Numerical Modelling of Mixing in a Microfluidic Droplet Using a Two-Phase Moving Frame of Reference Approach
Abstract
1. Introduction
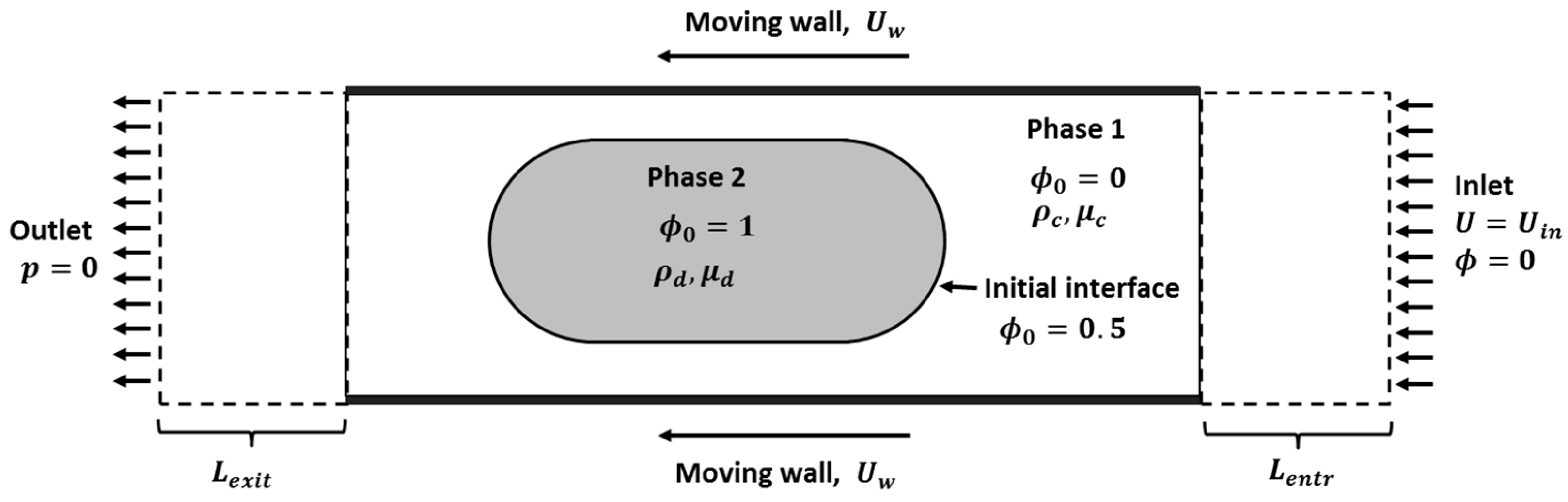
2. Model Description
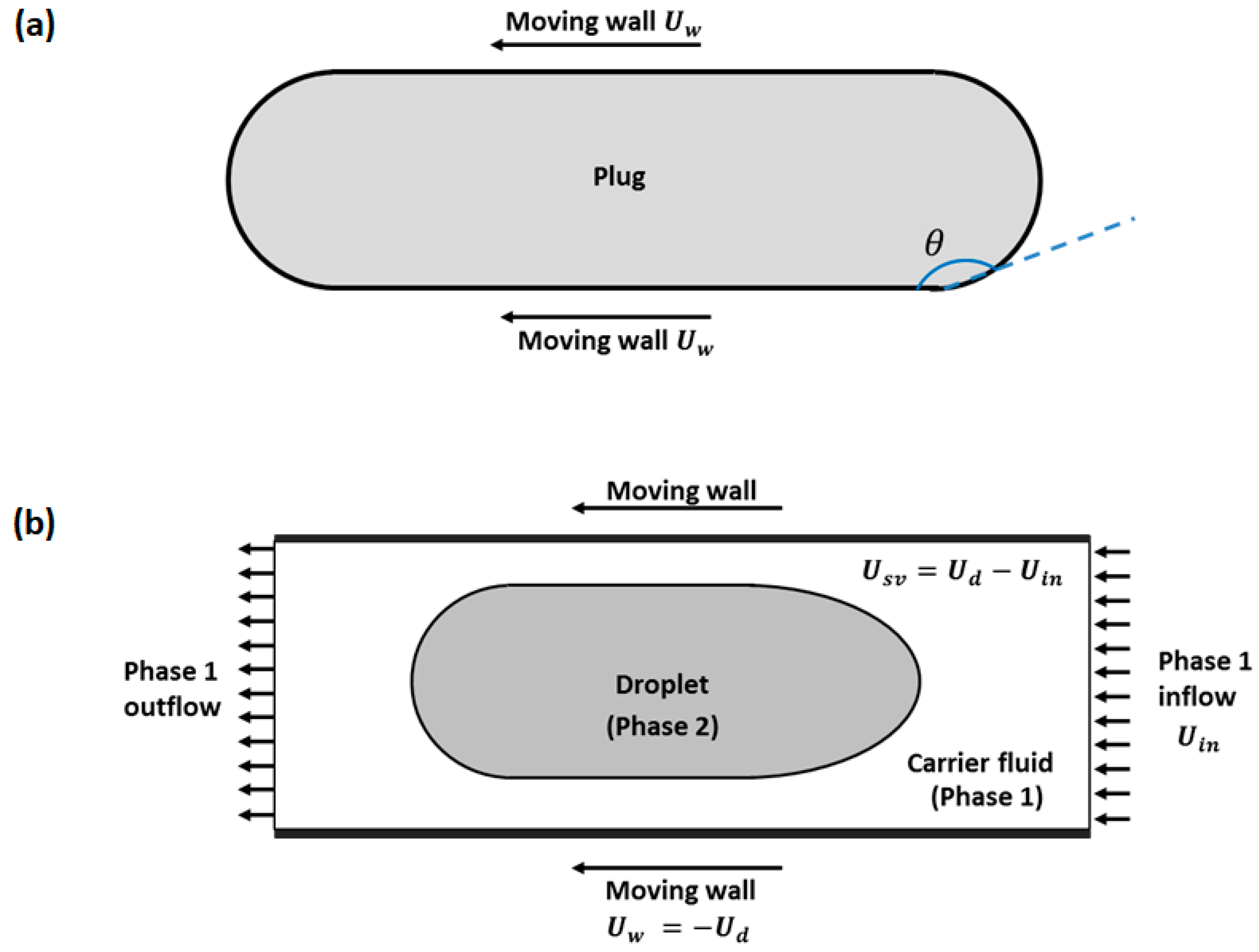
