in living organisms, metal–organic framework nanoparticles have unique physical and chemical properties with an easy and environmentally friendly hydrothermal route. The high affinity between Cu2+ ion and the porphyrin ligand in the structure of metal-organic framework nanoparticles can statically quench the fluorescence signal of the sensor with Cu2+ ion with high selectivity. The prepared sensor has an ultra-low limit of detection value of 220 pM [106]. Patir et al. reported nitrogen-doped carbon dots fluorescence sensor that was prepared by a one-step pyrolytic method utilizing urea and ethylenediaminetetraacetic acid for Cu2+ ion detection. The lowest detection limit for Cu2+ ion detection was 2.3 nM in an aqueous medium, which is close to the allowed levels of Cu2+ ion in drinking water. They loaded a paper-based microfluidic system loaded with nitrogen-doped carbon dots using candle wax channels on a paper. They mentioned that this sensor system is low-cost, simple and disposable paper-based platform will be very helpful for onsite detection [107].
2.3. Luminescence, Chemiluminescence, and Photoluminescence Sensors
Luminescence occurs when an excited molecule emits light as it returns to its lower energy level ground state. A few types of luminescence can be sundered base on the welding of the energy that cause an excited state. Chemiluminescence is an emission of light that is dependent on chemical reactions and is of the greatest interest to researchers [108]. As an example, Shi et al. depicted a luminescence sensor for Cu2+ ion determination. The nanoparticles selected for radiometric imaging were combined with a fluorescence sensor CYDAC16. The interaction between nanoparticles CYDAC16 and Cu2+ ion was explained in Figure 4A. The nanoparticle CYDAC16 ensures a ratiometric signal based on an up-conversion luminescence of 660 and 800 nm. The sensor performed an analysis of living mice and cells and could accomplish Cu2+ ion detection with the luminescence resonance energy transfer. The detection limit was calculated as 37 nmol/L [109]. Li et al. constructed a luminescence sensor offering high sensitivity and selectivity in the determination of Cu2+ ion. COF-JLU3 synthesized under solvothermal conditions has a porous framework structure that mediates the binding of Cu2+ ion. In the existence of Cu(NO3)2, the fluorescence lifetime decreased from 1.5 ns to 0.7 ns. The reason for this is the decrease in luminescence intensity [110].
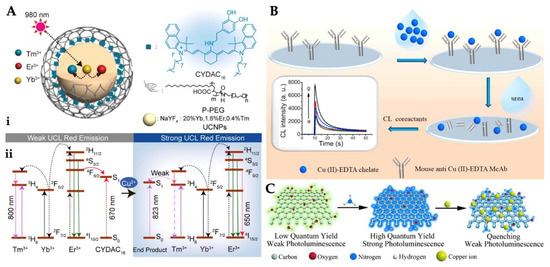
Figure 4.
Unification of nanoparticles and CYDAC16 (A), scheme of the protocol for Cu2+ ion detection (B) and representation of amino-functionalized quantum dots and their quenching by Cu2+ ion (C). Republished with permission from [109,115,116].
Chemiluminescence transition is a chemical reaction excited with the emission of light in the upper state while returning to the ground state. These transitions are defined as electromagnetic radiation dissipated from the near-ultraviolet to the near-infrared [111,112]. Chemiluminescence, which is the production of light from a chemical reaction, has many advantages, such as simplicity, rapidity, and sensitivity of detection [113,114]. For instance, Ouyang et al. reported a chemiluminescence sensor for facile, fast, sensitive, and affordable cost for the determination of Cu2+. As depicted in Figure 4B, Cu(II)-EDTA was used as a chelate and then immobilized onto the microplate. They computed the detection limit value as 0.33 ng/mL, and this value obtained a wide range of 1.0–1000 ng/mL [115].
To show the precise detection of Cu2+ ion and a stronger enhancing effect, gold nanostars were utilized for chemiluminescence sensor preparation by Amjadi and Abolghasemi-Fakhri. To increase density, nanostars were produced using seed-mediated growth. Attaching the tips of nanostars to surface plasmons causes an increase in emission intensity. The gold nanostar-based sensor system can detect very low levels in the sensitive and fast detection of Cu2+ ion [117]. Moreover, Chan et al. presented a photoluminescence sensor for highly sensitive Cu2+ ion detection. 16-Mercaptohexadecanoic acid-modified CdSe quantum dots were prepared as a sensor. Research on the presence of very low concentrations of Cu2+ ion showed a reduction in the sensor of quantum dots. They calculated the limit of detection as 5 nM in the dynamic range of up to 100 µM, and also the reaction time was observed at 5 min [118]. Wang et al.’s photoluminescence sensor was developed for determining Cu2+ ion. A cellulose nanofibril was produced by the in situ synthesis procedure. Fluorescence spectrometer, Fourier transforms infrared spectroscopy, scanning electron microscopy, and X-ray diffraction were used for the characterization of nanofibril film samples. Depending on the increment in the Cu2+ ion concentration, there was a gradual decrease in fluorescence intensity of cellulose nanofibril, and a linear relationship was determined between them and the Cu2+ ion [119].
Wang et al. prepared carbon dots with different solutions for Cu2+ ion detection by the hydrothermal method. The concentration-dependent multicolor photoluminescence emitted different colors with decreasing concentration, exhibiting the three strongest peaks. This sensor was highly selective and sensitive to detect Cu2+ ion at ppm limits [120]. Sun et al. designed a fluorescence sensor for the determination of Cu2+ ion in living cells depending on the hydrothermal treatment of graphene quantum dots. Following this process, the greenish-yellow fluorescent graphene quantum dots were transformed into amino-functionalized graphene quantum dots (Figure 4C). Compared with other metal ions, Cu2+ ion have a greater affinity for N and O on the amino-functionalized graphene quantum dots’ surface, thus, the chelating kinetics is faster. Amino-functionalized graphene quantum dots also showed higher selectivity towards Cu2+ ion [116]. Ganiga et al. designed nitrogen-rich carbon dots to detect Cu2+ ions. Their study was the first to employ the interaction of Cu2+ ion with NCD-based fluorescent nanomaterials to unravel the photoluminescence conduct. As a result of the exhaustive analyzes made, the steady-state photoluminescence emission of NCDs was revealed from the direct recombination of excitons and the involvement of defect states. The detection limit was calculated as 10 μM in 10 μM–0.4 mM dynamic range, respectively [121].
