Microgripper Using Soft Microactuators for Manipulation of Living Cells
Abstract
:1. Introduction
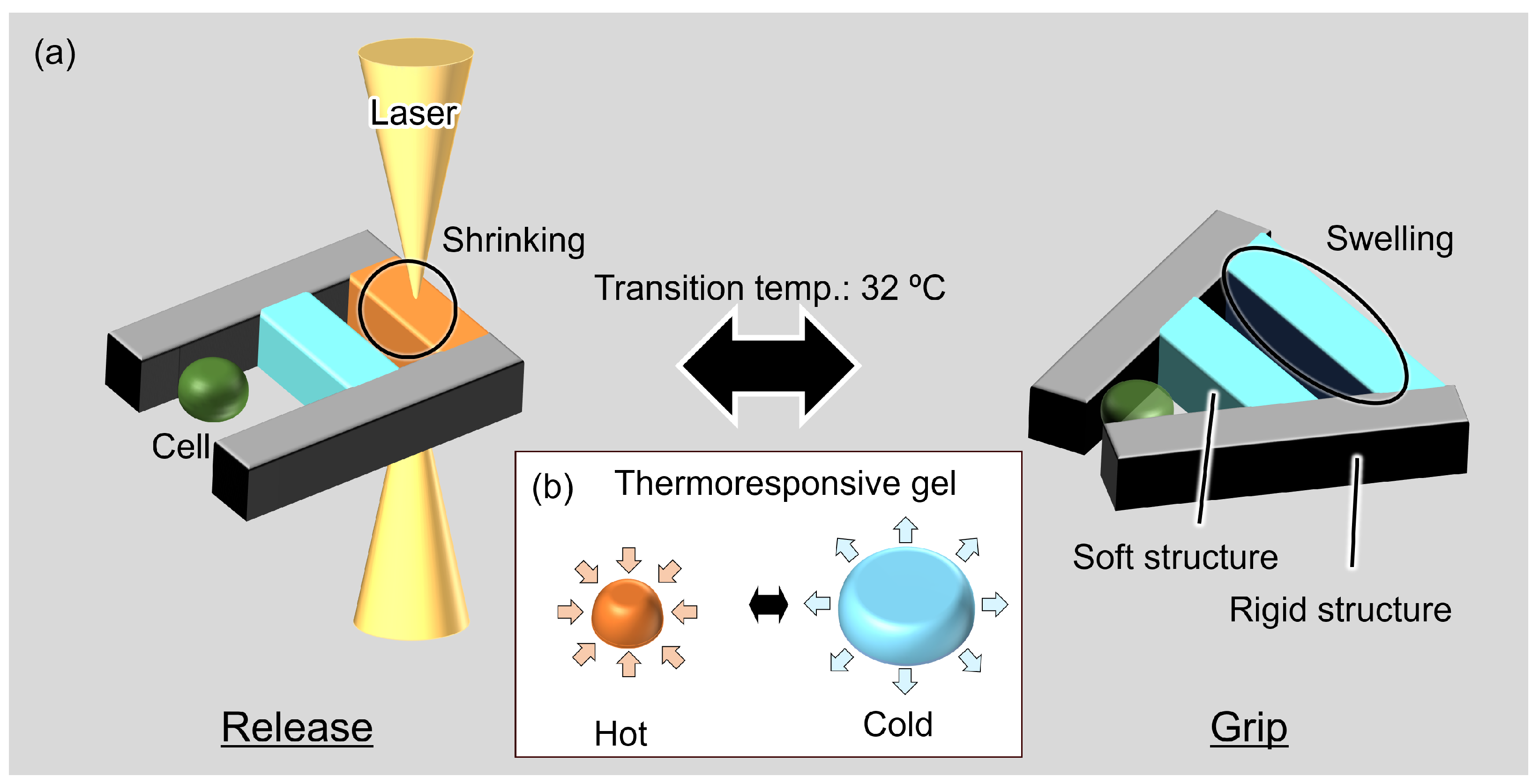
2. Concept
3. Design of the Microgripper
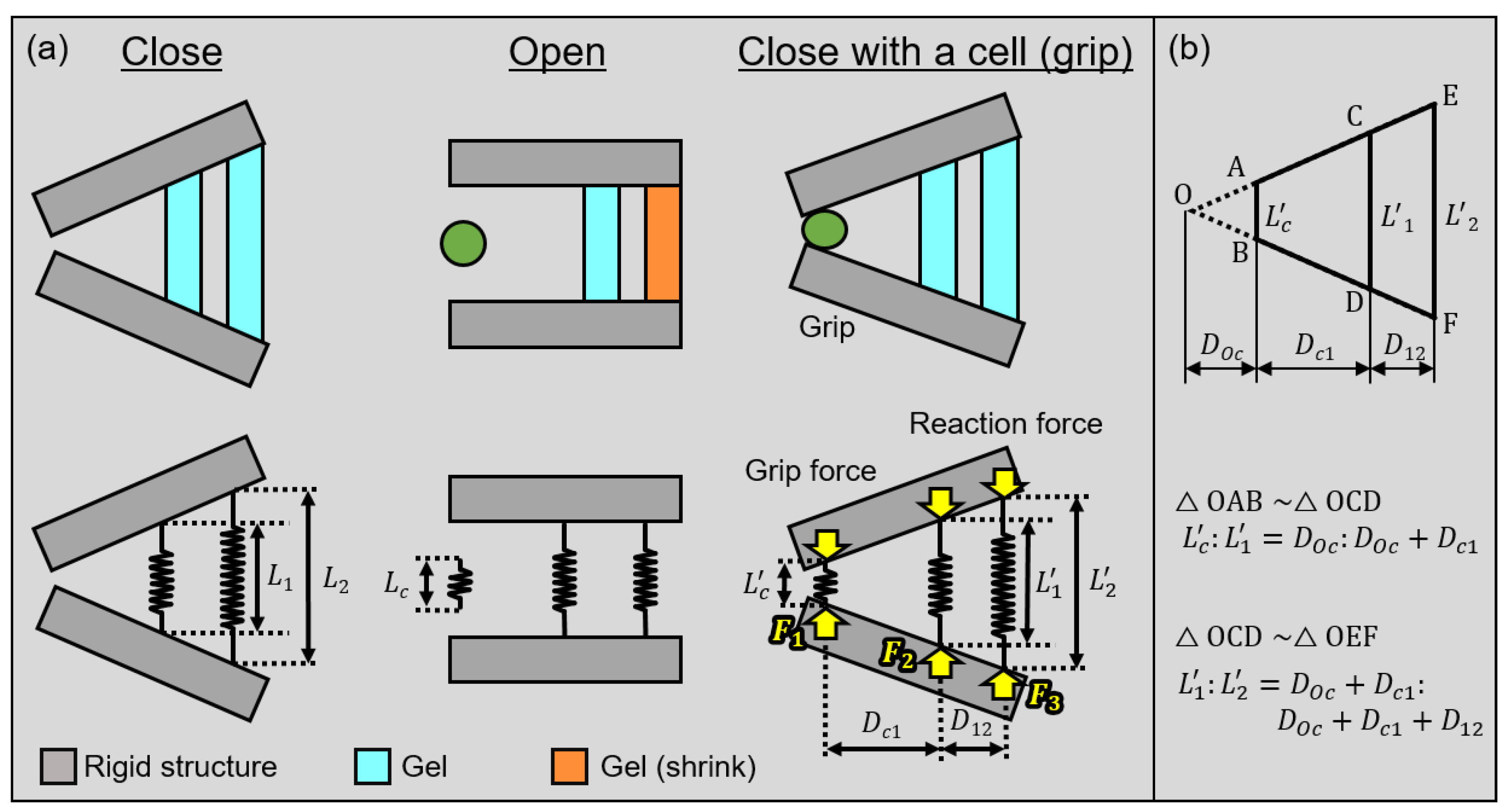
3.1. Theoretical Analysis of the Microgripper
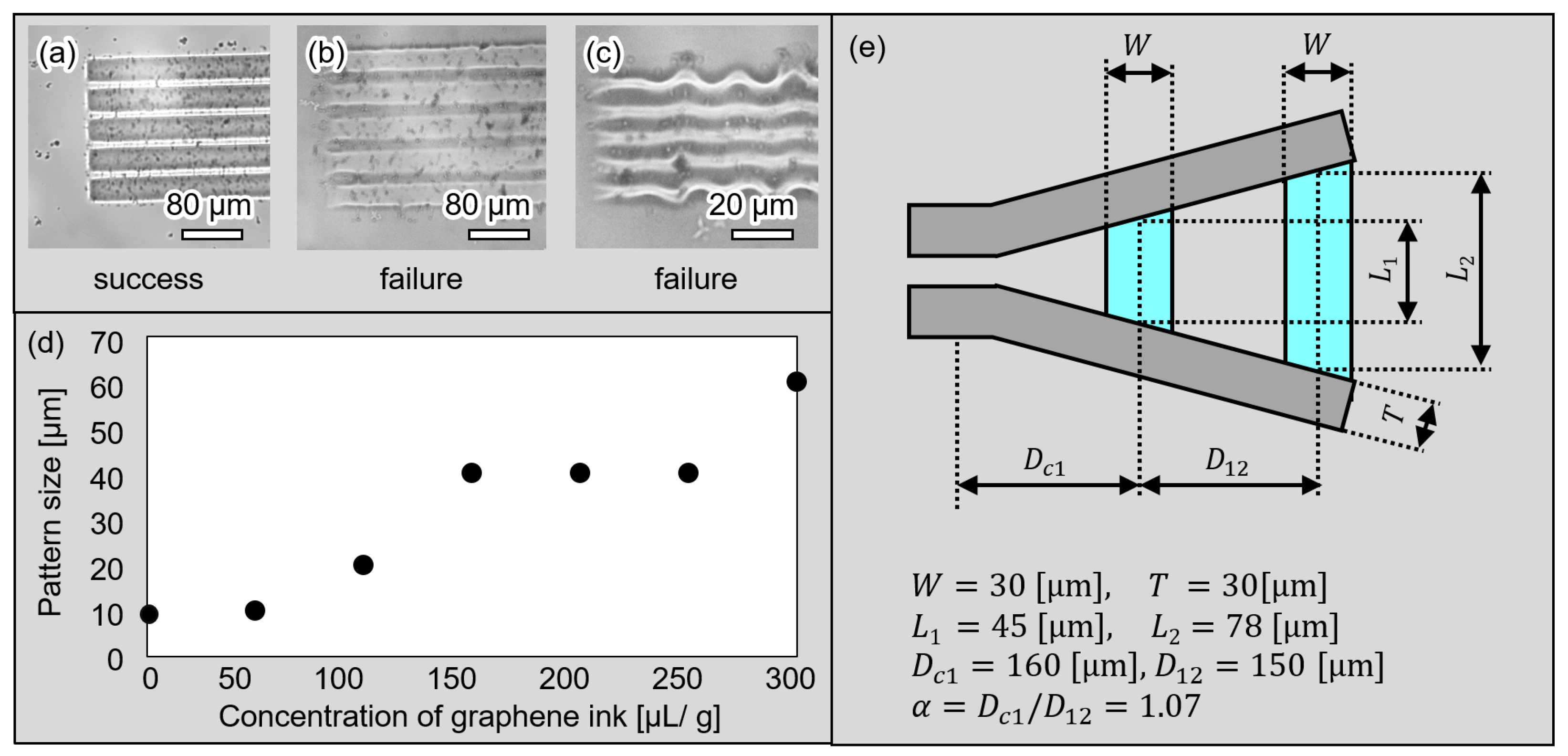
3.2. Design of the Microgripper
4. Experiments
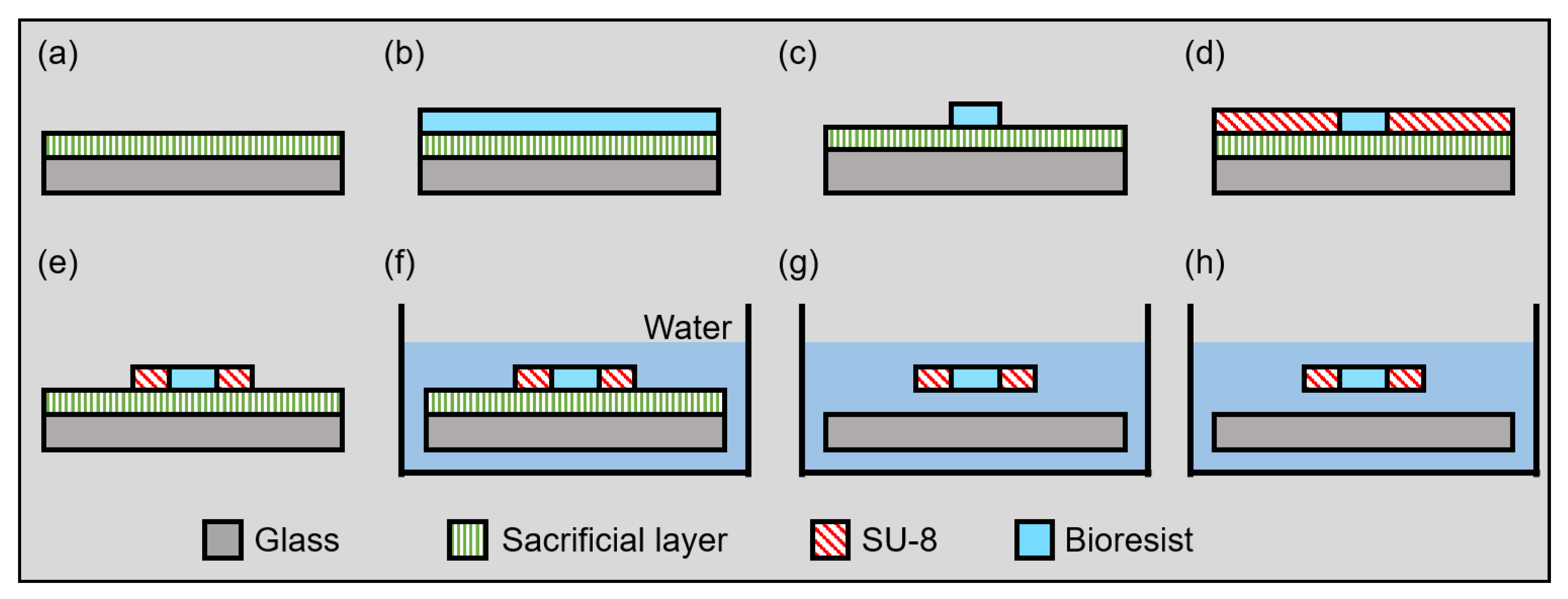
4.1. Fabrication Process of the Microgripper
- (a)
- Spin-coating dextran to a glass substrate;
- (b)
- Spin-coating BioResist to the glass substrate;
- (c)
- Patterning BioResist;
- (d)
- Spin-coating SU-8 to the glass substrate;
- (e)
- Patterning BioResist;
- (f)
- Immersing the substrate in water;
- (g)
- Releasing the fabricated pattern in the water;
- (h)
- Removing the substrate from the water.
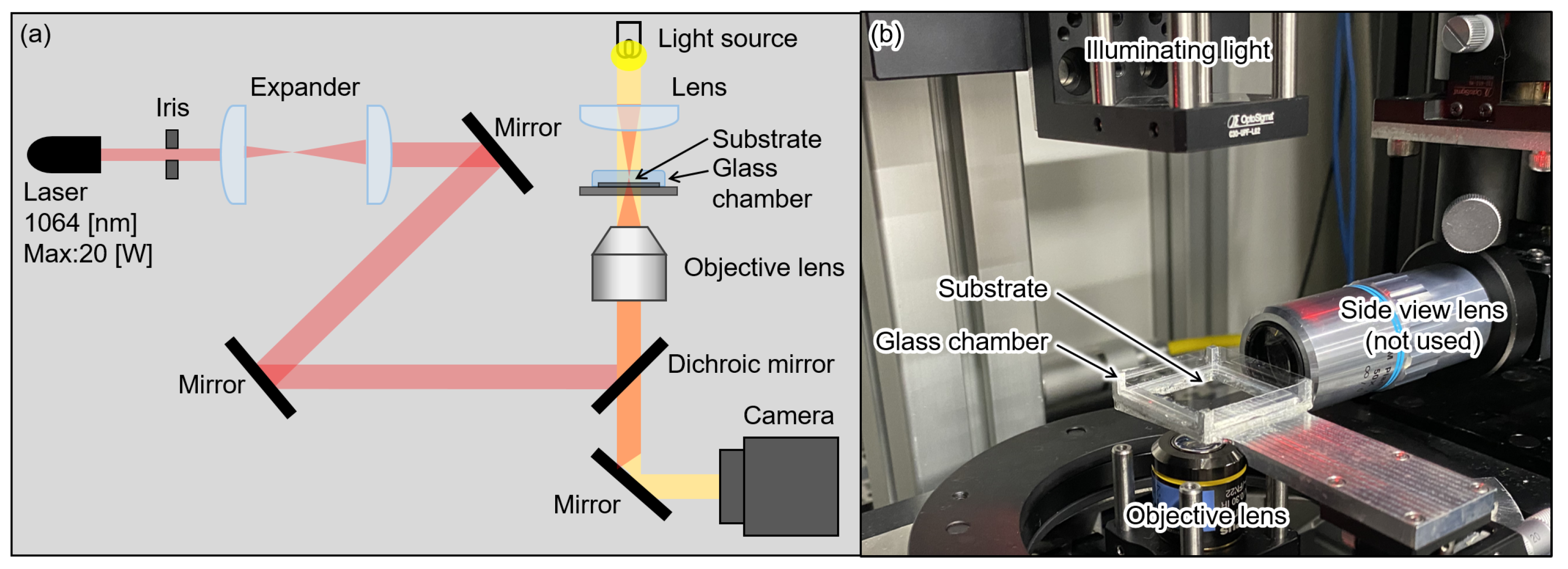
4.2. Experimental System
5. Results
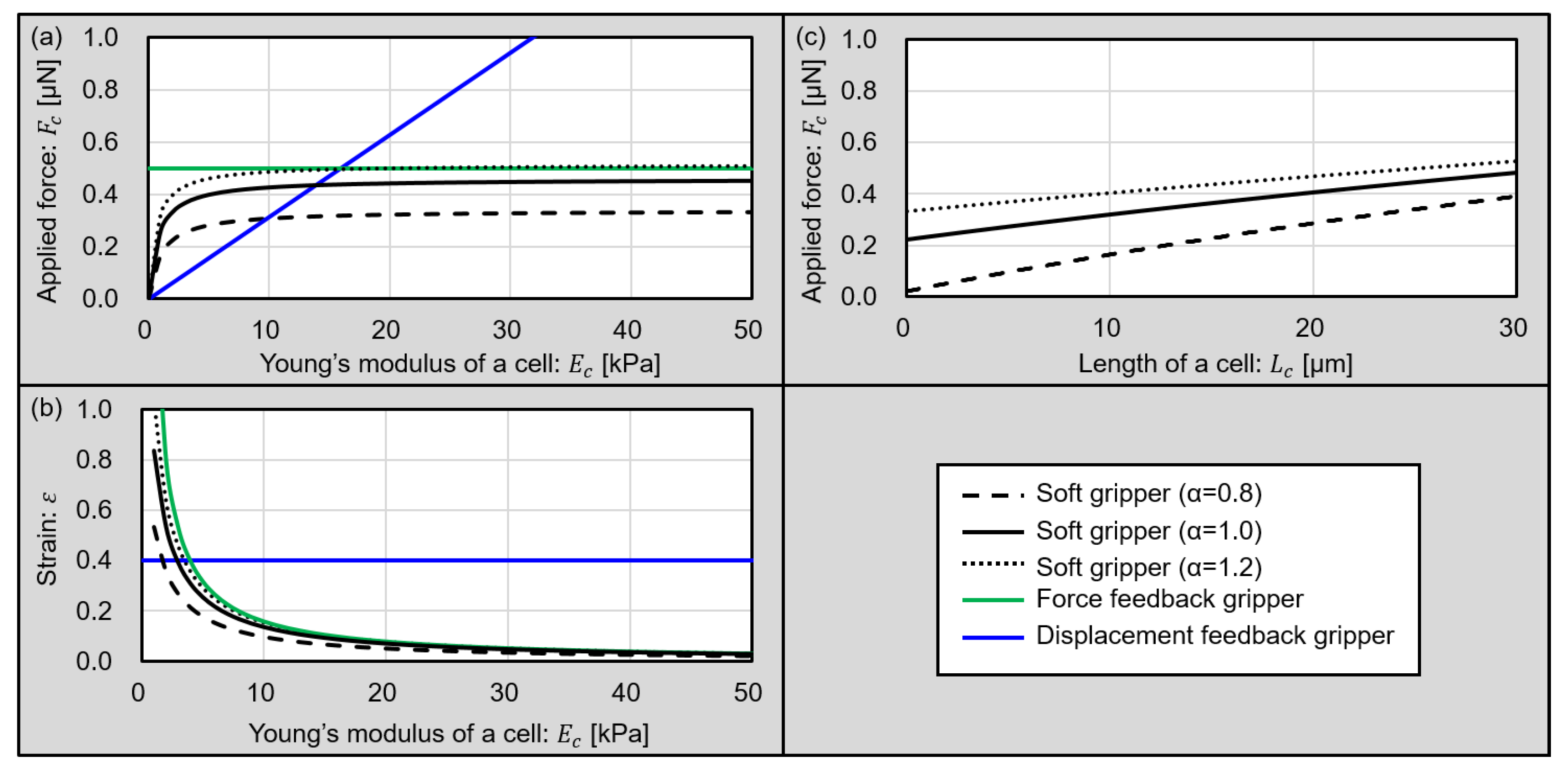
5.1. Driving Characteristics of the Microgripper
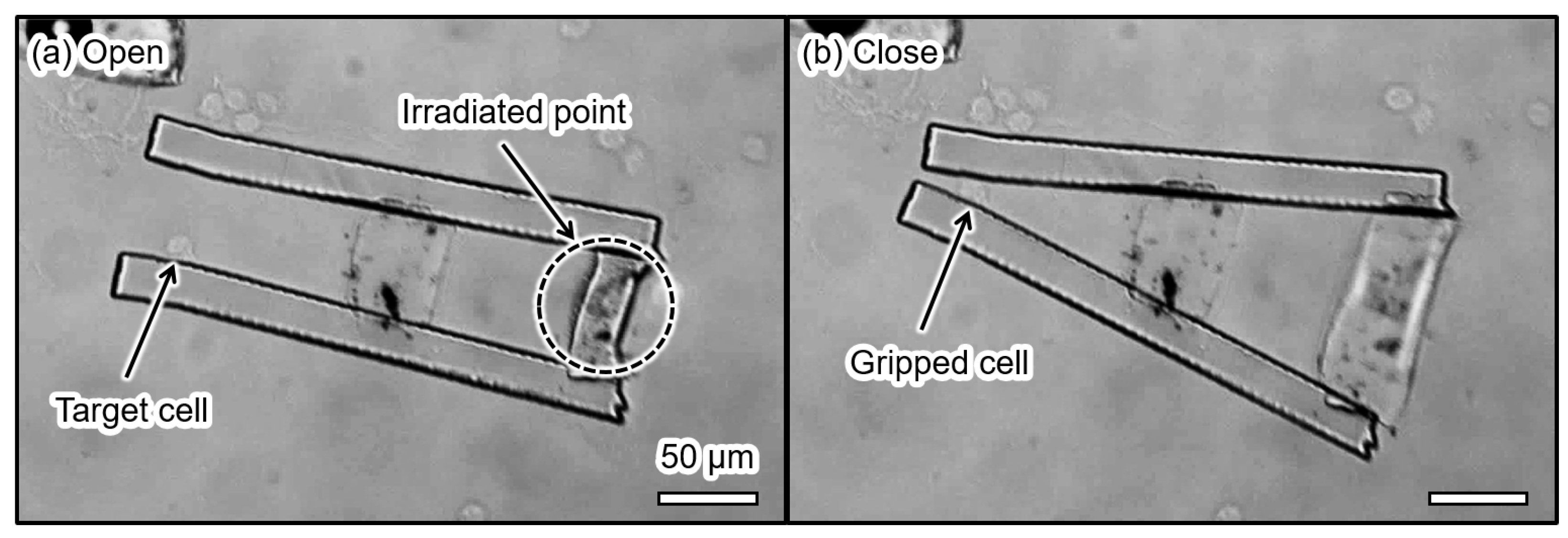
5.2. Demonstration of the Gripping a Living Single Cell