2.1. Two-Phase Moving Frame of Reference
2.2. Numerical Tools
2.3. Governing Equations
Two-Phase Flow
- The flow is Newtonian and isothermal.
- There are zero gradients for interfacial tension and hence, negligible Marangoni effects.
- The system is single droplet system (the flow field is a result of a single droplet).
- Viscosity and density are constant in each phase and are not functions of concentration of the species.
- Mass transport is confined within the droplet phase and is no interfacial mass transfer.
2.4. Mass Transport
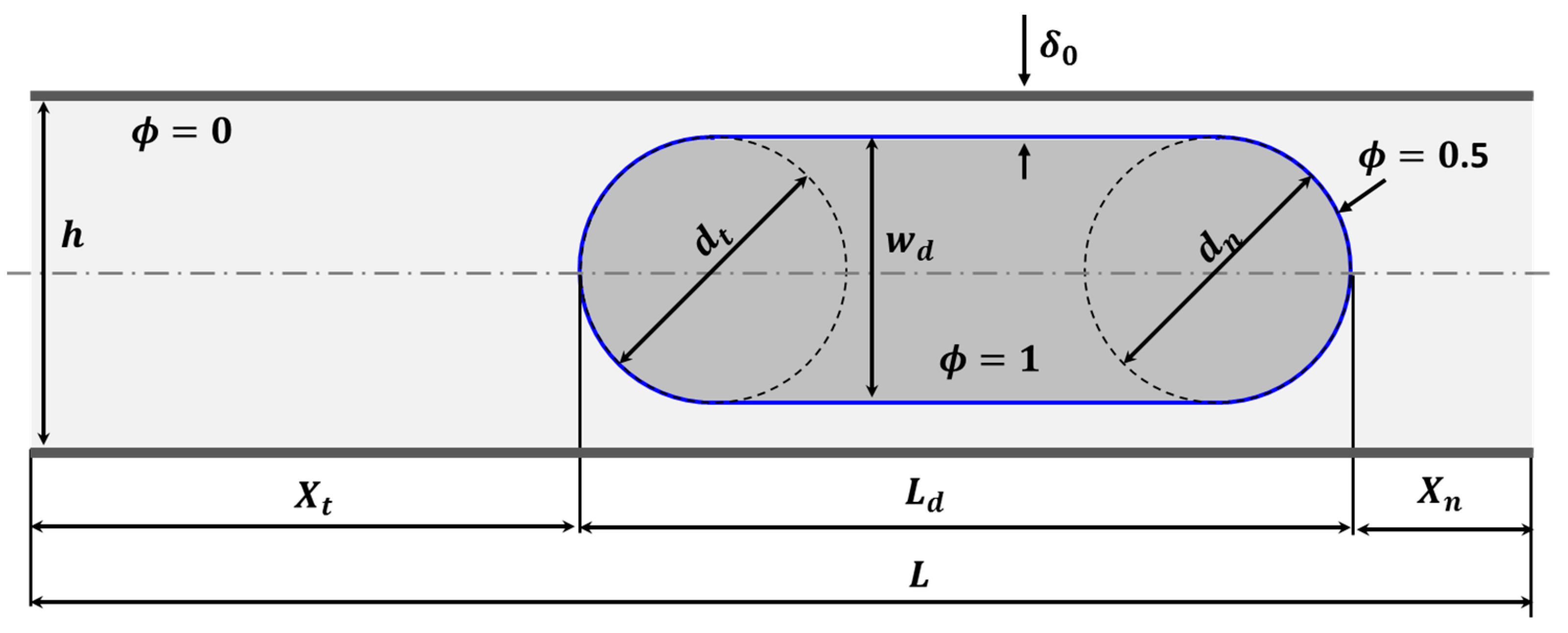
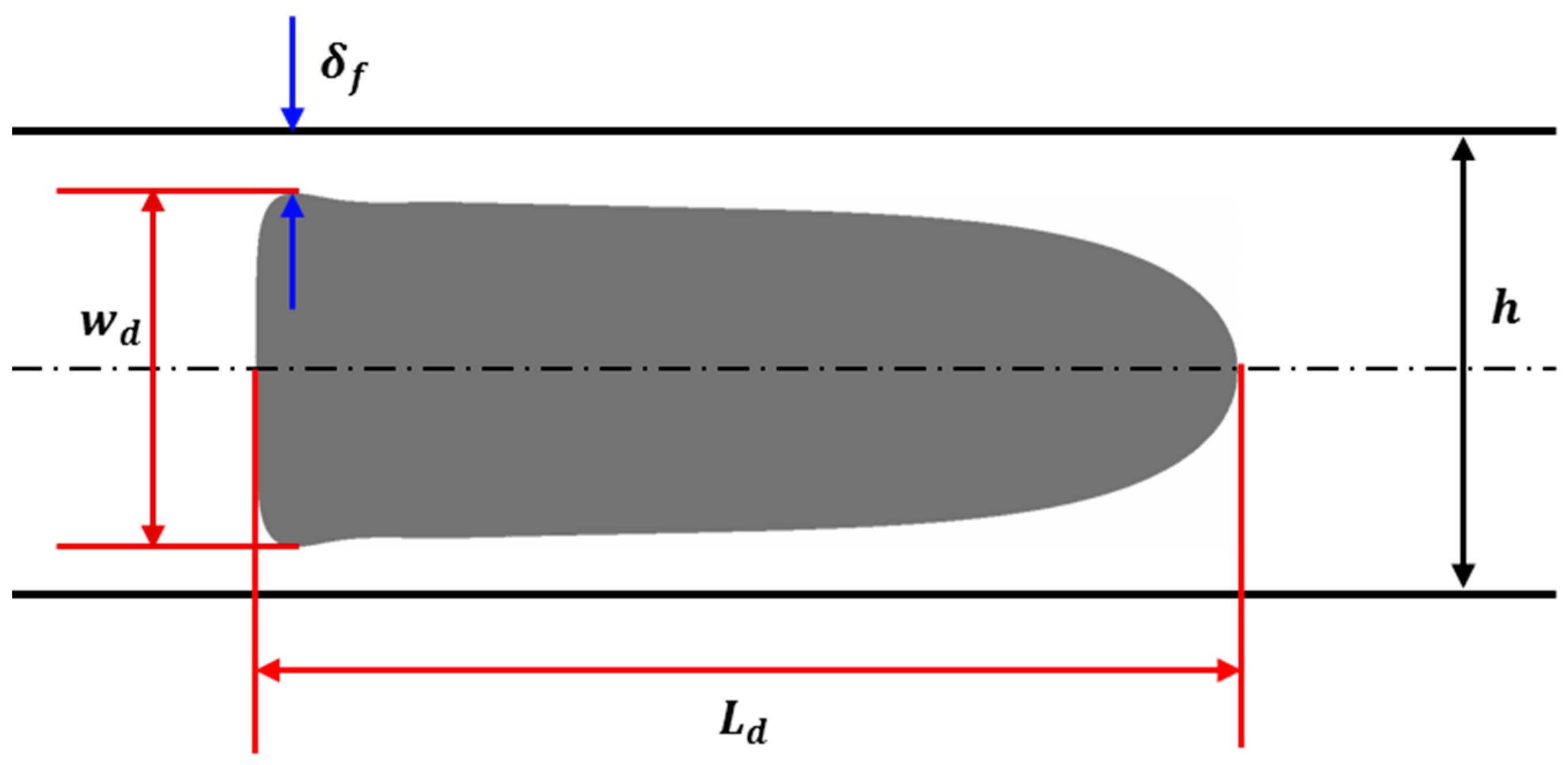
2.5. Model Geometry
2.6. Dimensionless Numbers
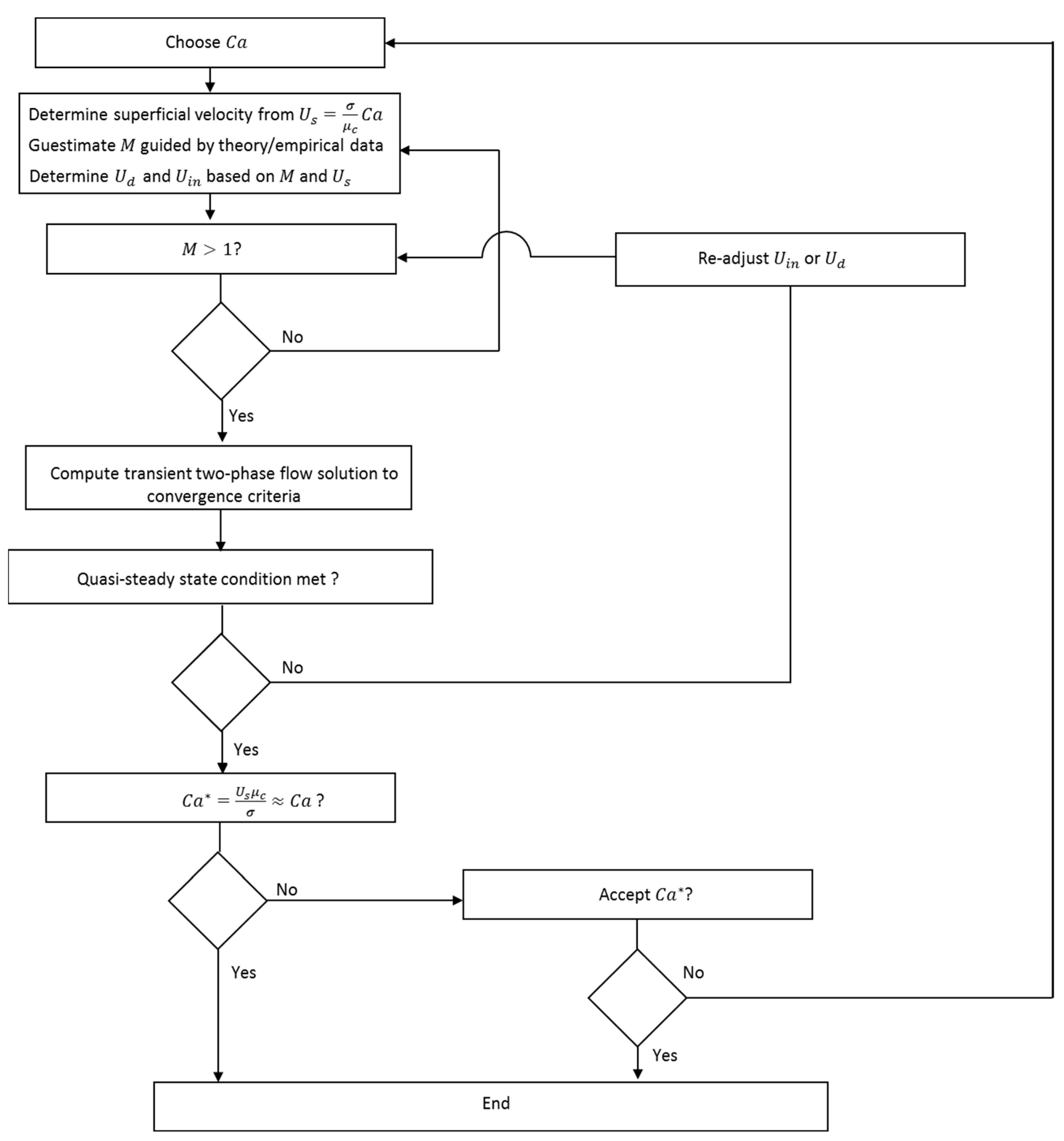
2.7. Numerical Implementation
2.7.1. Computation of the Flow Field