Zhao et al. prepared a selective fluorescence sensor determination for Cu2+ ion. The photoluminescence intensity of the sensor prepared using polydopamine, which does not have any photoluminescence properties, increases even more in the existence of Cu2+ ion. The sensor detection limit was determined to be as low as 1 nM [122]. A sensor was designed for Cu2+ ion detection by coating carbon dots synthesized by Liu et al. hydrothermal route with branched polyethylenimine. Photoluminescence emission spectra of the carbon quantum dots with an increment of excitation peaks from 365 to 525 nm, emission peaks are red-shifted from 440 to 540 nm. Photoluminescence intensities were diminished. The fluorescence sensor with high selectivity detected Cu2+ ion to a low detection limit of 115 nM [123]. Liu et al. developed pristine graphene quantum dots that were produced with oxidation of pitch graphite fibers to detect Cu2+ ion. The results showed the photoluminescence properties of these quantum dots could be removed by different metals during additional cysteine that can only cause recovery of the photoluminescence of graphene quantum dots removed by Cu2+ ion [124].
2.4. Surface Plasmon Resonance
Surface plasmon resonance (SPR) is an important optical sensor type that depends on the power of reflected light from a prism that is covered with a metal film [125,126]. SPR sensors use surface plasmon (SP) waves to research molecular interactions occurring on the sensor surface [127]. The SP is an electromagnetic wave induced by p-polarized light; this wave is spread along the surface of nanoparticles or layers [128,129]. The area vector of this wave achieves its highest value at the interface and it, therefore, decomposes in both the dielectric and the metal, and it decays exponentially in both environments. This wave generates SP polaritons propagating along the interface [130,131]. SP is not formed directly by light excitation of a flat metal surface. The Kretschmann configuration is commonly used to stimulate plasmons. In this configuration, the prism is in contact with a thin plasmonic metal surface to measure dielectric permittivity [132,133,134].
SPR sensors have many advantages, such as low cost [135], no labeling [136], require a low sample volume, real-time measurement [137], high sensitivity and specificity, and allow fast measurement [138,139,140,141]. They have increasing applications in the detection of various analytes in medical diagnosis, environmental monitoring, food safety, and so on [142,143,144,145,146]. Recently, Gerdan et al. prepared a molecularly imprinted nanofilm-based SPR sensor to detect Cu2+ ion in buffer and also artificial plasma and urine samples. The SPR sensor was comprehensively characterized and then used for Cu2+ ion detection from solutions with a wide range (0.04–5 μM) in a high correlation coefficient (Figure 5A). They calculated the SPR sensor detected Cu2+ ion with a low limit of detection of 0.027 µM. Other kinetic experiments, such as reusability, selectivity, and storage stability, were also performed to show all properties of the sensor [147]. Safran et al. determined Cu2+ ion using an SPR sensor. Firstly, they modified a gold sensor surface with poly(hydroxyethyl methacrylate-N-metacryloyl-(L)-cysteine methyl ester and the modified surface was used for the immobilization of Cu2+ ion. Characterization measurements of the sensor surface were carried out with different methods. The SPR sensor was also used for the demonstration of the selectivity and sensitivity in aqueous solutions [148]. Figure 5B depicts the %ΔR values of the SPR sensor at different concentrations.
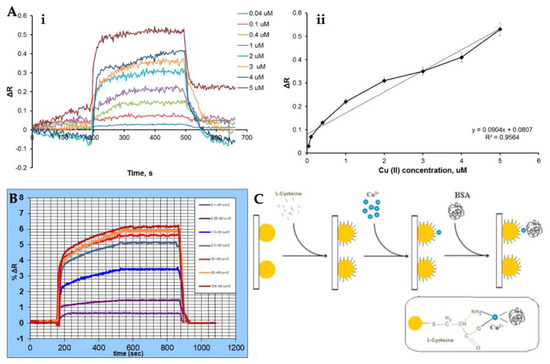
Figure 5.
Real-time detection by Cu2+-imprinted SPR sensor (A), %ΔR values of SPR sensor versus time at several concentrations of Cu2+ ion (B), and the illustration for LSPR-based Cu2+ ion detection (C). Republished with permission from [147,148,152].
Forzani et al. also reported SPR sensor to detect Cu2+ ion. The surface was separated into reference and detection areas. From these areas, the various angles were measured with a quadrant cell photodetector. The response was changed in the existence of the Cu2+ ion. Selective detection of Cu2+ ion in the broad range was achieved. Drinking water was analyzed with this sensor [149]. Daniyal and co-workers used the SPR spectroscopy for sensing Cu2+ ion. This sensor was made by adding graphene oxide and modifying it on the surface. The detection of the Cu2+ ion to the sensor surface was observed by SPR and values were calculated, such as the detection limit and signal-to-noise ratio. The optical sensor detection range was 0.01 until 0.5 ppm [150]. Chen et al. presented a sensor for the detection of Cu2+ ion. This sensor is dependent on the conformational change of Cu2+-specific peptides. Peptides that bind specifically to the Cu2+ ion were modified on the surface. Then, selectivity analyzes of the sensor were performed and the peptide showed good selectivity towards Cu2+ ion. The detection limit was calculated as 0.44 pM, and the detection range was 1 × 10−12 M to 1 × 10−6 M [151]. Finally, Ding et al. designed a sensor that determined Cu2+ ion in a real sample. With the self-assembled method, indium tin oxide film-coated gold nanoparticles were prepared (Figure 5C) and then the characterization process was carried out. The strong chelation between Cu2+ and Cys, which allows the formation of a stable Cys-Cu2+ complex, was formed by modifying the Cys onto the gold surface. Thereby it causes a shift in the LSPR absorption band. The alteration occurring with the red-shift at the peak of the LSPR band is the basis for Cu2+ ion detection [152].
3. Conclusions
Optical sensors are generally utilized in biomedical and pharmaceutical research, environmental applications, and health care to determine different biomolecules for disease diagnosis. In this review, we overviewed recent and different optical sensing technologies as state-of-the-art Cu2+ ion detection applications. Early diagnosis of diseases that develop due to deficiency or excess of Cu2+ ion at very low concentrations is very important. The optical sensors are thus attractive because of the advantages of simple and naked eye recognition require less equipment in analysis, real-time detection, high reversibility, environmental stability, durability, and practicability. Cu2+ ion detection systems still have some challenges in terms of point-of-care diagnostic procedures for special laboratories and are open to improving optical performance and chemical and physical properties toward more extensive applications in various fields. Moreover, microfluidic systems-integrated optical sensors are proposed as complementary platforms, which refers to conventional methods for the detection of several molecules with rapid response and convenience of usage. In summary, the detection of Cu2+ ion is very crucial because it is an important metal for the human body. As depicted in Table 1, there has been an increase in studies on several optical sensors with many parameters, including polymer types, detection range, and limit of detection (LOD) values. The results show that they can be combined with other methods, technologies, and platforms. Among these detection systems, some of them aim at developing the quality and enabling reliability to detect diseases in their early stages and measure food quality for human health.

Table 1.
Comparison of different optical sensors for Cu2+ ion detection.
