Gold Nanocone Array with Extensive Electromagnetic Fields for Highly Reproducible Surface-Enhanced Raman Scattering Measurements
Abstract
:1. Introduction
2. Materials and Methods
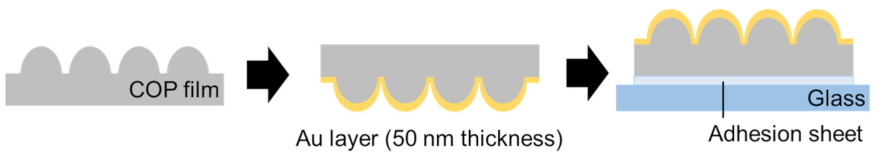
2.1. Fabrication of Au NCA
2.2. Microscopy
2.3. Optical Characterization
2.4. Evaluation of Au NCA Performance as SERS Substrate
3. Results and Discussion
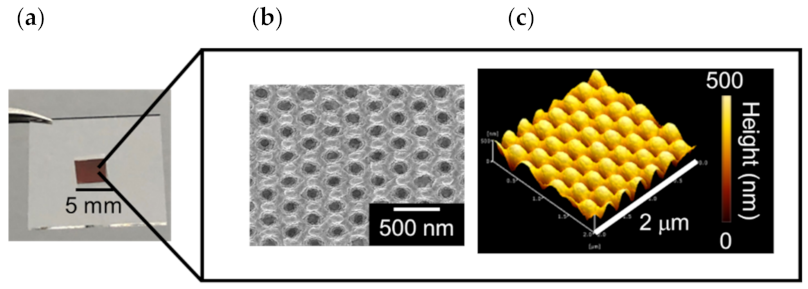
3.1. Fabrication and Optical Characterization of Au NCA
3.2. Evaluation of Au NCA Performance as SERS Substrate
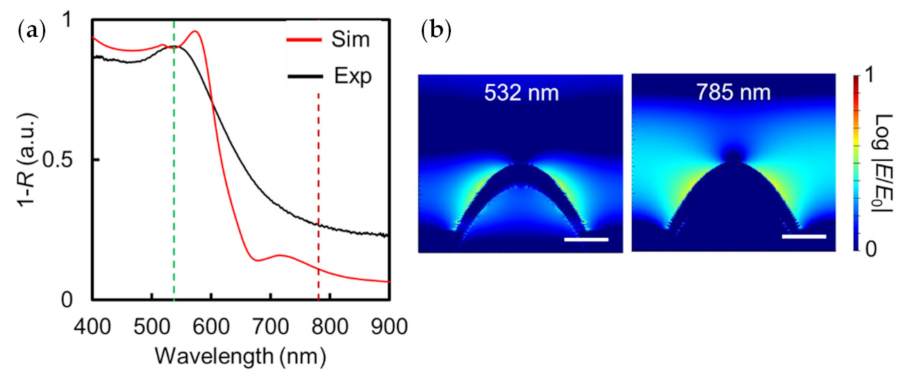
3.2.1. Optimization of Excitation Laser Wavelength
3.2.2. Theoretical Consideration of the SERS Enhancement Effect of Au Thickness on Au NCA by FDTD Simulation
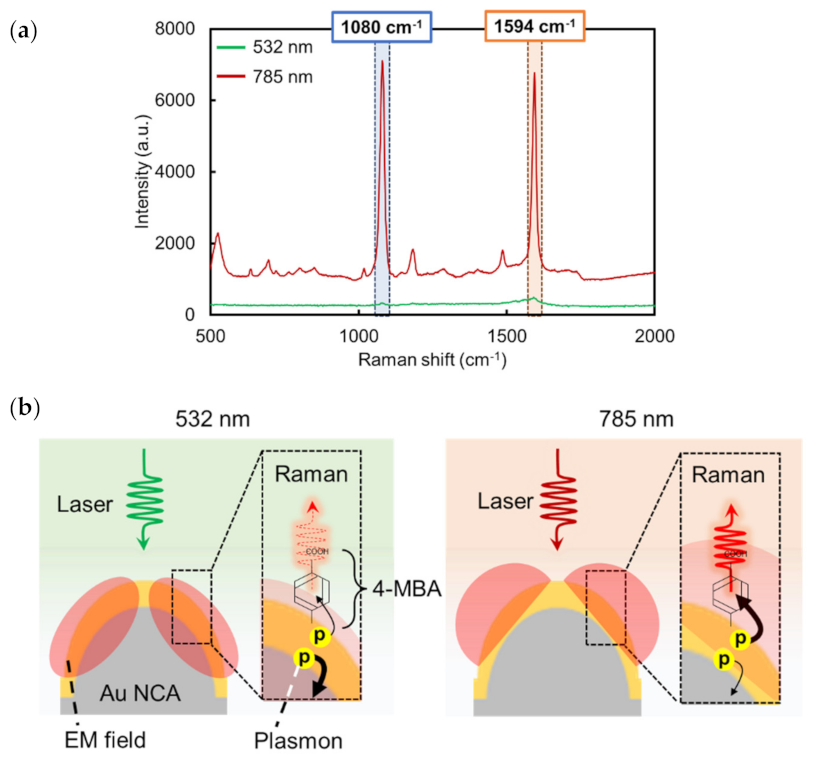
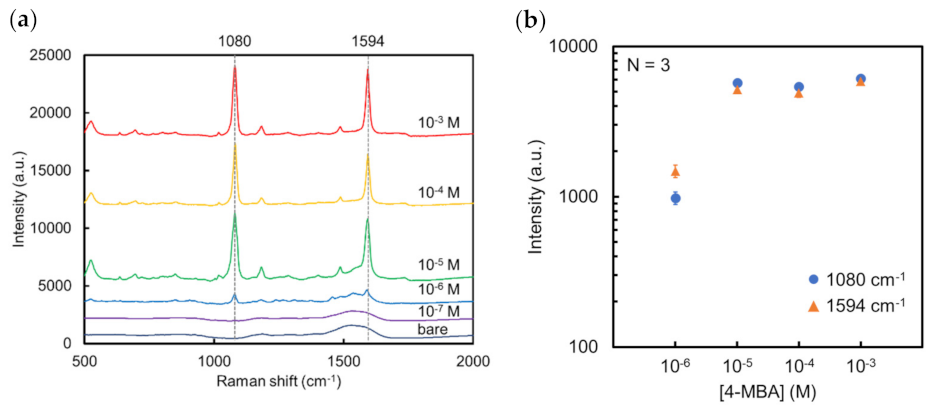
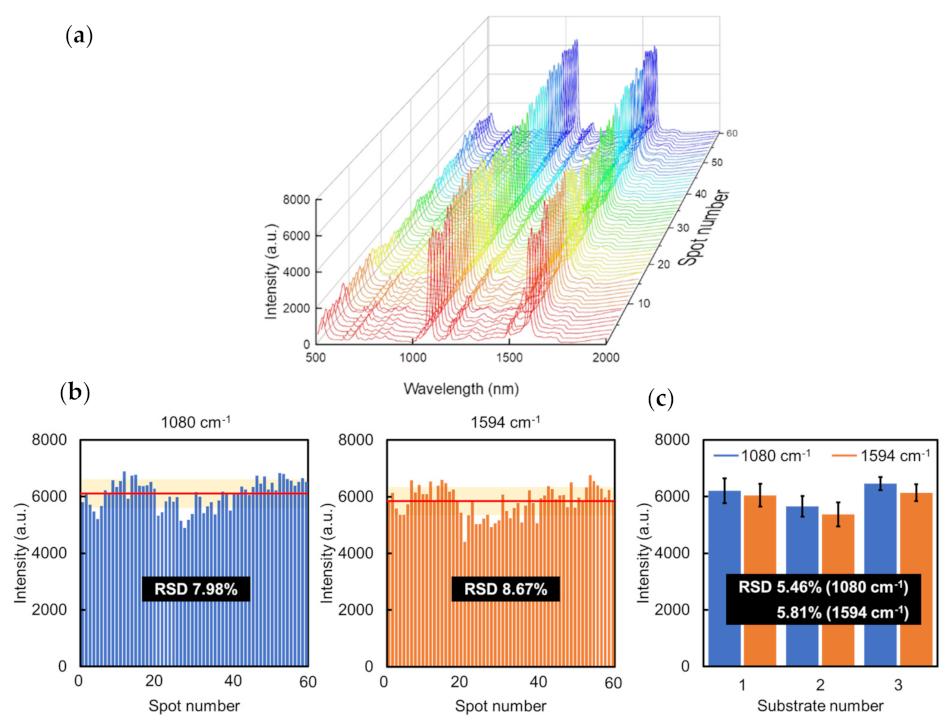
3.2.3. Evaluation of the Sensitivity and Reproducibility of Au NCA SERS Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raghu, K.; Valerie, S.L. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Leonid, M.; Yoel, S. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019, 17, e3000363. [Google Scholar] [CrossRef]
- Reza, M.M.; Justin, D.B.; Adam, M.; Brenda, G.; Laili, M.; Thaddeus, G.; Ivaylo, I.; Anahita, M.; Jessica, S.; Alison, L.A.; et al. Nanoparticle-Mediated Binning and Profiling of Heterogeneous Circulating Tumor Cell Subpopulations. Angew. Chem. Int. Ed. 2015, 54, 139–143. [Google Scholar] [CrossRef]
- Francesc, C.-G.; Nicola, A. Tracking cancer progression: From circulating tumor cells to metastasis. Genome Med. 2020, 12, 31. [Google Scholar] [CrossRef] [Green Version]
- Xiao, P.; Richard, W.V. Membrane protein structures and interactions fromcovalent labeling coupled with mass spectrometry. Mass Spectrom. Rev. 2022, 41, 51–69. [Google Scholar] [CrossRef]
- Louise, J.W.; Jason, K.K.L.; Jacqueline, M.M.; Joel, P.M. The characterization of protein interactions—What, how and how much? Chem. Soc. Rev. 2021, 50, 12292–12307. [Google Scholar] [CrossRef]
- Bordanaba-Florit, G.; Royo, F.; Kruglik, S.G.; Falcón-Pérez, J.M. Using single-vesicle technologies to unravel the heterogeneity of extracellular vesicles. Nat. Protocol. 2021, 16, 3163–3185. [Google Scholar] [CrossRef]