2.7.2. Computation of the Concentration Field
3. Results
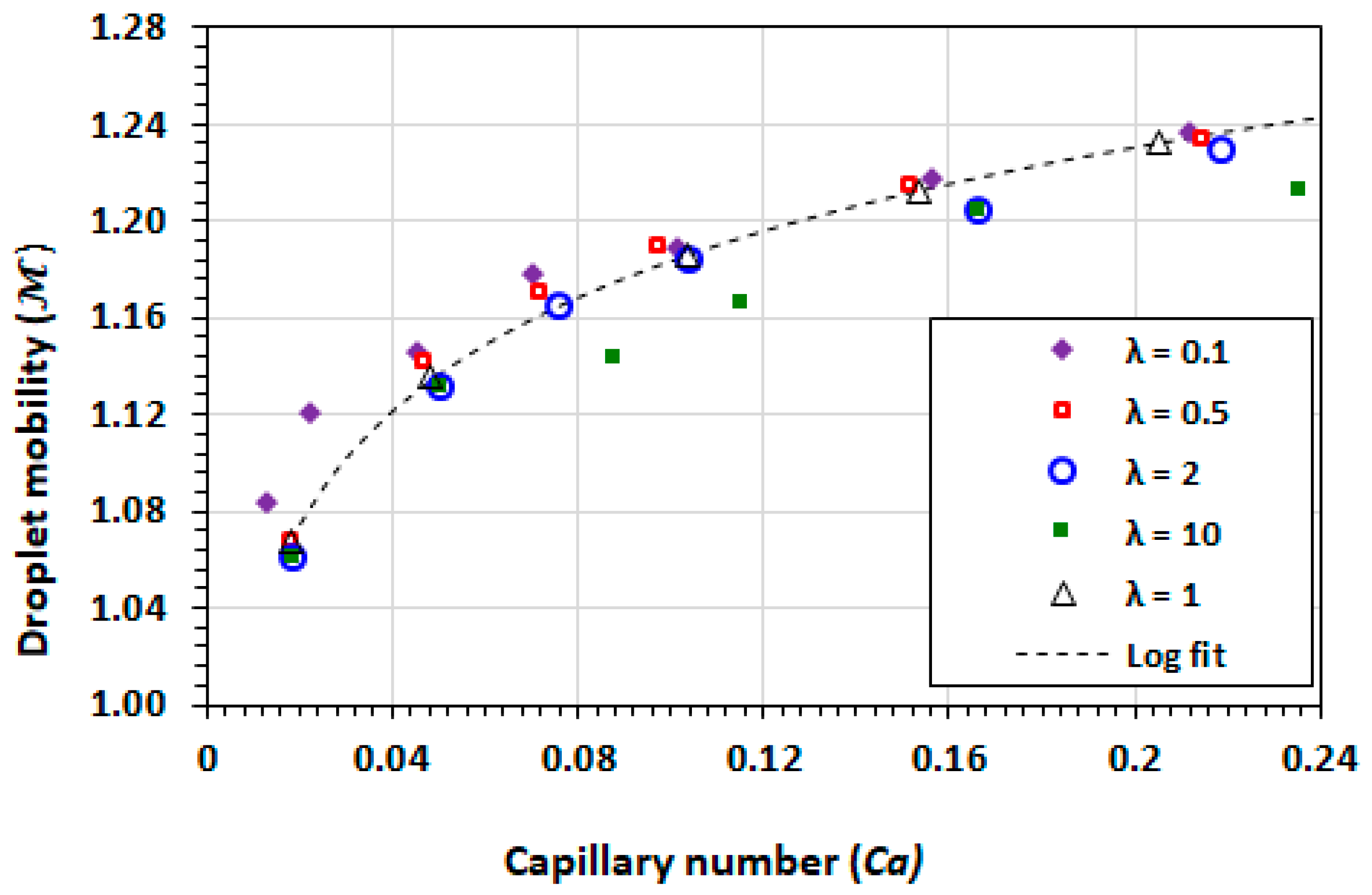
3.1. Droplet Mobility
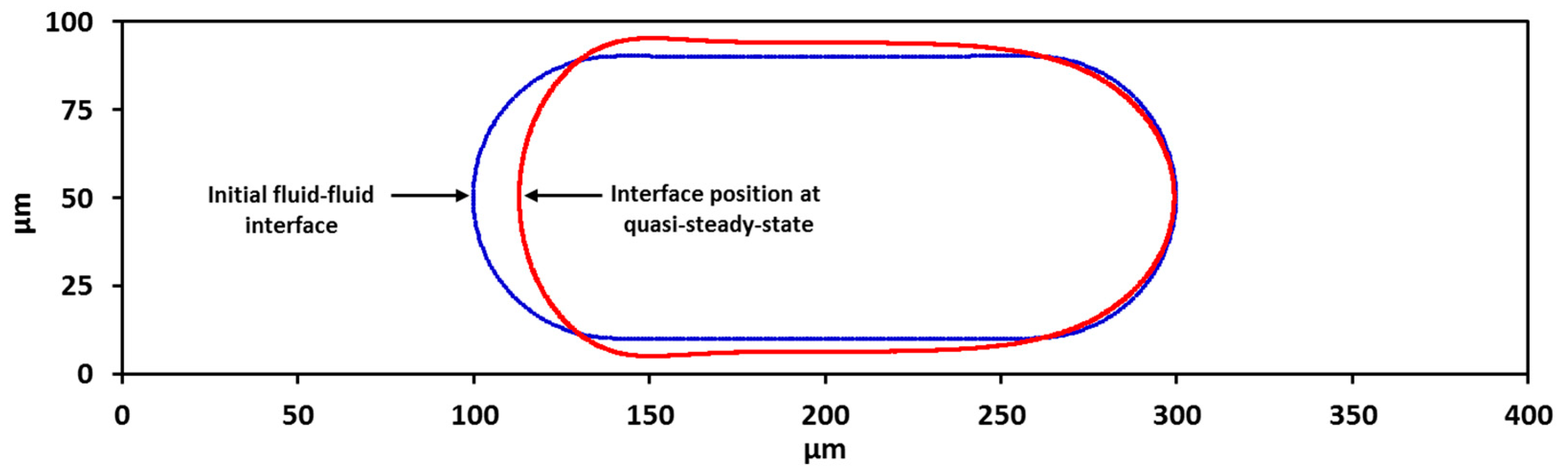
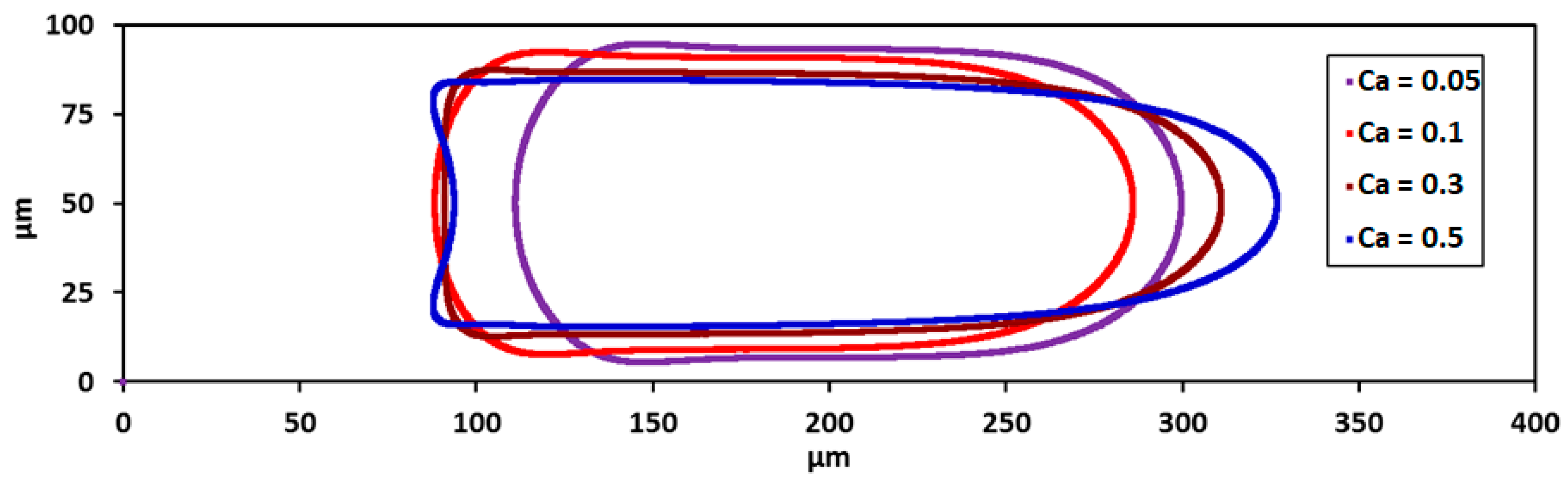
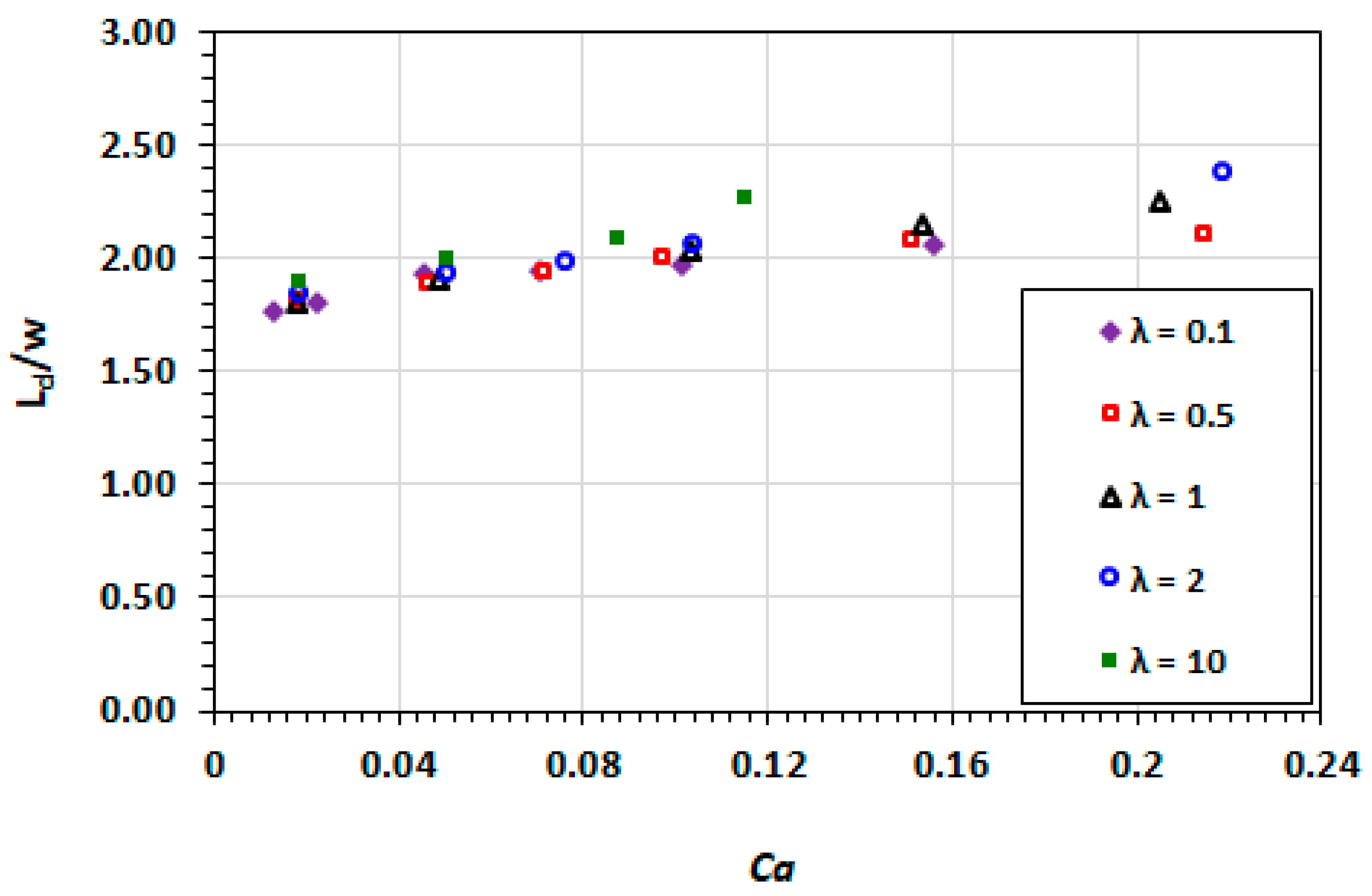