6. Discussions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ashkin, A.; Dziedzic, J.M. Optical trapping and manipulation of viruses and bacteria. Science 1987, 235, 1517–1520. [Google Scholar] [CrossRef]
- Zhong, M.C.; Wei, X.B.; Zhou, J.H.; Wang, Z.Q.; Li, Y.M. Trapping red blood cells in living animals using optical tweezers. Nat. Commun. 2013, 4, 1768. [Google Scholar] [CrossRef] [Green Version]
- Novotny, L.; Bian, R.X.; Xie, X.S. Theory of nanometric optical tweezers. Phys. Rev. Lett. 1997, 79, 645. [Google Scholar] [CrossRef] [Green Version]
- Morgan, H.; Hughes, M.P.; Green, N.G. Separation of submicron bioparticles by dielectrophoresis. Biophys. J. 1999, 77, 516–525. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Purdon, A.; Westervelt, R. Manipulation of biological cells using a microelectromagnet matrix. Appl. Phys. Lett. 2004, 85, 1063–1065. [Google Scholar] [CrossRef]
- Laurell, T.; Petersson, F.; Nilsson, A. Chip integrated strategies for acoustic separation and manipulation of cells and particles. Chem. Soc. Rev. 2007, 36, 492–506. [Google Scholar] [CrossRef]