- Marcel, I.R.; Maria, G.A.; Catarina, G.; Ivana, M.; Joshua, A.W.; Vanessa, M.F.; Muhammad, N.; Naveed, A.; Yvonne, C.; Laura, M.; et al. Technical challenges of working with extracellular vesicles. Nanoscale 2018, 10, 881–906. [Google Scholar] [CrossRef] [Green Version]
- Priyamvada, G.; Ashish, V.; Nilesh, R.; Anurag, K.S.; Santosh, K.S.; Brijesh, K.; Rajiv, K.; Vibhav, G. Mass Spectrometry-Based Technology and Workflows for Studying the Chemistry of Fungal Endophyte Derived Bioactive Compounds. ACS Chem. Biol. 2021, 16, 2068–2086. [Google Scholar] [CrossRef]
- Zeina, H.; Walid, A.; Raya, S.; Humam, K.; Massoud, L.K. Circulating Tumor Cell Detection Technologies and Clinical Utility: Challenges and Opportunities. Cancers 2020, 12, 1930. [Google Scholar] [CrossRef]
- Mads, S.B.; Michael, B.A.; Molly, M.S. Online quantitative monitoring of live cell engineered cartilage growth using diffuse fiber-optic Raman spectroscopy. Biomaterials 2017, 140, 128–137. [Google Scholar] [CrossRef]
- Zachary, J.S.; Changwon, L.; Tatu, R.; Randy, P.C.; Sidhartha, H.; Alisha, K.; Kit, L.; Heikki, S.; Elisa, L.; Ibañez, T.; et al. Single exosome study reveals subpopulations distributed among cell lines with variability related to membrane content. J. Extracell. Vesicles 2015, 4, 28533. [Google Scholar] [CrossRef] [Green Version]
- Rebecca., L.P.; Nicole., J.C.; Meron., G.; Katherine., E.C.; Eric, A.E. Diagnostic bacteriology: Raman spectroscopy. Methods Mol. Biol. 2017, 1616, 249. [Google Scholar] [CrossRef]
- Elvin, S.A.; Venera, V.K.; Bekzhan, D.K.; Elena, V.Z.; Oleg, V.R.; Tamila, V.M.; Georgy, V.M.; Saleh, A.; Tatsuya, T.; Suleyman, I.A. Raman Spectroscopy and Its Modifications Applied to Biological and Medical Research. Cells 2022, 11, 386. [Google Scholar] [CrossRef]
- Yuko, S.Y.; Yukihiro, O.; Tamitake, I. Recent progress and frontiers in the electromagnetic mechanism of surface-enhanced Raman scattering. J. Photochem. Photobio. C 2014, 21, 81–104. [Google Scholar] [CrossRef]
- Yuko, S.Y.; Tamitake, I. Why and how do the shapes of surface enhanced Raman scattering spectra change? Recent progress from mechanistic studies. J. Raman Spectrosc. 2016, 47, 78–88. [Google Scholar] [CrossRef]
- Cheng, Z.; Mengxi, X.; Li-Jia, X.; Ting, W.; Xin, M.; Xiao-Shan, Z.; Ren, H.; Bin, R. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef]