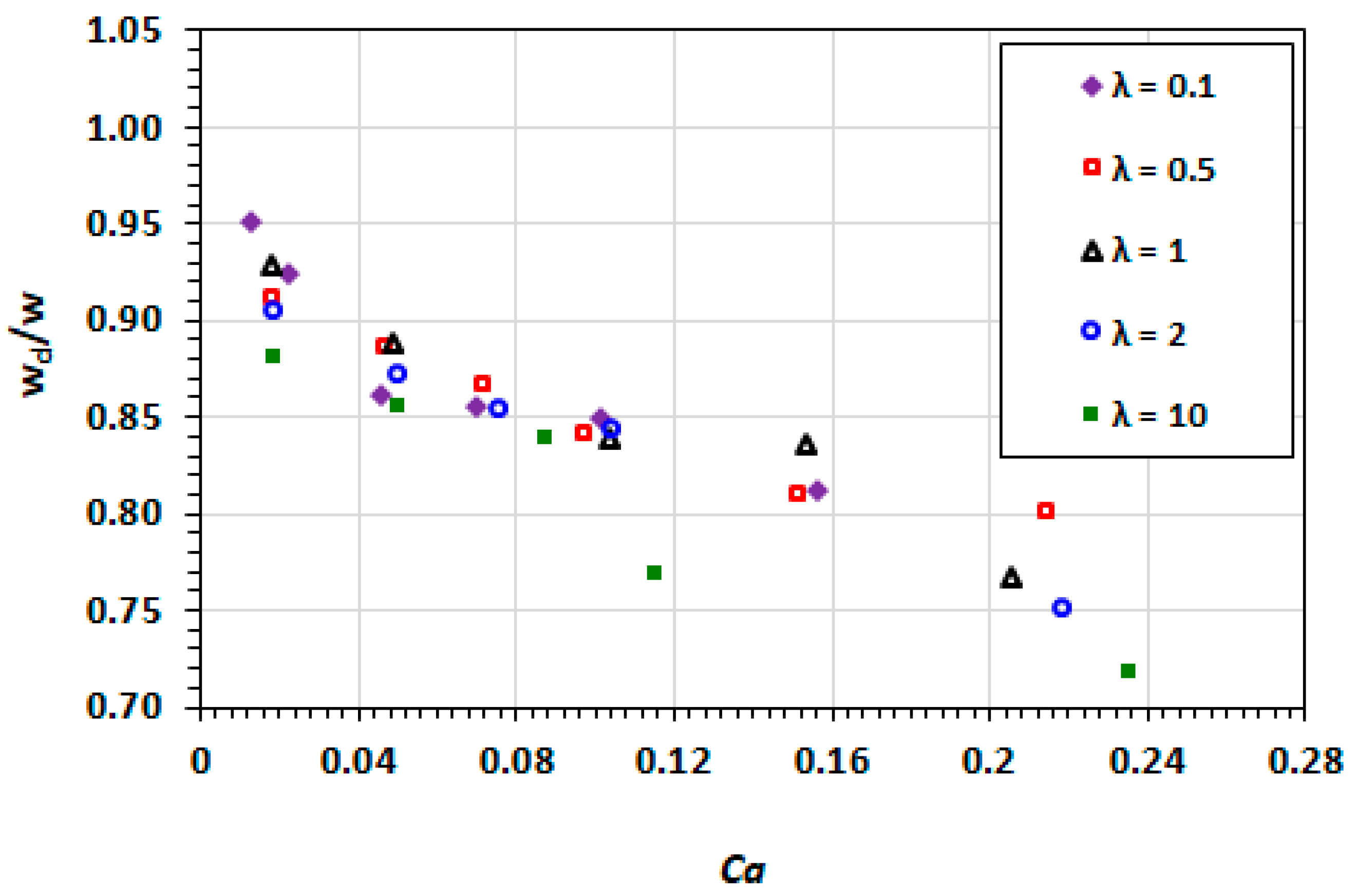
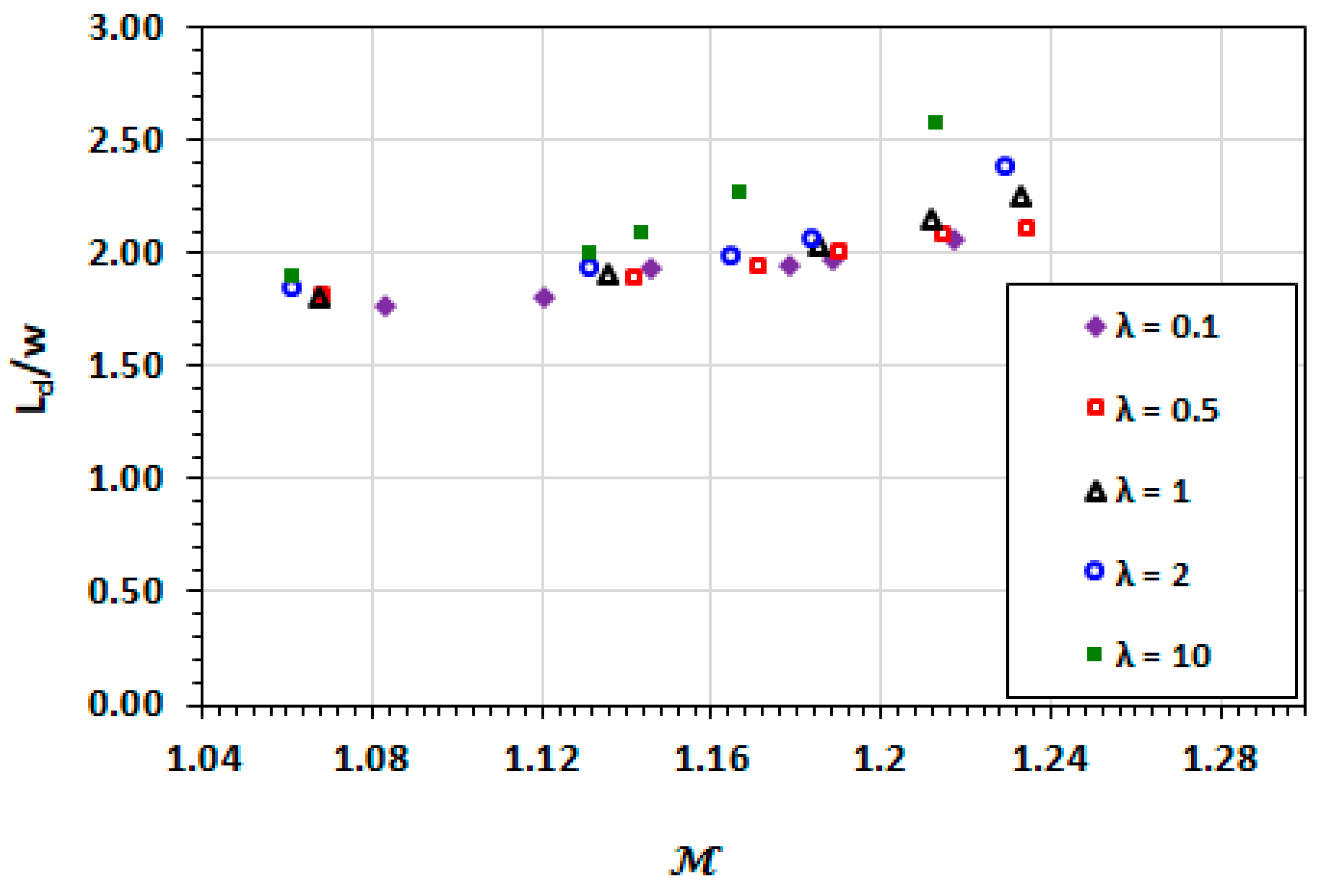
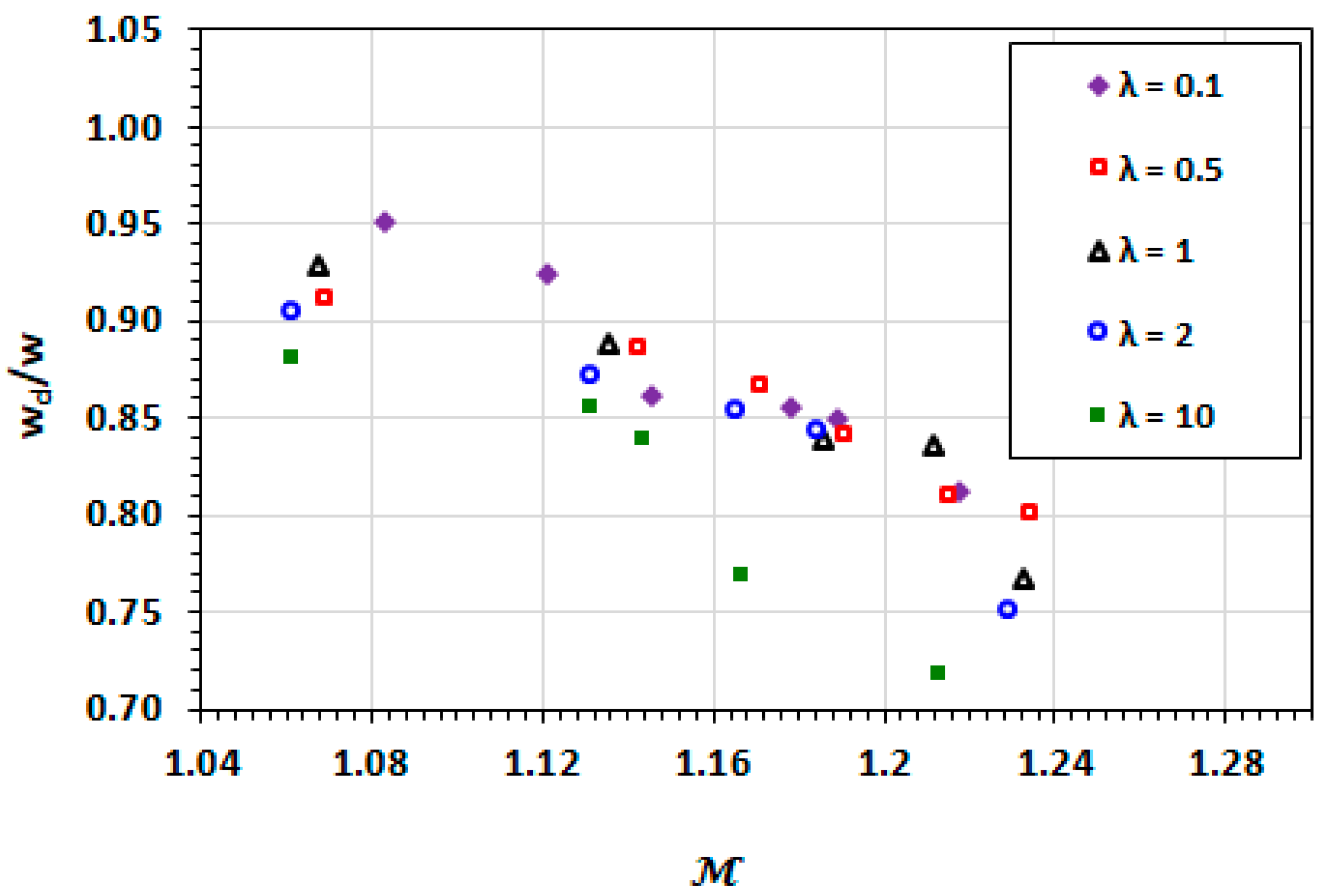
3.2. Droplet Shape and Size
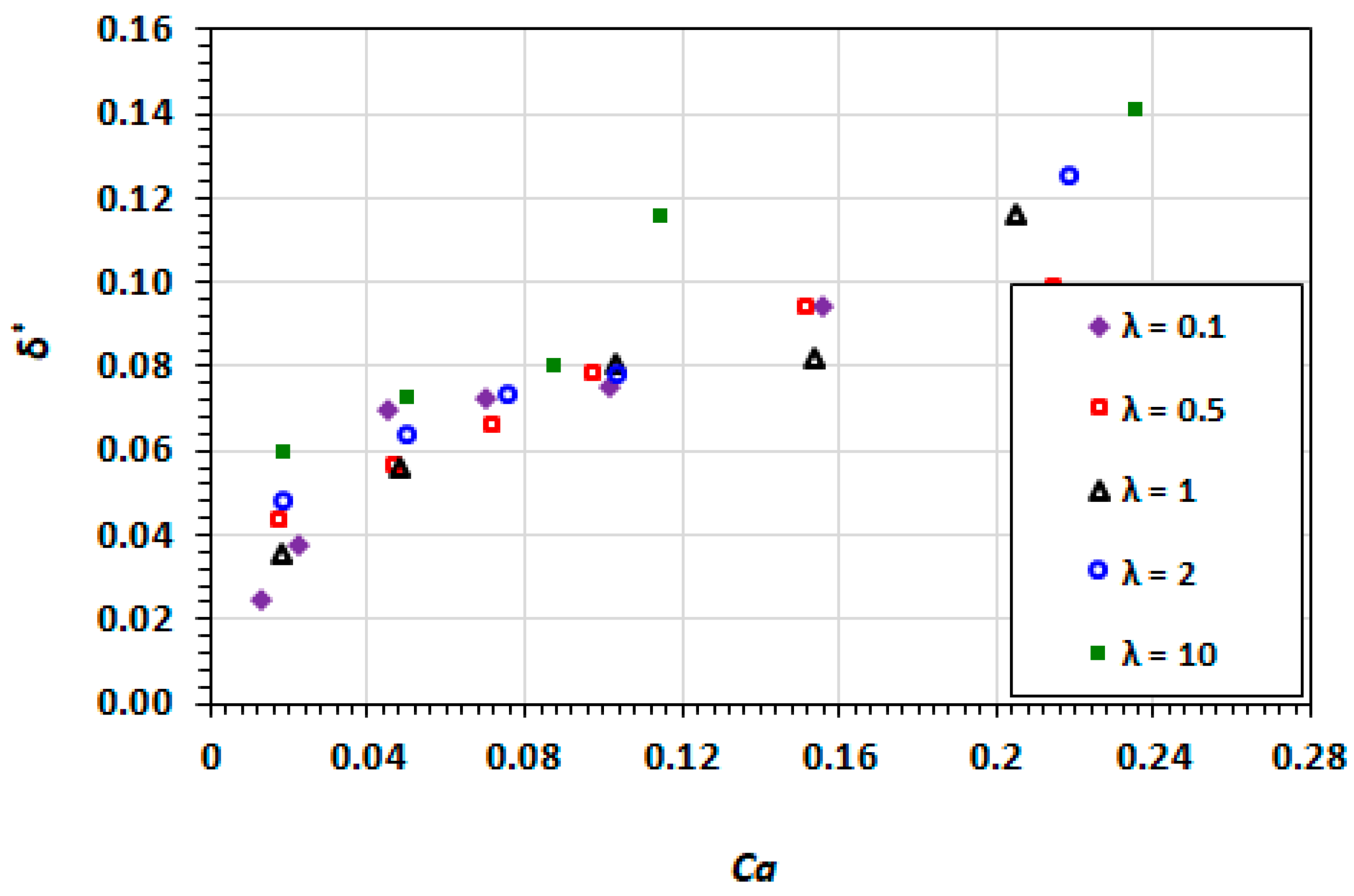
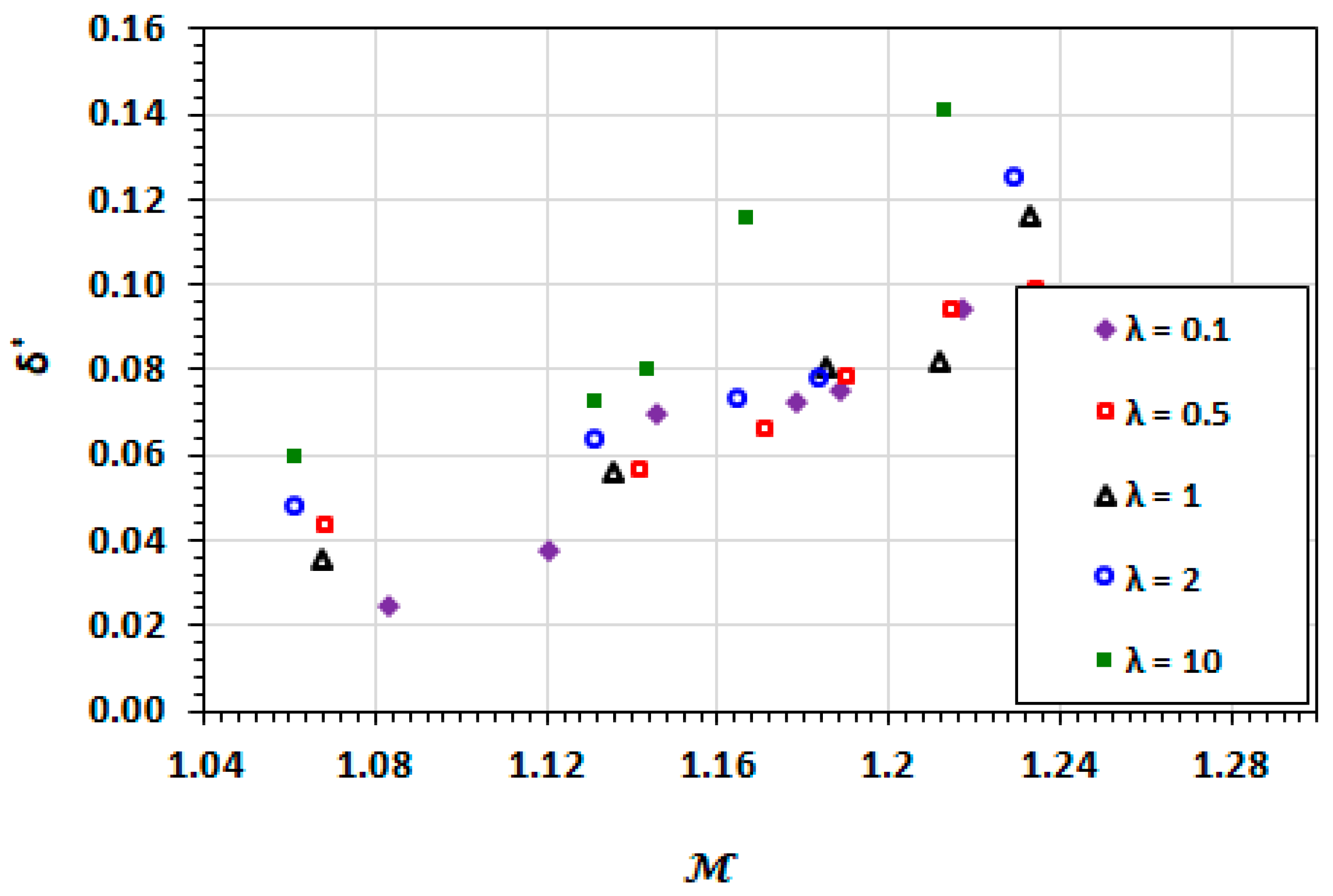
3.3. Film Thickness