Author Contributions
Conceptualization, Z.G. and Y.S.; writing—original draft preparation, Z.G.; writing—review and editing, Y.S. and A.D.; visualization, Y.S.; supervision, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript received no external funding.
Acknowledgments
Yeşeren Saylan gratefully acknowledges the support from The Scientific and Technological Research Council of Turkey (TÜBİTAK) - Directorate of Science Fellowships and Grant Programmes (BİDEB) 2247-D National Program of Young Researchers (Project No. 121C226).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karakuş, E. A rhodamine based fluorescent chemodosimeter for the selective and sensitive detection of copper (II) ions in aqueous media and living cells. J. Mol. Struct. 2021, 1224, 129037. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Zhao, M.; Ding, Y.; Li, Z.; Ma, Y.; Li, H.; Cui, H. Fabrication of the Ni-based composite wires for electrochemical detection of copper (II) ions. Anal. Chim. Acta 2021, 1143, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jou, M.J.; Lee, H.; Kou, S.; Lim, J.; Nam, S.W.; Park, S.; Kim, K.M.; Yoon, J. New fluorescent and colorimetric chemosensors bearing rhodamine and binaphthyl groups for the detection of Cu2+. Sens. Actuators B Chem. 2009, 137, 597–602. [Google Scholar] [CrossRef]
- Kumar, V.; Kalita, J.; Bora, H.K.; Misra, U.K. Relationship of antioxidant and oxidative stress markers in different organs following copper toxicity in a rat model. Toxicol. Appl. Pharmacol. 2016, 293, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zu, M.; Yang, S.; Zhang, S.; Zhou, W.; Mai, Z.; Ge, C.; Xu, Y.; Fang, Y.; Zhang, S. Visible-light-driven photoelectrochemical determination of Cu2+ based on CdS sensitized hydrogenated TiO2 nanorod arrays. Sens. Actuators B Chem. 2018, 270, 270–276. [Google Scholar] [CrossRef]
- Kaykhaii, M.; Khajeh, M.; Hashemi, S.H. Magnetic molecularly imprinted polymer nanoparticles for selective extraction of copper from aqueous solutions prior to its flame atomic absorption determination. J. Anal. Chem. 2015, 70, 1325–1329. [Google Scholar] [CrossRef]
- Duan, J.X.; Li, X.; Zhang, C.C. The synthesis and adsorption performance of polyamine Cu2+ imprinted polymer for selective removal of Cu2+. Polym. Bull. 2017, 74, 3487–3504. [Google Scholar] [CrossRef]
- Sakunkaewkasem, S.; Petdum, A.; Panchan, W.; Sirirak, J.; Charoenpanich, A.; Sooksimuang, T.; Wanichacheva, N. Dual-analyte fluorescent sensor based on [5]helicene derivative with super large stokes shift for the selective determinations of Cu2+ or Zn2+ in buffer solutions and its application in a living cell. ACS Sens. 2018, 3, 1016–1023. [Google Scholar] [CrossRef]
- Abu-Dalo, M.A.; Salam, A.A.; Nassory, N.S. Ion imprinted polymer based electrochemical sensor for environmental monitoring of copper (II). Int. J. Electrochem. Sci. 2015, 10, 6780–6793. [Google Scholar]
- Tarnowska, M.; Krawczyk, T. Click chemistry as a tool in biosensing systems for sensitive copper detection. Biosens. Bioelectron. 2020, 169, 112614. [Google Scholar] [CrossRef] [PubMed]
- Nadimetla, D.N.; Bhosale, S.V. Tetraphenylethylene AIEgen bearing thiophenylbipyridine receptor for selective detection of copper (II) ion. N. J. Chem. 2021, 45, 7614–7621. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, C.; Wang, S.; Li, M.; Guo, W. MOF-derived porous ZnO-Co3O4 nanocages as peroxidase mimics for colorimetric detection of copper(II) ions in serum. Analyst 2021, 146, 605–611. [Google Scholar] [CrossRef]
- Kuras, M.J.; Więckowska, E. Synthesis and characterization of a new copper (II) ion-imprinted polymer. Polym. Bull. 2015, 72, 3227–3240. [Google Scholar] [CrossRef]
- Shen, Y.J.; Zhang, K. A bifunctional optical probe based on ESIPT-triggered disalicylaldehyde with ratiometric detection of iron and copper ions. Polyhedron 2021, 193, 114883. [Google Scholar] [CrossRef]
- Bagheri, N.; Mazzaracchio, V.; Cinti, S.; Colozza, N.; Di Natale, C.; Netti, P.A.; Saraji, M.; Roggero, S.; Moscone, D.; Arduini, F. Electroanalytical sensor based on gold-nanoparticle-decorated paper for sensitive detection of copper ions in sweat and serum. Anal. Chem. 2021, 93, 5225–5233. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yu, G.; Wei, X.; Zhan, C.; Jeon, J.W.; Wang, X.; Jeffryes, C.; Guo, Z.; Wei, S.; Wujcik, E.K. Fabric/multi-walled carbon nanotube sensor for portable on-site copper detection in water. Adv. Compos. Mater. 2019, 2, 711–719. [Google Scholar] [CrossRef]
- Odetola, L.; Sills, S.; Morrison, S. A pilot study on the feasibility of testing residential tap water in North Carolina: Implications for environmental justice and health. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 972–978. [Google Scholar] [CrossRef]
- Jung, H.S.; Know, P.S.; Lee, J.W.; Kim, J.I.; Hong, C.S.; Kim, J.W.; Yan, S.; Lee, J.Y.; Lee, J.H.; Joo, T.; et al. Coumarin-derived Cu2+-selective fluorescence sensor: Synthesis, mechanisms, and applications in living cells. J. Am. Chem. Soc. 2009, 131, 2008–2012. [Google Scholar] [CrossRef]
- Frag, E.Y.Z.; Mohamed, M.E.; Ali, A.E.; Mohamed, G.G. Potentiometric sensors selective for Cu (II) determination in real water samples and biological fluids based on graphene and multi-walled carbon nanotubes modified graphite electrodes. NISCAIR-CSIR 2020, 59A, 162–173. [Google Scholar]
- Kirk, K.A.; Andreescu, S. Easy-to-use sensors for field monitoring of copper contamination in water and pesticide-sprayed plants. Anal. Chem. 2019, 91, 13892–13899. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency Office of Pesticide Programs; Copper Facts; EPA.gov: New York, NY, USA, 2008.