- Xiaoru, Z.; Chao, L.; Yujiao, P.; Weiling, S.; Shusheng, Z. Preparation of a Novel Raman Probe and Its Application in the Detection of Circulating Tumor Cells and Exosomes. ACS Appl. Mater. Interfaces 2019, 11, 28671–28680. [Google Scholar] [CrossRef]
- Jaena, P.; Miyeon, H.; ByeongHyeon, C.; Hyesun, J.; Jik-han, J.; Hyun, K.K.; Sunghoi, H.; Ji-ho, P.; Yeonho, C. Exosome Classification by Pattern Analysis of Surface-Enhanced Raman Spectroscopy Data for Lung Cancer Diagnosis. Anal. Chem. 2017, 89, 6695–6701. [Google Scholar] [CrossRef]
- Juhong, C.; Yinyong, L.; Kang, H.; Panxue, W.; Lili, H.; Kenneth, R.C.; Sam, R.N. Nanoimprinted Patterned Pillar Substrates for Surface-Enhanced Raman Scattering Applications. ACS Appl. Mater. Interfaces 2015, 7, 22106–22113. [Google Scholar] [CrossRef]
- Jing, W.; Kevin, M.K.; Yuling, W.; Matt, T. Engineering State-of-the-Art Plasmonic Nanomaterials for SERS-Based Clinical Liquid Biopsy Application. Adv. Sci. 2019, 6, 1900730. [Google Scholar] [CrossRef] [Green Version]
- Sara, A.-C.; Lei, W.; Alexandra, T.; Kevin, O.; Lorena, D. Multiplexing Liquid Biopsy with Surface-Enhanced Raman Scattering Spectroscopy. Adv. Optical Mater. 2021, 9, 2001171. [Google Scholar] [CrossRef]
- Yuying, Z.; Xue, M.; Xiaoyue, T.; Rong, X. Recent Progress on Liquid Biopsy Analysis using Surface-Enhanced Raman Spectroscopy. Theranostics 2019, 9, 491–525. [Google Scholar] [CrossRef]
- Xue, T.; Wang, S.; Ou, G.; Li, Y.; Ruan, H.; Li, Z.; Ma, Y.; Zou, R.; Qiu, J.; Shen, Z.; et al. Detection of circulating tumor cells based on improved SERS-active magnetic nanoparticles. Anal. Methods 2019, 11, 2918–2928. [Google Scholar] [CrossRef]
- Pang, W.; Wang, C.; Xiao, R.; Sun, Z. Dual-Selective and Dual-Enhanced SERS Nanoprobes Strategy for Circulating Hepatocellular Carcinoma Cells Detection. Chem. Eur. J. 2018, 24, 7060–7067. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Wang, L.; Tian, Y.; Yin, B.-C.; Ye, B.-C. Multiple and sensitive SERS detection of cancer-related exosomes based on gold–silver bimetallic nanotrepangs. Analyst 2020, 145, 2795–2804. [Google Scholar] [CrossRef]
- Tian, Y.-F.; Ning, C.-F.; He, F.; Yin, B.-C.; Ye, B.-C. Highly sensitive detection of exosomes by SERS using gold nanostar@Raman reporter@nanoshell structures modified with a bivalent cholesterol labeled DNA anchor. Analyst 2018, 143, 4915–4922. [Google Scholar] [CrossRef]