- Hayakawa, T.; Sakuma, S.; Arai, F. On-chip 3D rotation of oocyte based on a vibration-induced local whirling flow. Microsyst. Nanoeng. 2015, 1, 15001. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Tanikawa, T.; Arai, T. Micro-manipulation system with a two-fingered micro-hand and its potential application in bioscience. J. Biotechnol. 2008, 133, 219–224. [Google Scholar] [CrossRef]
- Hagiwara, M.; Kawahara, T.; Arai, F. Local streamline generation by mechanical oscillation in a microfluidic chip for noncontact cell manipulations. Appl. Phys. Lett. 2012, 101, 074102. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Mao, Z.; Chen, Y.; Xie, Z.; Lata, J.P.; Li, P.; Ren, L.; Liu, J.; Yang, J.; Dao, M.; et al. Three-dimensional manipulation of single cells using surface acoustic waves. Proc. Natl. Acad. Sci. USA 2016, 113, 1522–1527. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xu, Q. Design and analysis of a totally decoupled flexure-based XY parallel micromanipulator. IEEE Trans. Robot. 2009, 25, 645–657. [Google Scholar]
- Mita, M.; Kawara, H.; Toshiyoshi, H.; Ataka, M.; Fujita, H. An electrostatic 2-dimensional micro-gripper for nano structure. In Proceedings of the TRANSDUCERS’03 12th International Conference on Solid-State Sensors, Actuators and Microsystems, Digest of Technical Papers (Cat. No.03TH8664), Boston, MA, USA, 8–12 June 2003; Volume 1, pp. 272–275. [Google Scholar]