- Chereddy, N.R.; Janakipriya, S.; Korrapati, P.S.; Thennarasu, S.; Mandal, A.B. Solvent-assisted selective detection of sub-micromolar levels of Cu2+ ions in aqueous samples and live-cells. Analyst 2013, 138, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Normaya, E.; Baharu, N.A.; Ahmad, M.N. Synthesis of thiosemicarbazone-based colorimetric chemosensor for Cu2+ ions recognition in aqueous medium: Experimental and theoretical studies. J. Mol. Struct. 2020, 1212, 128094. [Google Scholar] [CrossRef]
- Yuan, Z.; Cai, N.; Du, Y.; He, Y.; Yeung, E.S. Sensitive and selective detection of copper ions with highly stable polyethyleneimine-protected silver nanoclusters. Anal. Chem 2014, 86, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Liu, J.; Liu, X.; Liu, J.; Xiang, J.; Wu, Y. A sensitive and selective sensing platform based on CdTe QDs in the presence of L-cysteine for detection of silver, mercury and copper ions in water and various drinks. Food Chem. 2016, 213, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.A.N.; Reddy, K.J.; Duk, L.K.; Reddy, A.V. Evaluation of 2,6-diacetylpyridinebis-4-phenyl-3-thiosemicarbazone as complexing reagent for zinc in food and environmental samples. J. Saudi Chem. Soc. 2016, 20, S271–S279. [Google Scholar] [CrossRef]
- Wannapob, R.; Kanatharana, P.; Limbut, W.; Numnuam, A.; Asawatreratanakul, P.; Thammakhet, C.; Thavarungkul, P. Affinity sensor using 3-aminophenylboronic acid for bacteria detection. Biosens. Bioelectron. 2010, 26, 357–364. [Google Scholar] [CrossRef]
- Kong, T.; Liu, G.W.; Li, X.B.; Wang, Z.; Zhang, Z.G.; Xie, G.H.; Zhang, Y.; Sun, J.; Xu, C. Synthesis and identification of artificial antigens for cadmium and copper. Food Chem. 2010, 123, 1204–1209. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Rahman, M.M.; Asiri, A.M. Novel composite material for selective copper(II) detection and removal from aqueous media. J. Mol. Liq. 2019, 283, 772–780. [Google Scholar] [CrossRef]
- Losev, V.N.; Buyko, O.V.; Trofimchuk, A.K.; Zuy, O.N. Silica sequentially modified with polyhexamethylene guanidine and Arsenazo I for preconcentration and ICP–OES determination of metals in natural waters. Microchem. J. 2015, 123, 84–89. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, H.; Zhang, H.; Ma, L.; Wang, Z. Polyacrylamide-phytic acid-polydopamine conducting porous hydrogel for rapid detection and removal of copper (II) ions. Biosens. Bioelectron. 2017, 91, 306–312. [Google Scholar] [CrossRef]
- Feng, H.; Fu, Q.; Du, W.; Zhu, R.; Ge, X.; Wang, C.; Li, Q.; Su, L.; Yang, H.; Song, J. Quantitative assessment of copper (II) in Wilson’s disease based on photoacoustic imaging and ratiometric surface-enhanced raman scattering. ACS Nano 2021, 15, 3402–3414. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Deng, P.; Li, J.; Tang, S. Fluorescent ion-imprinted sensor for selective and sensitive detection of copper (II) ions. Sens. Actuators B Chem. 2018, 255, 2095–2104. [Google Scholar] [CrossRef]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Wen, T.; Qu, F.; Li, N.B.; Luo, H.Q. A facile, sensitive, and rapid spectrophotometric method for copper (II) ion detection in aqueous media using polyethyleneimine. Arab. J. Chem. 2017, 10, S1680–S1685. [Google Scholar] [CrossRef]
- Derin, E.; Inci, F. Advances in biosensor technologies for acute kidney injury. ACS Sens. 2022, 7, 358–385. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Liu, X.; Li, P.; Wu, W. Nanocellulose as a promising substrate for advanced sensors and their applications. Int. J. Biol. Macromol. 2022, 218, 473–487. [Google Scholar] [CrossRef]
- Saylan, Y.; Akgönüllü, S.; Bereli, N.; Yavuz, H.; Denizli, A. Scaling up of biosensors for clinical applications and commercialization. In Advanced Biosensors for Virus Detection; Academic Press: Cambridge, MA, USA, 2022; pp. 407–421. [Google Scholar]
- Erdem, O.; Es, I.; Saylan, Y.; Inci, F. Unifying the efforts of medicine, chemistry, and engineering in biosensing technologies to tackle the challenges of the COVID-19 pandemic. Anal. Chem. 2021, 94, 3–25. [Google Scholar] [CrossRef]
- Rodríguez-Sevilla, E.; Rodríguez-Sevilla, E.; Ramírez-Silva, M.T.; Romero-Romo, M.; Ibarra Escutia, P.; Palomar-Pardavé, M. Electrochemical quantification of the antioxidant capacity of medicinal plants using biosensors. Sensors 2014, 14, 14423–14439. [Google Scholar] [CrossRef]
- Algieri, C.; Drioli, E.; Guzzo, L.; Donato, L. Bio-mimetic sensors based on molecularly imprinted membranes. Sensors 2014, 14, 13863–13912. [Google Scholar] [CrossRef]
- Altıntaş, Z.; Tothill, I. Biomarkers and biosensors for the early diagnosis of lung cancer. Sens. Actuators B Chem. 2013, 188, 988–998. [Google Scholar] [CrossRef]
- Roda, A.; Mirasoli, M.; Michelini, E.; Di Fusco, M.; Zangheri, M.; Cevenini, L.; Roda, B.; Simoni, P. Progress in chemical luminescence-based biosensors: A critical review. Biosens. Bioelectron. 2016, 76, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Baryeh, K.; Takalkar, S.; Lund, M.; Liu, G. Introduction to medical biosensors for point of care applications. In Medical Biosensors for Point of Care (POC) Applications; Narayan, R.J., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 3–25. [Google Scholar]
- Marazuela, M.; Moreno-Bondi, M. Fiber-optic biosensors–an overview. Anal. Bioanal. Chem. 2002, 372, 664–682. [Google Scholar] [CrossRef] [PubMed]
- Healy, D.A.; Healy, D.A.; Hayes, C.J.; Leonard, P.; McKenna, L.; O’Kennedy, R. Biosensor developments: Application to prostate-specific antigen detection. Trends Biotechnol. 2007, 25, 125–131. [Google Scholar] [CrossRef]
- Motaharian, A.; Naseri, K.; Mehrpour, O.; Shoeibi, S. Electrochemical determination of atypical antipsychotic drug quetiapine using nano-molecularly imprinted polymer modified carbon paste electrode. Anal. Chim. Acta 2020, 1097, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Purohit, B.; Vernekar, P.R.; Shetti, N.P.; Chandra, P. Biosensor nanoengineering: Design, operation, and implementation for biomolecular analysis. Sens. Int. 2020, 1, 100040. [Google Scholar] [CrossRef]