- Darya, R.; Helmuth, M. Prospects for plasmonic hot spots in single molecule SERS towards the chemical imaging of live cells. Phys. Chem. Chem. Phys. 2015, 17, 21072–21093. [Google Scholar] [CrossRef] [Green Version]
- Xi-bin, X.; Jiang-shan, L.; Miao, L.; Yu-ying, W.; Zao, Y.; Xi-bo, L.; You-gen, Y.; Yong-jian, T. The influence of edge and corner evolution on plasmon properties and resonant edge effect in gold nanoplatelets. Phys. Chem. Chem. Phys. 2015, 17, 2641–2650. [Google Scholar] [CrossRef]
- Shan, C.; Zhen, W.; Wenbin, G.; Zhigang, C.; Weibang, L.; John, R.L.; Zhigang, Z. Electrochromic semiconductors as colorimetric SERS substrates with high reproducibility and renewability. Nat. Commun. 2019, 10, 678. [Google Scholar] [CrossRef]
- Ying, F.; Nak-Hyun, S.; Dana, D.D. Measurement of the Distribution of Site Enhancements in Surface-Enhanced Raman Scattering. Science 2008, 321, 388. [Google Scholar] [CrossRef]
- Prasanta, M.; Bhagya, S.T. Progress in surface enhanced Raman scattering molecular sensing: A review. Surf. Interfaces 2022, 28, 101655. [Google Scholar] [CrossRef]
- Vasyl, S.; Gregor, F.; Janez, Z.; Ibrahim, A.; Uros, C. Surface-enhanced Raman spectroscopy for chemical and biological sensing using nanoplasmonics: The relevance of interparticle spacing and surface morphology. Appl. Phys. Rev. 2020, 7, 031307. [Google Scholar] [CrossRef]
- Wenyao, H.; Ling, X.; Yufei, H.; Gongke, L. Recent progress on three-dimensional substrates for surface-enhanced Raman spectroscopic analysis. Microchem. J. 2022, 172, 106908. [Google Scholar] [CrossRef]
- Hiang, K.L.; Yih, H.L.; Charlynn, S.L.K.; Gia, C.P.-Q.; Xuemei, H.; Chee, L.L.; Howard, Y.F.S.; Ya-Chuan, K.; Qi, A.; Xing, Y.L. Designing surface-enhanced Raman scattering (SERS) platforms beyond hotspot engineering: Emerging opportunities in analyte manipulations and hybrid materials. Chem. Soc. Rev. 2019, 48, 731–756. [Google Scholar] [CrossRef]
- Jayakumar, P.; Yusong, W.; Amalina, B.E.A.; Dinish, U.S.; Malini, O. Towards a point-of-care SERS sensor for biomedical and agri-food analysis applications: A review of recent advancements. Nanoscale 2021, 13, 553–580. [Google Scholar] [CrossRef]
- Limin, L.; Wee, S.C. Rapid Fabrication of a Flexible and Transparent Ag Nanocubes@PDMS Film as a SERS Substrate with High Performance. ACS Appl. Mater. Interfaces 2020, 12, 37538–37548. [Google Scholar] [CrossRef]