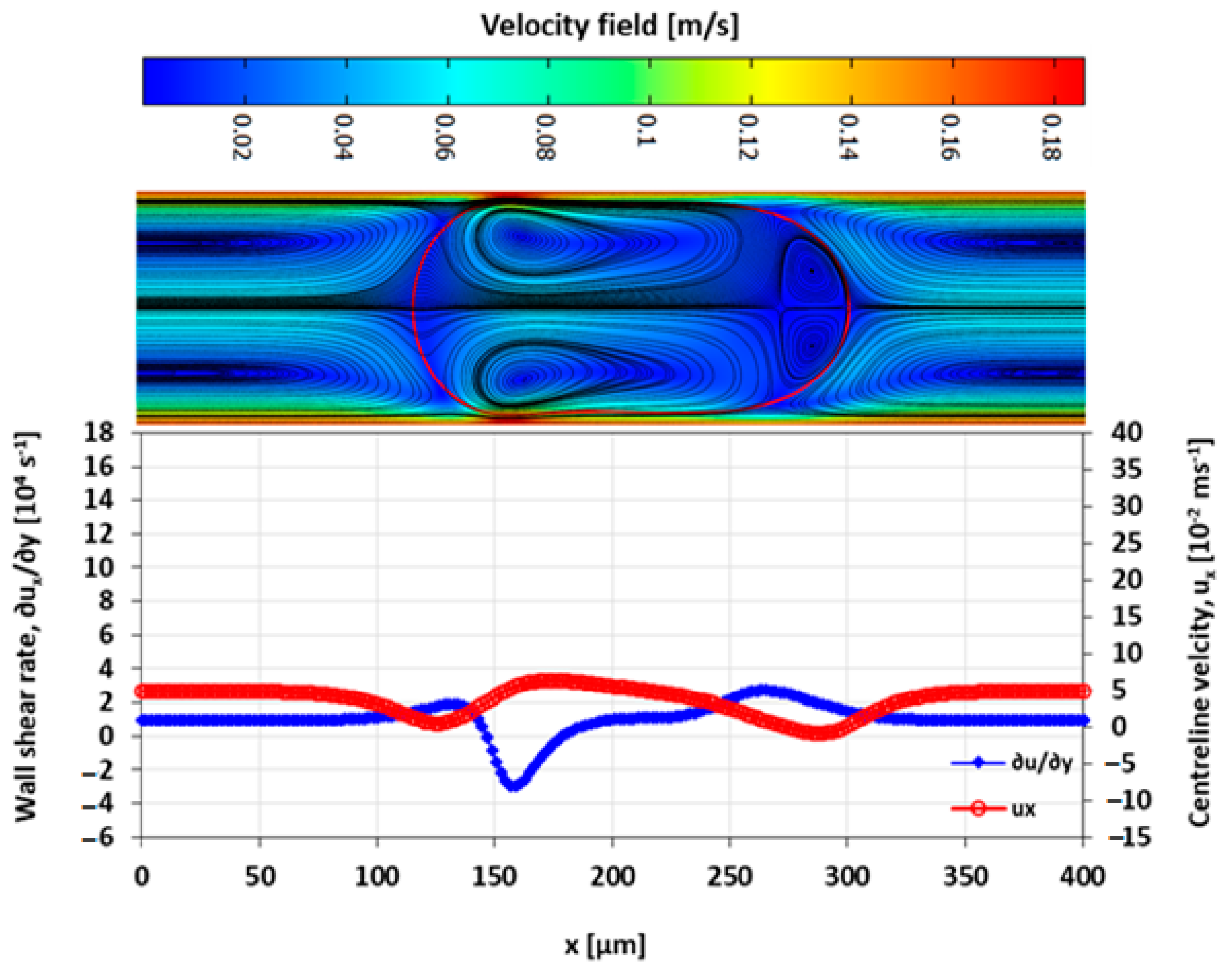
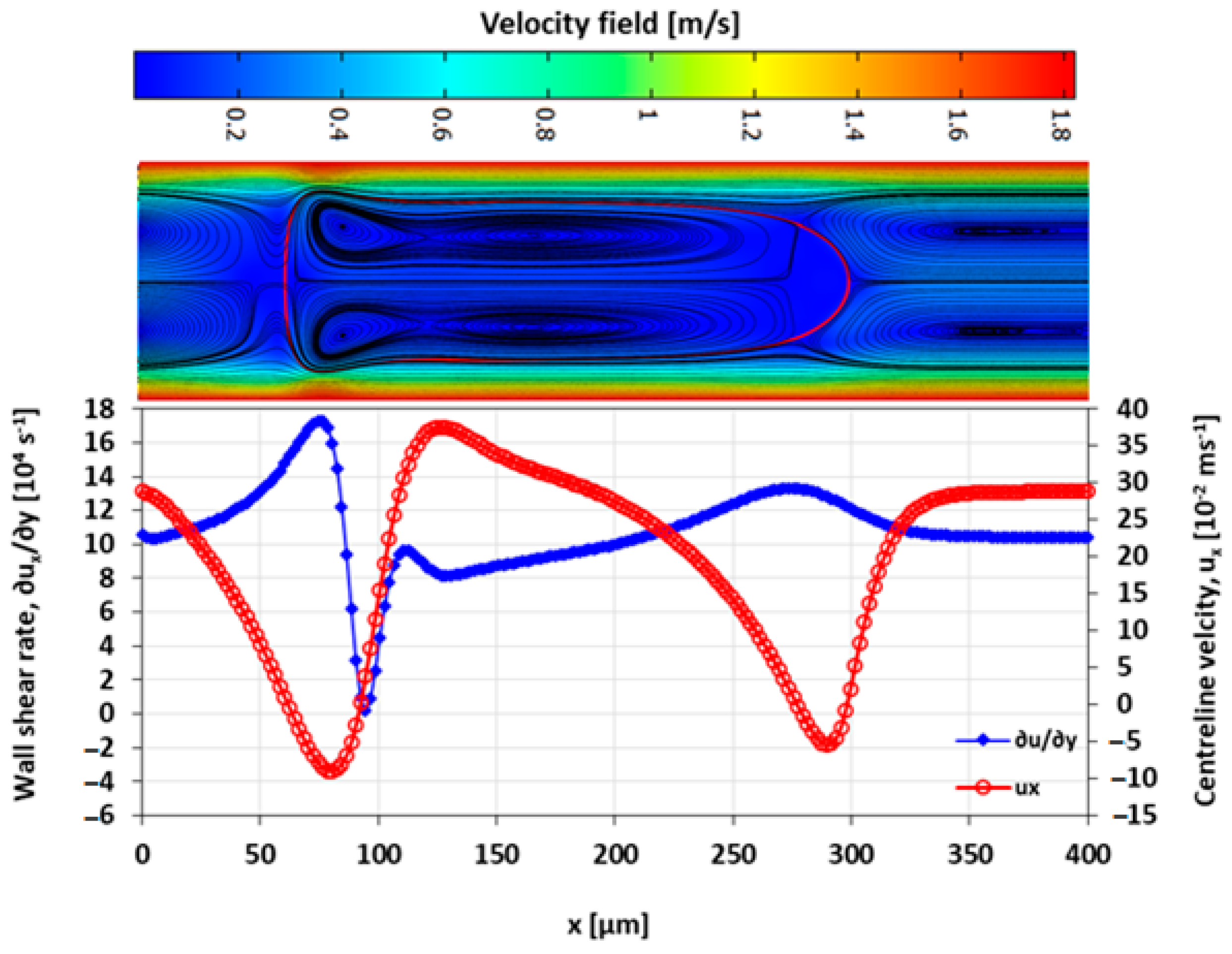
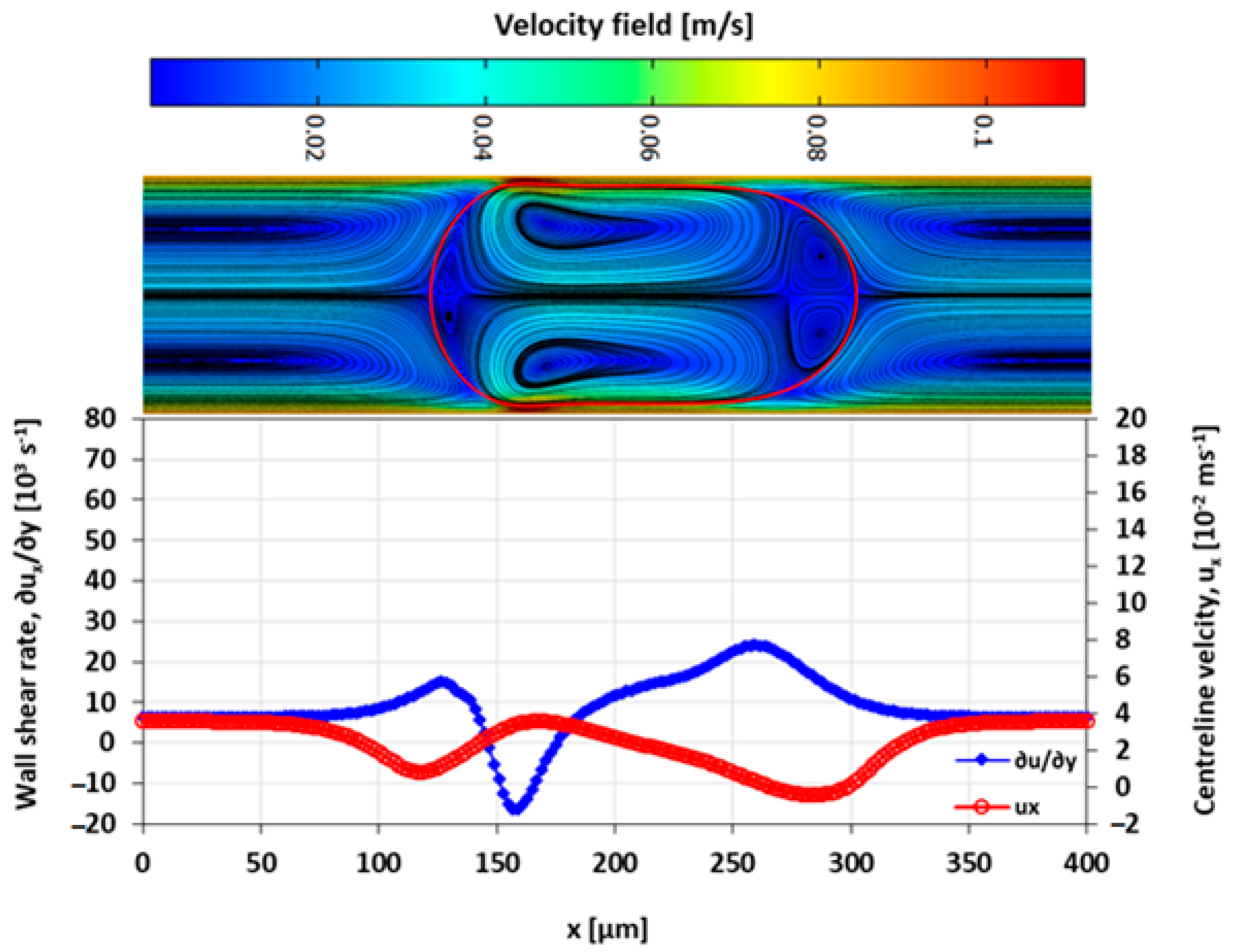
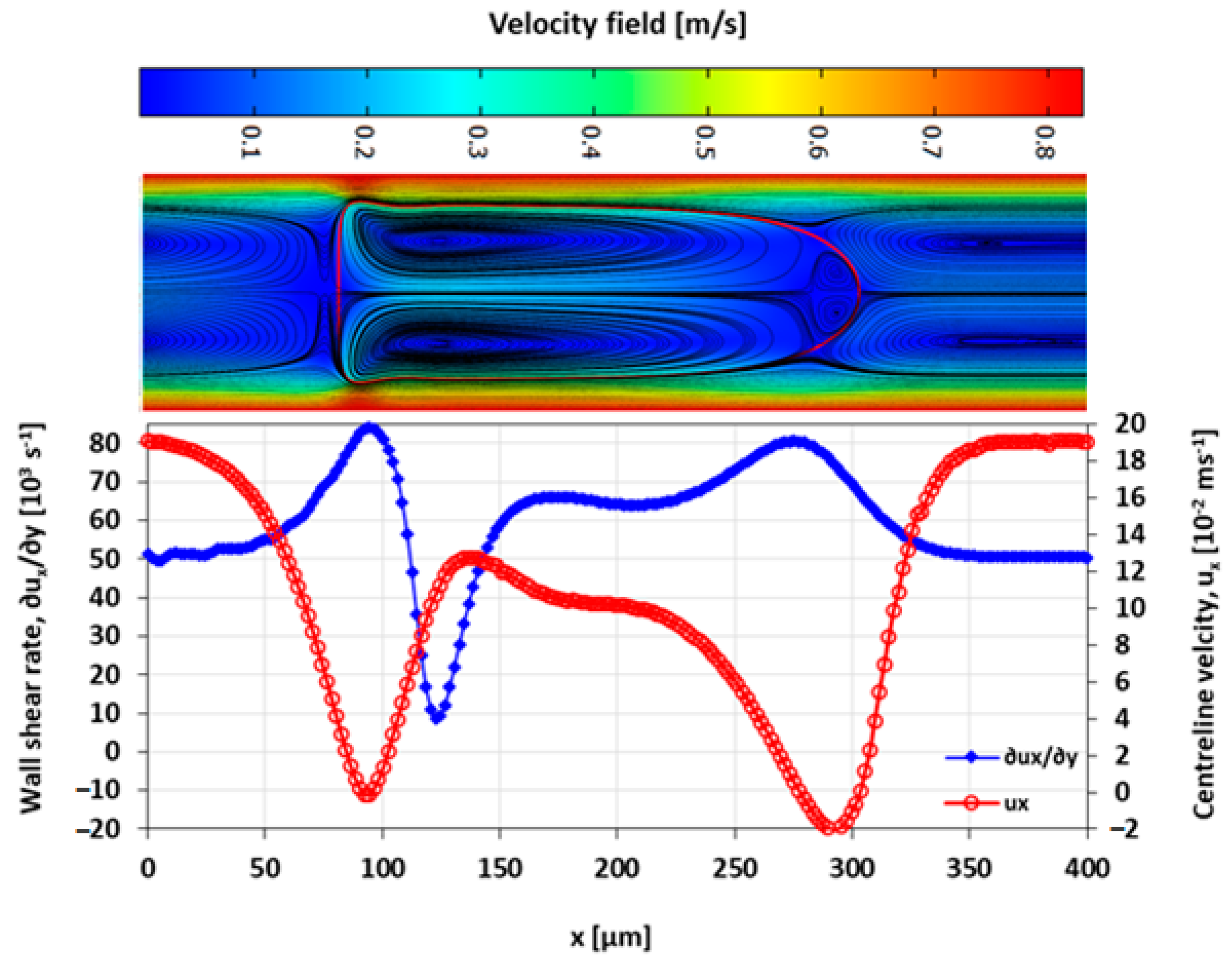
3.4. Velocity Field
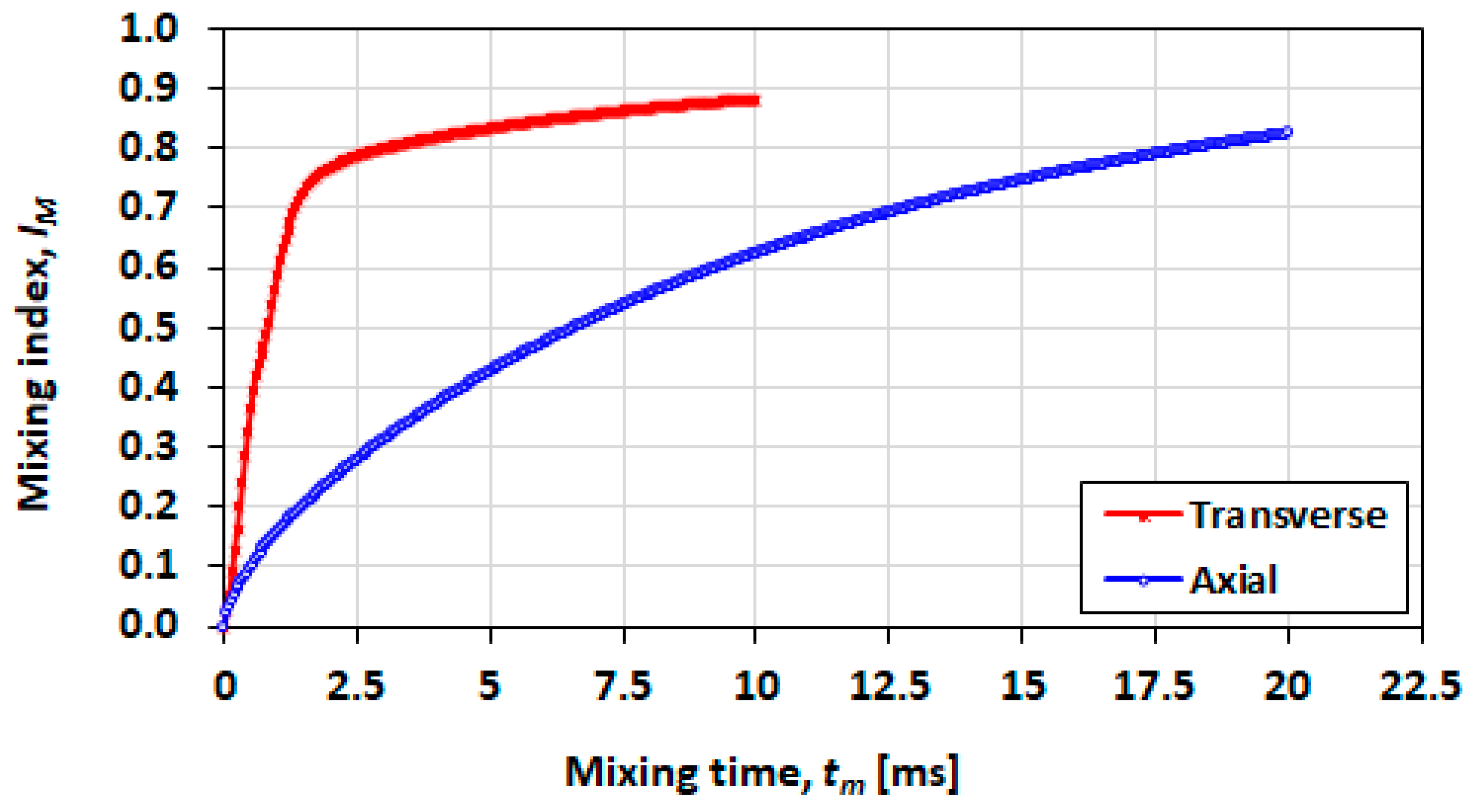