- Grazon, C.; Baer, R.C.; Kuzmanović, U.; Nguyen, T.; Chen, M.; Zamani, M.; Chern, M.; Aquino, P.; Zhang, X.; Lecommandoux, S.; et al. A progesterone biosensor derived from microbial screening. Nat. Commun. 2020, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, H. Biosensors: Enzyme immobilization chemistry. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; pp. 64–71. [Google Scholar]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R.; Rab, S. Biosensors applications in medical field: A brief review. Sens. Int. 2021, 2, 100100. [Google Scholar] [CrossRef]
- Saylan, Y.; Akgönüllü, S.; Denizli, A. Plasmonic sensors for monitoring biological and chemical threat agents. Biosensors 2020, 10, 142. [Google Scholar] [CrossRef]
- Pirzada, M.; Altintas, Z. Recent progress in optical sensors for biomedical diagnostics. Micromachines 2020, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Khansili, N.; Rattu, G.; Krishna, P.M. Label-free optical biosensors for food and biological sensor applications. Sens. Actuators B Chem. 2018, 265, 35–49. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, Y.; Lv, C.; Zhang, Z.; Peng, X. Copper-based metal–organic xerogels on paper for chemiluminescence detection of dopamine. Anal. Methods 2020, 12, 4191–4198. [Google Scholar] [CrossRef]
- Emir Diltemiz, S.; Yağmuroğlu, O. Development of reflectometric interference spectroscopy based sensor for paraokson determination. Eskiseh. Tech. Univ. J. Sci. Technol. C-Life Sci. Biotechnol. 2019, 8, 12–22. [Google Scholar]
- Zhang, H.; Dong, X.; Wang, J.; Guan, R.; Cao, D.; Chen, Q. Fluorescence emission of polyethylenimine-derived polymer dots and its application to detect copper and hypochlorite ions. ACS Appl. Mater. Interfaces 2019, 11, 32489–32499. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Mergu, N.; Kumawat, L.K. A new multifunctional rhodamine-derived probe for colorimetric sensing of Cu(II) and Al(III) and fluorometric sensing of Fe(III) in aqueous media. Sens. Actuators B Chem. 2016, 223, 101–113. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, T.; Gil, Y.G.; Kim, G.H.; Park, C.; Jang, H.; Min, J. Fabrication of bioprobe self-assembled on Au–Te nanoworm structure for SERS biosensor. Materials 2020, 13, 3234. [Google Scholar] [CrossRef] [PubMed]
- Dibekkaya, H.; Saylan, Y.; Yılmaz, F.; Derazshamshir, A.; Denizli, A. Surface plasmon resonance sensors for real-time detection of cyclic citrullinated peptide antibodies. J. Macromol. Sci. A 2016, 53, 585–594. [Google Scholar] [CrossRef]
- Safran, V.; Göktürk, I.; Bakhshpur, M.; Yılmaz, F.; Denizli, A. Development of molecularly imprinted polymer-based optical sensor for the sensitive penicillin G detection in milk. ChemistrySelect 2021, 6, 11865–11875. [Google Scholar] [CrossRef]
- Saylan, Y.; Erdem, Ö.; Ünal, S.; Denizli, A. An alternative medical diagnosis method: Biosensors for virus detection. Biosensors 2019, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Balaji, A.; Zhang, J. Electrochemical and optical biosensors for early-stage cancer diagnosis by using graphene and graphene oxide. Cancer Nanotechnol. 2017, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Arshad, F.; Nabi, F.; İkbal, S.; Khan, R.H. Applications of graphene-based electrochemical and optical biosensors in early detection of cancer biomarkers. Colloids Surf. B Biointerfaces 2022, 212, 112356. [Google Scholar] [CrossRef] [PubMed]
- Çimen, D.; Bereli, N.; Günaydın, S.; Denizli, A. Detection of cardiac troponin-I by optic biosensors with immobilized anti-cardiac troponin-I monoclonal antibody. Talanta 2020, 219, 121259. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.J.; Pollard, T.D.; Goyanes, A.; Gaisford, S.; Elbadawi, M.; Basit, A.W. Optical biosensors—Illuminating the path to personalized drug dosing. Biosens. Bioelectron. 2021, 188, 113331. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Ultrathin transition-metal dichalcogenide nanosheet-based colorimetric sensor for sensitive and label-free detection of DNA. Sens. Actuators B Chem. 2019, 290, 565–572. [Google Scholar] [CrossRef]
- Tonyushkina, K.; Nichols, J.H. Glucose meters: A review of technical challenges to obtaining accurate results. J. Diabetes Sci. Technol. 2009, 3, 971–980. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Saylan, Y.; Denizli, A. Molecularly imprinted polymer-based microfluidic systems for point-of-care applications. Micromachines 2019, 10, 766. [Google Scholar] [CrossRef]
- Manz, A.; Harrison, D.J.; Verpoorte, E.M.J.; Fettinger, J.C.; Paulus, A.; Lüdi, H.; Widmer, H.M. Planar chips technology for miniaturization and integration of separation techniques into monitoring systems: Capillary electrophoresis on a chip. J. Chromatog. A 1992, 593, 253–258. [Google Scholar] [CrossRef]
- Chin, C.D.; Linder, V.; Sia, S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip. 2012, 12, 2118–2134. [Google Scholar] [CrossRef]
- Teh, S.Y.; Lin, R.; Hung, L.H.; Lee, A.P. Droplet microfluidics. Lab Chip. 2008, 8, 198–220. [Google Scholar] [CrossRef]
- Shende, P.; Prabhakar, B.; Patil, A. Color changing sensors: A multimodal system for integrated screening. TrAC Trends Anal. Chem. 2019, 121, 115687. [Google Scholar] [CrossRef]
- VS, A.P.; Joseph, P.; Daniel, K.; Susithra, L.; Kinoshita, T.; Muthusamy, S. Colorimetric sensors for rapid detection of various analytes- review. Mater. Sci. Eng. C 2017, 78, 1231–1245. [Google Scholar]
- Liu, Y.; Han, X.; He, L.; Yin, Y. Thermoresponsive assembly of charged gold nanoparticles and their reversible tuning of plasmon coupling. Angew. Chem. Int. Ed. 2012, 51, 6373–6377. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, Y.; Yin, Y. Colorimetric stress memory sensor based on disassembly of gold nanoparticle chains. Nano Lett. 2014, 14, 2466–2470. [Google Scholar] [CrossRef]