- Lee, C.; Carney, R.P.; Hazari, S.; Smith, Z.J.; Knudson, A.; Robertson, C.S.; Lam, K.S.; Wachsmann-Hogiu, S. 3D plasmonic nanobowl platform for the study of exosomes in solution. Nanoscale 2015, 7, 9290–9297. [Google Scholar] [CrossRef]
- Ji, S.; Lin, G.; Zhengjun, G.; Dongmei, W.; Xiaosi, Y.; Meikun, F. Facile fabrication of a large-area and cost-effective PDMS-SERS substrate by sandpaper template assisted lithography. Anal. Methods 2019, 11, 4917–4922. [Google Scholar] [CrossRef]
- Dewen, X.; Wei, S.; Hanwen, L.; Yinlong, L.; Tianan, Y.; Jiau, W.; Hong, W.; Cheng, Y.; Bingyan, C. A gold nanoparticle doped flexible substrate for microplastic SERS detection. Phys. Chem. Chem. Phys. 2022, 24, 12036–12042. [Google Scholar] [CrossRef]
- Rongke, G.; Xuefei, S.; Changbiao, Z.; Chaogui, W.; Sheng, C.; Kai, G.; Ning, M.; Huafang, C.; Zhongyi, G.; Lin-Bao, L.; et al. Light trapping induced flexible wrinkled nanocone SERS substrate for highly sensitive explosive detection. Sens. Actuators B 2020, 314, 128081. [Google Scholar] [CrossRef]
- Kawasaki, D.; Yamada, H.; Maeno, K.; Sueyoshi, K.; Hisamoto, H.; Endo, T. Core−Shell-Structured Gold Nanocone Array for Label-Free DNA Sensing. ACS Appl. Nano Mater. 2019, 2, 4983–4990. [Google Scholar] [CrossRef]
- Kawasaki, D.; Maeno, K.; Yamada, H.; Sueyoshi, K.; Hisamoto, H.; Endo, T. TiN-contained polymer-metal core-shell structured nanocone array: Engineering of sensor performance by controlling plasmonic properties. Sens. Actuators B 2019, 299, 126932. [Google Scholar] [CrossRef]
- Lim, S.; Kim, H.-K. Deposition Rate Effect on Optical and Electrical Properties of Thermally Evaporated WO3−x/Ag/WO3−x Multilayer Electrode for Transparent and Flexible Thin Film Heaters. Sci. Rep. 2020, 10, 8357. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Pimentel, A.C.; Mateus, T.; Leitao, J.P.; Soares, J.; Falacao, B.P.; Araujo, A.; Vicente, A.; Filonovich, S.A.; Águas, H.; et al. Influence of the layer thickness in plasmonic gold nanoparticles produced by thermal evaporation. Sci. Rep. 2013, 3, 1469. [Google Scholar] [CrossRef]