- Lofroth, M.; Avci, E. Development of a novel modular compliant gripper for manipulation of micro objects. Micromachines 2019, 10, 313. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Meng, X.; Zhang, H.; Sun, L. Development of a Magnetically Driven Microgripper for PicoNewton Force-Controlled Microscale Manipulation and Characterization. IEEE Trans. Ind. Electron. 2019, 67, 2065–2075. [Google Scholar] [CrossRef]
- Boudaoud, M.; Haddab, Y.; Le Gorrec, Y. Modeling and optimal force control of a nonlinear electrostatic microgripper. IEEE/ASME Trans. Mechatron. 2012, 18, 1130–1139. [Google Scholar] [CrossRef] [Green Version]
- Avci, E.; Ohara, K.; Nguyen, C.N.; Theeravithayangkura, C.; Kojima, M.; Tanikawa, T.; Mae, Y.; Arai, T. High-speed automated manipulation of microobjects using a two-fingered microhand. IEEE Trans. Ind. Electron. 2014, 62, 1070–1079. [Google Scholar] [CrossRef]
- Nah, S.; Zhong, Z. A microgripper using piezoelectric actuation for micro-object manipulation. Sens. Actuators A Phys. 2007, 133, 218–224. [Google Scholar] [CrossRef]
- Kohl, M.; Just, E.; Pfleging, W.; Miyazaki, S. SMA microgripper with integrated antagonism. Sens. Actuators A Phys. 2000, 83, 208–213. [Google Scholar] [CrossRef]
- Kim, K.; Liu, X.; Zhang, Y.; Sun, Y. Nanonewton force-controlled manipulation of biological cells using a monolithic MEMS microgripper with two-axis force feedback. J. Micromech. Microeng. 2008, 18, 055013. [Google Scholar] [CrossRef]
- Diller, E.; Sitti, M. Three-dimensional programmable assembly by untethered magnetic robotic micro-grippers. Adv. Funct. Mater. 2014, 24, 4397–4404. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, J.; Salehizadeh, M.; Onaizah, O.; Diller, E. Millimeter-scale flexible robots with programmable three-dimensional magnetization and motions. Sci. Robot. 2019, 4, eaav4494. [Google Scholar] [CrossRef]
- Ichikawa, A.; Sakuma, S.; Sugita, M.; Shoda, T.; Tamakoshi, T.; Akagi, S.; Arai, F. On-chip enucleation of an oocyte by untethered microrobots. J. Micromech. Microeng. 2014, 24, 095004. [Google Scholar] [CrossRef] [Green Version]
- Shintake, J.; Cacucciolo, V.; Floreano, D.; Shea, H. Soft robotic grippers. Adv. Mater. 2018, 30, 1707035. [Google Scholar] [CrossRef] [Green Version]