- Liu, D.; Fang, L.; Zhou, F.; Li, H.; Zhang, T.; Li, C.; Cai, W.; Deng, Z.; Li, L.; Li, Y. Ultrasensitive and stable Au dimer-based colorimetric sensors using the dynamically tunable gap-dependent plasmonic coupling optical properties. Adv. Funct. Mater. 2018, 28, 1707392. [Google Scholar] [CrossRef]
- Kaur, B.; Kaur, N.; Kumar, S. Colorimetric metal ion sensors-A comprehensive review of the years 2011–2016. Coord. Chem. Rev. 2018, 358, 13–69. [Google Scholar] [CrossRef]
- Milindanuth, P.; Pisitsak, P. A novel colorimetric sensor based on rhodamine-B derivative and bacterial cellulose for the detection of Cu(II) ions in water. Mater. Chem. Phys. 2018, 216, 325–331. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, B.; Wei, G. Two-dimensional material-based colorimetric biosensors: A review. Biosensors 2021, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Gangapuram, R.B.; Bandi, R.; Dadigala, R.; Kotu, G.M.; Guttena, V. Facile green synthesis of gold nanoparticles with carboxymethyl gum karaya, selective and sensitive colorimetric detection of copper (II) ions. J. Clust. Sci. 2017, 28, 2873–2890. [Google Scholar] [CrossRef]
- Park, G.J.; Hwang, I.H.; Song, E.J.; Kim, H.; Kim, C. A colorimetric and fluorescent sensor for sequential detection of copper ion and cyanide. Tetrahedron 2014, 70, 2822–2828. [Google Scholar] [CrossRef]
- Deng, H.H.; Li, G.W.; Liu, A.L.; Chen, W.; Lin, X.H.; Xia, X.H. Thermally treated bare gold nanoparticles for colorimetric sensing of copper ions. Microchim. Acta 2014, 181, 911–916. [Google Scholar] [CrossRef]
- Abdulazeez, I.; Basheer, C.; Al-Saadi, A.A. A selective detection approach for copper(II) ions using a hydrazone-based colorimetric sensor: Spectroscopic and DFT study. RSC Adv. 2018, 8, 39983–39991. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Z.; Qu, W.; Shao, H.; Jiang, X. Colorimetric detection of mercury, lead and copper ions simultaneously using protein-functionalized gold nanoparticles. Biosens. Bioelectron. 2011, 26, 4064–4069. [Google Scholar] [CrossRef] [PubMed]
- Lou, T.; Chen, L.; Wang, Y.; Chen, L.; Li, J. Colorimetric detection of trace copper ions based on catalytic leaching of silver-coated gold nanoparticles. ACS Appl. Mater. Interfaces 2011, 3, 4215–4220. [Google Scholar] [CrossRef]
- Xie, G.L.; Yu, H.; Deng, M.H.; Zhao, X.L.; Yu, P. A colorimetric microfluidic sensor made by a simple instrumental-free prototyping process for sensitive quantitation of copper. Chem. Pap. 2019, 73, 1509–1517. [Google Scholar] [CrossRef]
- Maddali, H.; Mmiles, C.E.; Kohn, J.; O’Carroll, D.M. Optical biosensors for virus detection: Prospects for SARS-CoV-2/COVID-19. ChemBioChem 2021, 22, 1176–1189. [Google Scholar] [CrossRef]
- Velasco-Garcia, M.N. Optical biosensors for probing at the cellular level: A review of recent progress and future prospects. Semin. Cell Dev. Biol. 2009, 20, 27–33. [Google Scholar] [CrossRef]
- Girigoswami, K.; Akhtar, N. Nanobiosensors and fluorescence based biosensors: An overview. Int. J. Nano Dimens. 2019, 10, 1–17. [Google Scholar]
- Sharma, A.; Khan, R.; Catanante, G.; Sherazi, T.A.; Bhand, S.; Hayat, A.; Marty, J.L. Designed strategies for fluorescence-based biosensors for the detection of mycotoxins. Toxins 2018, 10, 197. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, H.; Wu, F.; Hao, G.; Chen, Y.; Tan, C.; Tan, Y.; Jiang, Y. A dual-response quinoline-based fluorescent sensor for the detection of copper (II) and iron(III) ions in aqueous medium. Sens. Actuators B Chem. 2017, 243, 765–774. [Google Scholar] [CrossRef]
- Verwilst, P.; Sunwoo, K.; Kim, J.S. The role of copper ions in pathophysiology and fluorescent sensors for the detection thereof. Chem. Commun. 2015, 51, 5556–5571. [Google Scholar] [CrossRef]
- Cotruvo, J.J.A.; Aron, A.T.; Ramos-Torres, K.M.; Chang, C.J. Synthetic fluorescent probes for studying copper in biological systems. Chem. Soc. Rev. 2015, 44, 4400–4414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.C.; Qi, J.W.; Hu, C.; Zhang, L.; Song, W.; Liang, R.P.; Qiu, J.D. Cu nanoclusters-based ratiometric fluorescence probe for ratiometric and visualization detection of copper ions. Anal. Chim. Acta 2015, 895, 95–103. [Google Scholar] [CrossRef]
- Xie, Y.F.; Jiang, Y.J.; Zou, H.Y.; Wang, J.; Huang, C.Z. Discrimination of copper and silver ions based on the label-free quantum dots. Talanta 2020, 220, 121430. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Ding, T.; Liu, J.; Zhang, G.; Tong, L.; Cheng, X.; Yang, Y.; Chen, Z.; Tang, B. Fluorescence switch of gold nanoclusters stabilized with bovine serum albumin for efficient and sensitive detection of cysteine and copper ion in mice with Alzheimer’s disease. Talanta 2021, 223, 121745. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Wu, S.P. A pyrene-based highly selective turn-on fluorescent sensor for copper (II) ions and its application in living cell imaging. Sens. Actuators B Chem. 2013, 181, 743–748. [Google Scholar] [CrossRef]
- Fu, Y.; Ding, C.; Zhu, A.; Deng, Z.; Tian, Y.; Jin, M. Two-photon ratiometric fluorescent sensor based on specific biomolecular recognition for selective and sensitive detection of copper ions in live cells. Anal. Chem. 2013, 85, 11936–11943. [Google Scholar] [CrossRef]
- Peng, J.; Ling, J.; Zhang, X.Q.; Bai, H.P.; Zheng, L.; Cao, Q.E.; Ding, Z.T. Sensitive detection of mercury and copper ions by fluorescent DNA/Ag nanoclusters in guanine-rich DNA hybridization. Spectrochim. Acta A Mol. Biomol. 2015, 137, 1250–1257. [Google Scholar] [CrossRef]
- Du, T.; Wang, J.; Zhang, T.; Zhang, L.; Yang, C.; Yue, T.; Sun, J.; Li, T.; Zhou, M.; Wang, J. An integrating platform of ratiometric fluorescent adsorbent for unconventional real-time removing and monitoring of copper ions. ACS Appl. Mater. Interfaces 2020, 12, 13189–13199. [Google Scholar] [CrossRef]