- Guowei, S.; Chen, F.; Mengmeng, D.; Guangxia, J.; Qijun, S. The finite-difference time-domain (FDTD) guided preparation of Ag nanostructures on Ti substrate for sensitive SERS detection of small molecules. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 269, 120743. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Y.; Zhou, N.; Zhang, L.Y.; Hu, Q.; Ma, C.Y. FDTD simulation of Ag-decorated ZnO nanorods for optimization of 3D SERS substrates. Appl. Surf. Sci. 2021, 565, 150524. [Google Scholar] [CrossRef]
- Lee, K.-S.; El-Sayed, A.M. Gold and Silver Nanoparticles in Sensing and Imaging: Sensitivity of Plasmon Response to Size, Shape, and Metal Composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef]
- Ameer, S.F.; Pittman, U.C.; Zhang, D. Quantification of Resonance Raman Enhancement Factors for Rhodamine 6G (R6G) in Water and on Gold and Silver Nanoparticles: Implications for Single-Molecule R6G SERS. J. Phys. Chem. C 2013, 117, 27096–27104. [Google Scholar] [CrossRef]
- Shorie, M.; Kumar, V.; Kaur, H.; Singh, K.; Tomer, K.V.; Sabherwal, P. Plasmonic DNA hotspots made from tungsten disulfide nanosheets and gold nanoparticles for ultrasensitive aptamer-based SERS detection of myoglobin. Microchim. Acta 2018, 185, 158. [Google Scholar] [CrossRef]
- Vignesh, S.; Lu, D.; Ah, B.C.; Fung, L.Y. Fabrication of Large-Area Flexible SERS Substrates by Nanoimprint Lithography. ACS Appl. Nano Mater. 2018, 1, 886–893. [Google Scholar] [CrossRef]
- Marlitt, V.; Anil, H.T.; Marco, M.; Kaiyu, W.; Darmin, C.; Michael, S.; Schmidt, K.Z.; Anja, B. Injection-Molded Microfluidic Device for SERS Sensing Using Embedded Au-Capped Polymer Nanocones. ACS Appl. Mater. Interfaces 2018, 10, 37417–37425. [Google Scholar] [CrossRef]
- Wenning, Z.; Yiyao, W.; Xiaoguang, L.; Yebin, X.; Shuangbao, W.; Zhimou, X. The fabrication of polymer-nanocone-based 3D Au nanoparticle array and its SERS performance. Appl. Phys. A 2017, 123, 45. [Google Scholar] [CrossRef]
- Chengpeng, Z.; Peiyun, Y.; Linfa, P.; Xinmin, L.; Jie, C.; Meizhen, H.; Jun, N. Continuous fabrication of nanostructure arrays for flexible surface enhanced Raman scattering Substrate. Sci. Rep. 2016, 7, 39814. [Google Scholar] [CrossRef]
- Michota, A.; Bukowska, J. Surface-enhanced Raman scattering (SERS) of 4-mercaptobenzoic acid on silver and gold substrates. J. Raman Spectrosc. 2003, 34, 21–25. [Google Scholar] [CrossRef]
- Simona, E.H.; Catherine, J.M. Bimetallic silver–gold nanowires: Fabrication and use in surface-enhanced Raman scattering. J. Mater. Chem. 2006, 16, 3929–3935. [Google Scholar] [CrossRef]