- Scheggi, S.; Chandrasekar, K.K.T.; Yoon, C.; Sawaryn, B.; van de Steeg, G.; Gracias, D.H.; Misra, S. Magnetic motion control and planning of untethered soft grippers using ultrasound image feedback. In Proceedings of the 2017 IEEE International Conference on Robotics and Automation (ICRA), Singapore, 29 May–3 June 2017; pp. 6156–6161. [Google Scholar]
- Gao, W.; Wang, L.; Wang, X.; Liu, H. Magnetic driving flowerlike soft platform: Biomimetic fabrication and external regulation. ACS Appl. Mater. Interfaces 2016, 8, 14182–14189. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Mailand, E.; Zhou, J.; Huang, Z.; Dietler, G.; Kolinski, J.M.; Wang, X.; Sakar, M.S. Universal soft robotic microgripper. Small 2019, 15, 1803870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konishi, S. Small, Soft, Safe Micromachine as Multi-scale Interface for BME. In Proceedings of the 2008 International Symposium on Micro-NanoMechatronics and Human Science, Nagoya, Japan, 6–9 November 2008; pp. 184–187. [Google Scholar]
- Yokoyama, Y.; Umezaki, M.; Kishimura, T.; Tamiya, E.; Takamura, Y. Micro-and nano-fabrication of stimulus-responsive polymer using nanoimprint lithography. J. Photopolym. Sci. Technol. 2011, 24, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Yokoyama, Y.; Hayakawa, T. Structurally isolated photoactuation of graphene-mixed temperature-responsive hydrogels in soft-rigid series structure. Robomech J. 2019, 6, 11. [Google Scholar] [CrossRef]
- Koike, Y.; Yokoyama, Y.; Hayakawa, T. Light-driven hydrogel microactuators for on-chip cell manipulations. Front. Mech. Eng. 2020, 6, 2. [Google Scholar] [CrossRef]
- Matzelle, T.; Ivanov, D.; Landwehr, D.; Heinrich, L.A.; Herkt-Bruns, C.; Reichelt, R.; Kruse, N. Micromechanical properties of “smart” gels: Studies by scanning force and scanning electron microscopy of PNIPAAm. J. Phys. Chem. B 2002, 106, 2861–2866. [Google Scholar] [CrossRef]
- Pietuch, A.; Brückner, B.R.; Schneider, D.; Tarantola, M.; Rosman, C.; Sönnichsen, C.; Janshoff, A. Mechanical properties of MDCK II cells exposed to gold nanorods. Beilstein J. Nanotechnol. 2015, 6, 223–231. [Google Scholar] [CrossRef] [Green Version]

| Parameter | Value |
|---|---|
| : Length of a cell | 20 µm |
| : Length of the center gel | 69 µm |