- Xie, T.; Zhong, X.; Liu, Z.; Xie, C. Silica-anchored cadmium sulfide nanocrystals for the optical detection of copper (II). Microchim. Acta 2020, 187, 323. [Google Scholar] [CrossRef]
- Tan, Q.; Zhang, R.; Zhang, G.; Liu, X.; Qu, F.; Lu, L. Embedding carbon dots and gold nanoclusters in metal-organic frameworks for ratiometric fluorescence detection of Cu2+. Anal. Bioanal. Chem. 2020, 412, 1317–1324. [Google Scholar] [CrossRef]
- Chatterjee, S.; Gohil, H.; Raval, I.; Chatterjee, S.; Paital, A.R. An anthracene excimer fluorescence probe on mesoporous silica for dual functions of detection and adsorption of mercury (II) and copper (II) with biological in vivo applications. Small 2019, 15, 1804749. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, R.; Wang, J.; Zhang, Y.; Wen, C.; Tan, Y.; Yang, M. An ultrasensitive and selective fluorescent nanosensor based on porphyrinic metal–organic framework nanoparticles for Cu2+ detection. Analyst 2020, 145, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Patir, K.; Gogoi, S.K. Nitrogen-doped carbon dots as fluorescence ON–OFF–ON sensor for parallel detection of copper(II) and mercury(II) ions in solutions as well as in filter paper-based microfluidic device. Nanoscale Adv. 2019, 1, 592. [Google Scholar] [CrossRef]
- Blum, L.J.; Coulet, P.R. Luminescent biosensors. In Biosensors and Their Applications; Yang, V.C., Ngo, T.T., Eds.; Springer: New York, NY, USA, 2012; pp. 213–223. [Google Scholar]
- Shi, Y.; Liu, Q.; Yuan, W.; Xue, M.; Feng, W.; Li, F. Dye-assembled upconversion nanocomposite for luminescence ratiometric in vivo bioimaging of copper ions. ACS Appl. Mater. Interfaces 2018, 11, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Xia, H.; Mu, Y.; Liu, X. A robust and luminescent covalent organic framework as a highly sensitive and selective sensor for the detection of Cu2+ ions. Chem. Comm. 2016, 52, 6613–6616. [Google Scholar] [CrossRef]
- Rawal, R.; Kharangarh, P.R.; Dawra, S.; Tomar, M.; Gupta, V.; Pundir, C.S. A comprehensive review of bilirubin determination methods with special emphasis on biosensors. Process Biochem. 2020, 89, 165–174. [Google Scholar] [CrossRef]
- Gunn, J.A.; Gunn, J.A.; Shelley, C.; Lewis, S.W.; Toop, T.; Archer, M. The determination of morphine in the larvae of Calliphora stygia using flow injection analysis and HPLC with chemiluminescence detection. J. Anal. Toxicol. 2006, 30, 519–523. [Google Scholar] [CrossRef][Green Version]
- Hindson, B.J.; Barnett, N.W. Analytical applications of acidic potassium permanganate as a chemiluminescence reagent. Anal. Chim. Acta 2001, 445, 1–19. [Google Scholar] [CrossRef]
- Zhang, X.R.; Baeyens, W.R.G.; Van der Weken, G.; Calokerinos, A.C.; Nakashima, K. Chemiluminescence determination of captopril based on a Rhodamine B sensitized cerium (IV) method. Anal. Chim. Acta 1995, 303, 121–125. [Google Scholar] [CrossRef]
- Ouyang, H.; Shu, Q.; Wang, W.; Wang, Z.; Yang, S.; Wang, L.; Fu, Z. An ultra-facile and label-free immunoassay strategy for detection of copper (II) utilizing chemiluminescence self-enhancement of Cu (II)-ethylenediaminetetraacetate chelate. Biosens. Bioelectron. 2016, 85, 157–163. [Google Scholar] [CrossRef]
- Sun, H.; Gao, N.; Wu, L.; Ren, J.; Wei, W.; Qu, X. Highly photoluminescent amino-functionalized graphene quantum dots used for sensing copper ions. Chem. Eur. J. 2013, 19, 13362–13368. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Abolghasemi-Fakhri, Z. Gold nanostar-enhanced chemiluminescence probe for highly sensitive detection of Cu(II) ions. Sens. Actuators B Chem. 2018, 257, 629–634. [Google Scholar] [CrossRef]
- Chan, Y.H.; Chen, J.; Liu, Q.; Wark, S.E.; Son, D.H.; Batteas, J.D. Ultrasensitive copper (II) detection using plasmon-enhanced and photo-brightened luminescence of CdSe quantum dots. Anal. Chem. 2010, 82, 3671–3678. [Google Scholar] [CrossRef]
- Wang, H.; Pei, Y.; Qian, X.; An, X. Eu-metal organic framework@ TEMPO-oxidized cellulose nanofibrils photoluminescence film for detecting copper ions. Carbohydr. Polym. 2020, 236, 116030. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, Q.; Hu, S. Carbon dots with concentration-tunable multicolored photoluminescence for simultaneous detection of Fe3+ and Cu2+ ions. Sens. Actuators B Chem. 2017, 253, 928–933. [Google Scholar] [CrossRef]
- Ganiga, M.; Cyriac, J. Understanding the photoluminescence mechanism of nitrogen-doped carbon dots by selective interaction with copper ions. ChemPhysChem 2016, 17, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Kim, J. Highly sensitive and selective photoluminescence detection of copper ions via in situ formation of fluorescent polydopamine. J. Nanosci. Nanotechnol. 2017, 17, 5776–5779. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Zhang, Y. One-step green synthesized fluorescent carbon nanodots from bamboo leaves for copper(II) ion detection. Sens. Actuators B Chem. 2014, 196, 647–652. [Google Scholar] [CrossRef]
- Liu, X.; Gao, W.; Zhou, X.; Ma, Y. Pristine graphene quantum dots for detection of copper ions. J. Mater. Res. 2014, 29, 1401–1407. [Google Scholar] [CrossRef]
- Šípová, H.; Homola, J. Surface plasmon resonance sensing of nucleic acids: A review. Anal. Chim. Acta 2013, 773, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Bakhshpour, M.; Göktürk, I.; Bereli, N.; Yılmaz, F.; Denizli, A. selective detection of penicillin G antibiotic in milk by molecularly imprinted polymer-based plasmonic SPR sensor. Biomimetics 2021, 6, 72. [Google Scholar] [CrossRef]