- Jun, Y.; Xijiang, H.; Jiaojiao, H.; Leilei, K.; Bin, Z.; Yunchen, D.; Hongtao, Z.; Cunku, D.; Hsing-Lin, W.; Ping, X. Highly Sensitive Surface-Enhanced Raman Spectroscopy (SERS) Platforms Based on Silver Nanostructures Fabricated on Polyaniline Membrane Surfaces. ACS Appl. Mater. Interfaces 2012, 4, 2752–2756. [Google Scholar] [CrossRef]
- Fortuni, B.; Inose, T.; Uezono, S.; Toyouchi, S.; Umemoto, K.; Sekine, S.; Fujita, Y.; Ricci, M.; Lu, G.; Masuhara, A.; et al. In situ synthesis of Au-shelled Ag nanoparticles on PDMS for flexible, long-life, and broad spectrum sensitive SERS substrates. Chem. Commun. 2017, 53, 11298–11301. [Google Scholar] [CrossRef]
| SERS Substrate | SERS Condition | LOD | EF | RSD |
|---|---|---|---|---|
| Au NCA (This work) | 785 nm(1 mW, 60 s) | 1 μM | 1.15 × 105 | 5.46% |
| Nanocone polycarbonate [51] | 785 nm (35 mW, 5 s) | 1 μM | 1 × 105 | - * |
| Au-capped polymer nanocones [52] | 780 nm (5 mW, 0.05 s) | 0.1 μM | 5 × 106 | - * |
| Polymer-nanocone-based 3D Au nanoparticle array [53] | 532 nm (5 mW, 10 s) | 1 pM | 1.3 × 108 | <11% |
| Au covered polymer nanostructure array [54] | 785 nm (1 mW, 10 s) | - * | 1.21 × 107 | 7.93% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiwara, S.; Kawasaki, D.; Sueyoshi, K.; Hisamoto, H.; Endo, T. Gold Nanocone Array with Extensive Electromagnetic Fields for Highly Reproducible Surface-Enhanced Raman Scattering Measurements. Micromachines 2022, 13, 1182. https://doi.org/10.3390/mi13081182
Fujiwara S, Kawasaki D, Sueyoshi K, Hisamoto H, Endo T. Gold Nanocone Array with Extensive Electromagnetic Fields for Highly Reproducible Surface-Enhanced Raman Scattering Measurements. Micromachines. 2022; 13(8):1182. https://doi.org/10.3390/mi13081182
Chicago/Turabian StyleFujiwara, Satoko, Daiki Kawasaki, Kenji Sueyoshi, Hideaki Hisamoto, and Tatsuro Endo. 2022. "Gold Nanocone Array with Extensive Electromagnetic Fields for Highly Reproducible Surface-Enhanced Raman Scattering Measurements" Micromachines 13, no. 8: 1182. https://doi.org/10.3390/mi13081182
APA StyleFujiwara, S., Kawasaki, D., Sueyoshi, K., Hisamoto, H., & Endo, T. (2022). Gold Nanocone Array with Extensive Electromagnetic Fields for Highly Reproducible Surface-Enhanced Raman Scattering Measurements. Micromachines, 13(8), 1182. https://doi.org/10.3390/mi13081182