| : Length of the rear gel | 157 µm |
| : Cross-section area of a cell | 314 µm |
| : Cross-section area of the center gel | 1800 µm |
| : Cross-section area of the rear gel | 1800 µm |
| : Young’s modulus of a gel | 2.83 kPa [31] |
| : Ratio of and | 0.8, 1.0, 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodera, S.; Watanabe, T.; Yokoyama, Y.; Hayakawa, T. Microgripper Using Soft Microactuators for Manipulation of Living Cells. Micromachines 2022, 13, 794. https://doi.org/10.3390/mi13050794
Kodera S, Watanabe T, Yokoyama Y, Hayakawa T. Microgripper Using Soft Microactuators for Manipulation of Living Cells. Micromachines. 2022; 13(5):794. https://doi.org/10.3390/mi13050794
Chicago/Turabian StyleKodera, Shunnosuke, Tomoki Watanabe, Yoshiyuki Yokoyama, and Takeshi Hayakawa. 2022. "Microgripper Using Soft Microactuators for Manipulation of Living Cells" Micromachines 13, no. 5: 794. https://doi.org/10.3390/mi13050794
APA StyleKodera, S., Watanabe, T., Yokoyama, Y., & Hayakawa, T. (2022). Microgripper Using Soft Microactuators for Manipulation of Living Cells. Micromachines, 13(5), 794. https://doi.org/10.3390/mi13050794