- Saylan, Y.; Erdem, Ö.; Cihangir, N.; Denizli, A. Detecting fingerprints of waterborne bacteria on a sensor. Chemosensors 2019, 7, 33. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Yavuz, H.; Denizli, A. SPR nanosensor based on molecularly imprinted polymer film with gold nanoparticles for sensitive detection of aflatoxin B1. Talanta 2020, 219, 121219. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, A.P.; Dorofeenko, A.V.; Pukhov, A.A.; Lisyansky, A.A. Exciting surface plasmon polaritons in the Kretschmann configuration by a light beam. Phys. Rev. B 2018, 97, 235407. [Google Scholar] [CrossRef]
- Zhou, X.L.; Yang, Y.; Wang, S.; Liu, X.W. Surface plasmon resonance microscopy: From single-molecule sensing to single-cell imaging. Angew. Chem. Int. Ed. 2020, 59, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zeng, X.; Liang, J. Surface plasmon resonance: An introduction to a surface spectroscopy technique. J. Chem. Educ. 2010, 87, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Kretschmann, E.; Raether, H. Notizen: Radiative decay of non radiative surface plasmons excited by light. Z. Naturforsch. A 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Das, C.M.; Ouyang, Q.; Dinh, X.Q.; Coquet, P.; Yong, K.T. A theoretical insight into the use of anti-reflective coatings for the upliftment of sensitivity of surface plasmon resonance sensors. Opt. Commun. 2020, 458, 124748. [Google Scholar] [CrossRef]
- Cyago, A.; Advincula, R. Surface plasmon resonance spectroscopy and molecularly imprinted polymer (MIP) sensors. In Handbook of Spectroscopy; Gauglitz, G., Moore, S.D., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2014; pp. 1229–1258. [Google Scholar]
- Mohammadzadeh-Asl, S.; Aghanejad, A.; de la Guardia, M.; Ezzati Nazhad Dolatabadi, J.; Keshtkar, A. Surface plasmon resonance signal enhancement based on erlotinib loaded magnetic nanoparticles for evaluation of its interaction with human lung cancer cells. Opt. Laser Technol. 2021, 133, 106521. [Google Scholar] [CrossRef]
- Wei, T.; Ren, P.; Huang, L.; Ouyang, Z.; Wang, Z.; Kong, X.; Li, T.; Yin, Y.; Wu, Y.; He, Q. Simultaneous detection of aflatoxin B1, ochratoxin A, zearalenone and deoxynivalenol in corn and wheat using surface plasmon resonance. Food Chem. 2019, 300, 125176. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wu, Y.; Chang, S.J.; Chen, C.J.; Liu, J.T. Investigation of the recognition interaction between glycated hemoglobin and its aptamer by using surface plasmon resonance. Talanta 2021, 222, 121466. [Google Scholar] [CrossRef]
- Saylan, Y.; Akgönüllü, S.; Çimen, D.; Derazshamshir, A.; Bereli, N.; Yılmaz, F.; Denizli, A. Development of surface plasmon resonance sensors based on molecularly imprinted nanofilms for sensitive and selective detection of pesticides. Sens. Actuators B Chem. 2017, 241, 446–454. [Google Scholar] [CrossRef]
- Peng, D.; Kavanagh, O.; Gao, H.; Zhang, X.; Deng, S.; Chen, D.; Liu, Z.; Xie, C.; Situ, C.; Yuan, Z. Surface plasmon resonance biosensor for the determination of 3-methyl-quinoxaline-2-carboxylic acid, the marker residue of olaquindox, in swine tissues. Food Chem. 2020, 302, 124623. [Google Scholar] [CrossRef]
- Shibayama, J.; Mitsutake, K.; Yamauchi, J.; Nakano, H. Kretschmann- and Otto-type surface plasmon resonance waveguide sensors in the terahertz regime. Microw. Opt. Technol. Lett. 2021, 63, 103–106. [Google Scholar] [CrossRef]
- Dong, J.; Gao, N.; Peng, Y.; Guo, C.; Lv, Z.; Wang, Y.; Zhou, C.; Ning, B.; Liu, M.; Gao, Z. Surface plasmon resonance sensor for profenofos detection using molecularly imprinted thin film as recognition element. Food Control 2012, 25, 543–549. [Google Scholar] [CrossRef]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-enhanced optical sensors: A review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef]
- Inci, F.; Saylan, Y.; Kojouri, A.M.; Ogut, M.G.; Denizli, A.; Demirci, U. A disposable microfluidic-integrated hand-held plasmonic platform for protein detection. Appl. Mater. Today 2020, 18, 100478. [Google Scholar] [CrossRef]
- Rahtuvanoğlu, A.; Akgönüllü, S.; Karacan, S.; Denizli, A. Biomimetic nanoparticles based surface plasmon resonance biosensors for histamine detection in foods. ChemistrySelect 2020, 5, 5683–5692. [Google Scholar] [CrossRef]
- Atay, N.Ö.; Osman, B.; Akgöl, S.; Karagözler, A.; Denizli, A. Preparation of molecularly imprinted spr nanosensor for myoglobin detection. Curr. Appl. Polym. Sci. 2018, 2, 102–111. [Google Scholar] [CrossRef]
- Maisonneuve, M.; Valsecchi, C.; Wang, C.; Brolo, A.G.; Meunier, M. Leukemic marker detection using a spectro-polarimetric surface plasmon resonance platform. Biosens. Bioelectron. 2015, 63, 80–85. [Google Scholar] [CrossRef]
- Gerdan, Z.; Saylan, Y.; Uğur, M.; Denizli, A. Ion-imprinted polymer-on-a-sensor for copper detection. Biosensors 2022, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Safran, V.; Göktürk, I.; Derazshamshir, A.; Yılmaz, F.; Sağlam, N.; Denizli, A. Rapid sensing of Cu+2 in water and biological samples by sensitive molecularly imprinted based plasmonic biosensor. Microchem. J. 2019, 148, 141–150. [Google Scholar] [CrossRef]
- Forzani, E.S.; Zhang, H.; Chen, W.; Tao, N. Detection of heavy metal ions in drinking water using a high-resolution differential surface plasmon resonance sensor. Environ. Sci. Technol. 2005, 39, 1257–1262. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S. Exploration of surface plasmon resonance for sensing copper ion based on nanocrystalline cellulose-modified thin film. Opt. Express 2018, 26, 34880–34893. [Google Scholar] [CrossRef]
- Chen, H.; Jia, S.; Zhang, J.; Jang, M.; Chen, X.; Koh, K.; Wang, Z. Sensitive detection of copper(II) ions based on the conformational change of peptides by surface plasmon resonance spectroscopy. Anal. Methods 2015, 7, 8942–8946. [Google Scholar] [CrossRef]
- Ding, L.; Gao, Y.; Di, J. A sensitive plasmonic copper(II) sensor based on gold nanoparticles deposited on ITO glass substrate. Biosens. Bioelectron. 2016, 83, 9–14. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).