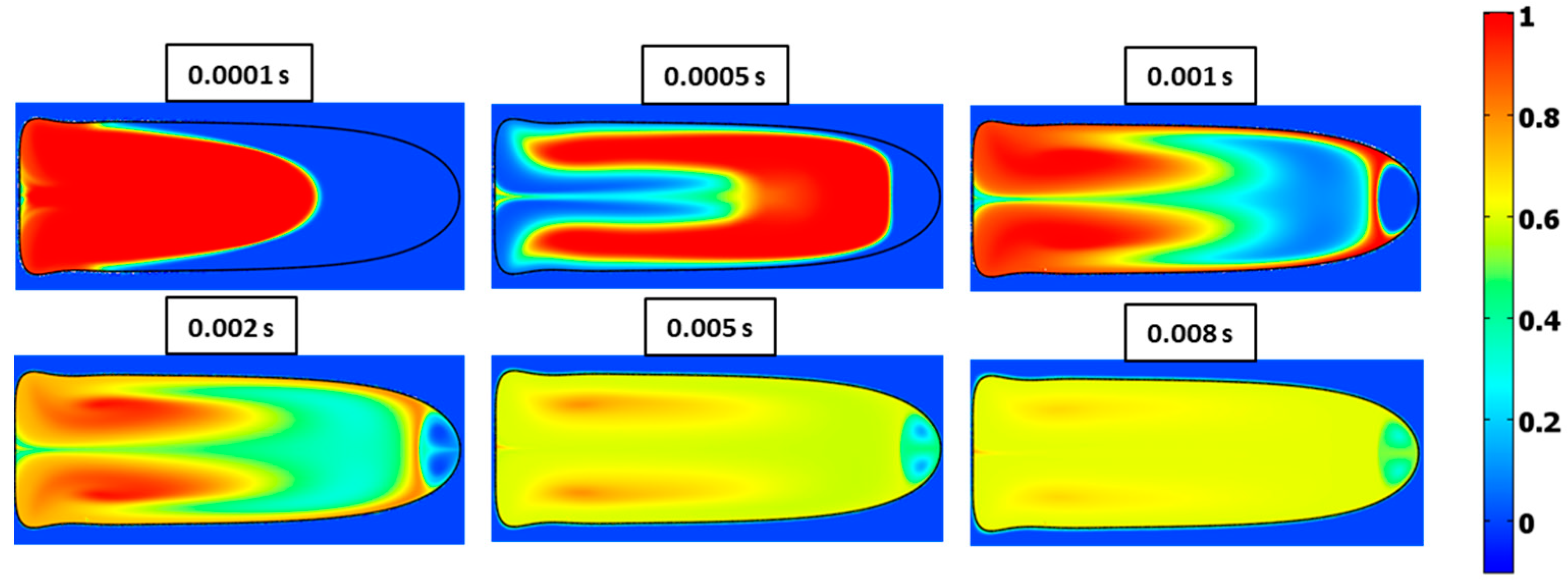
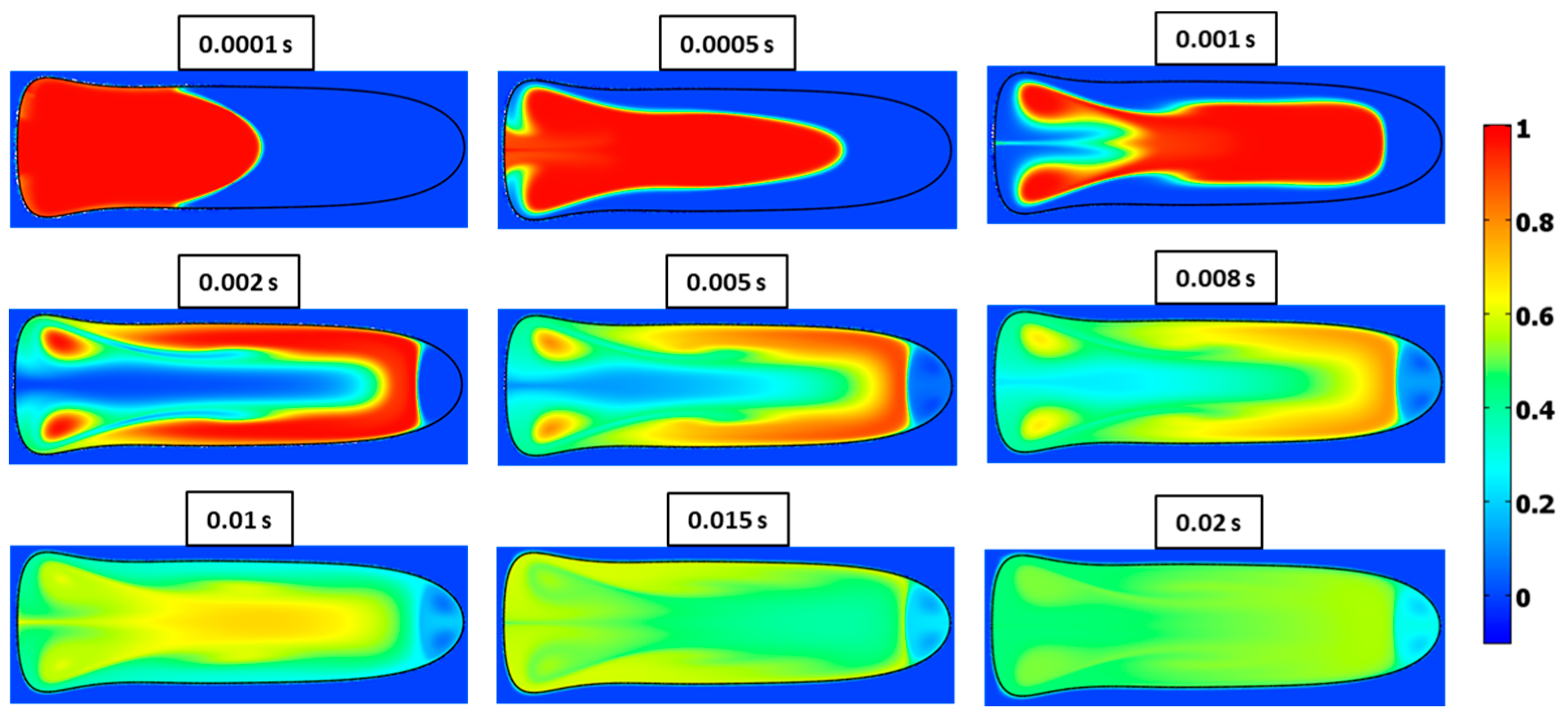
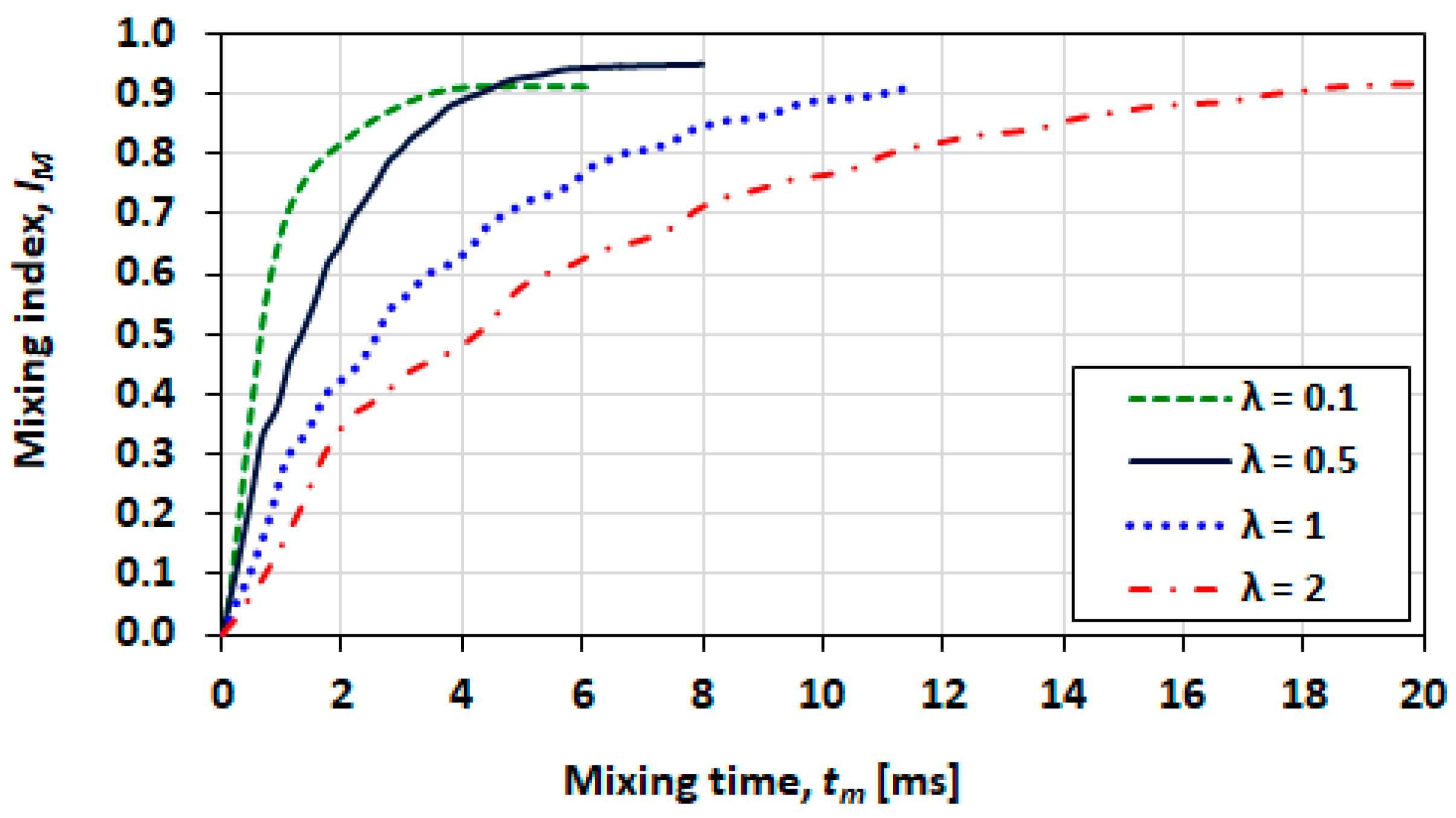
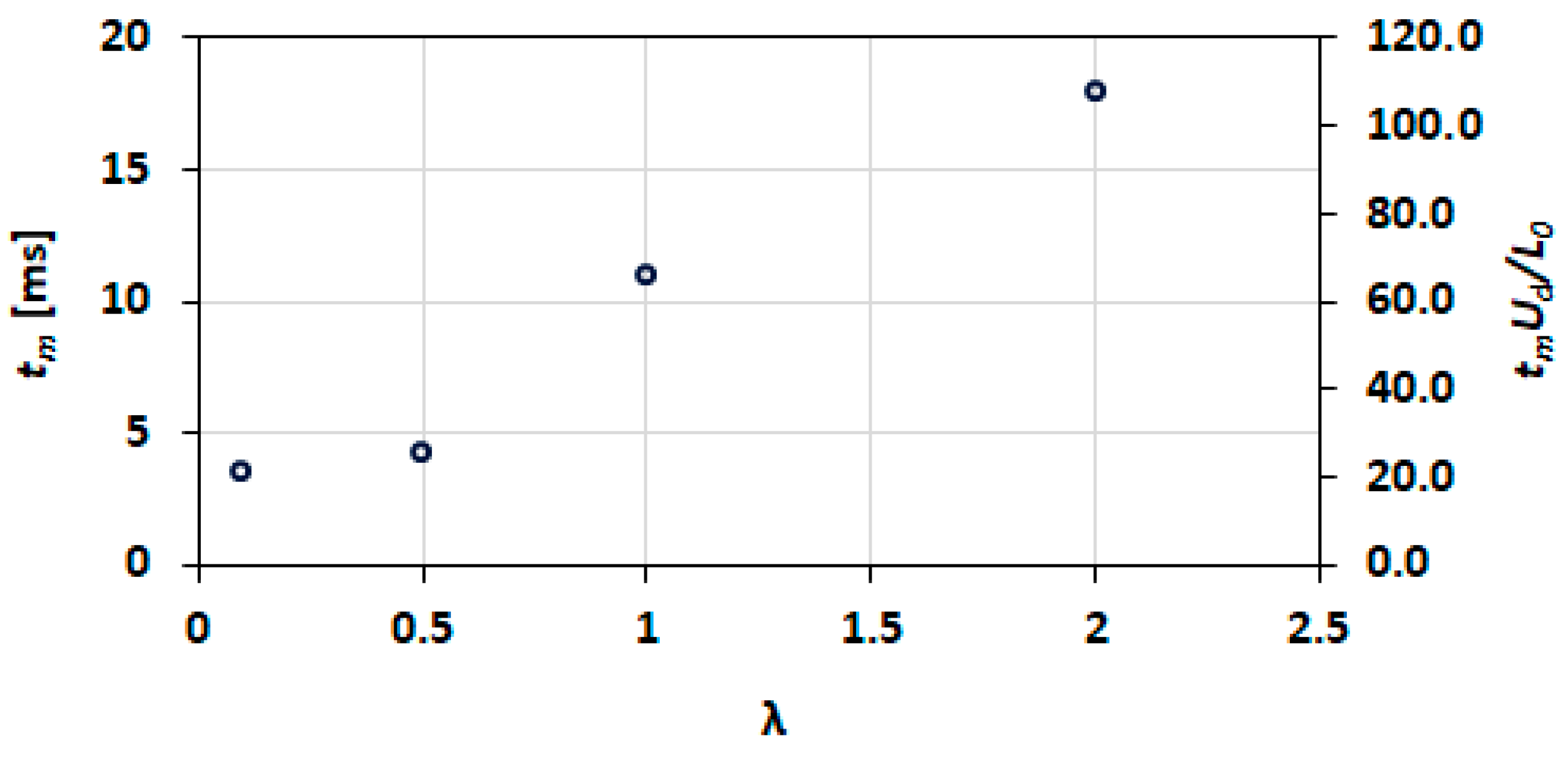
3.5. Mixing Index
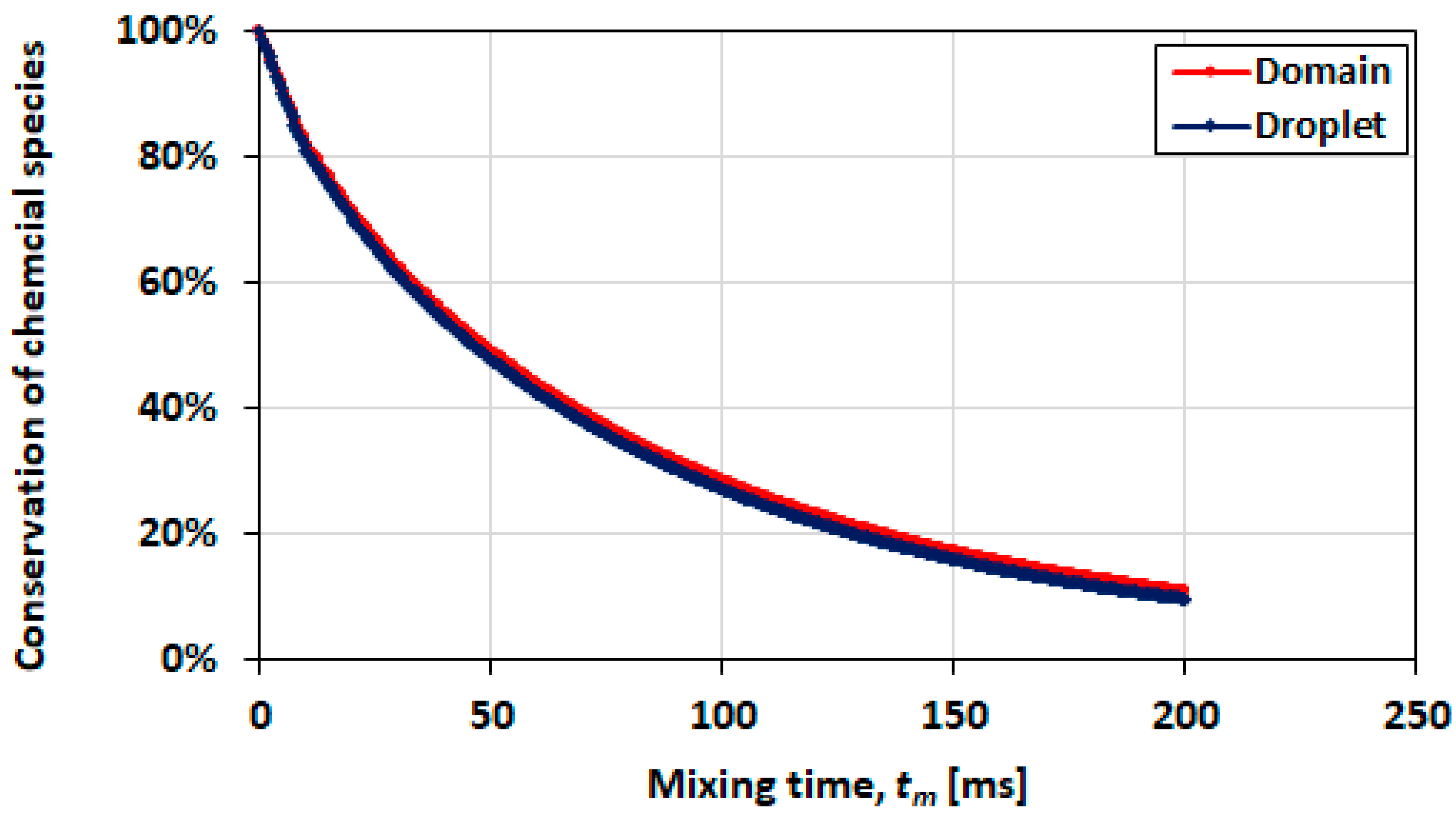
3.6. Limitation of the Droplet Mixing Model
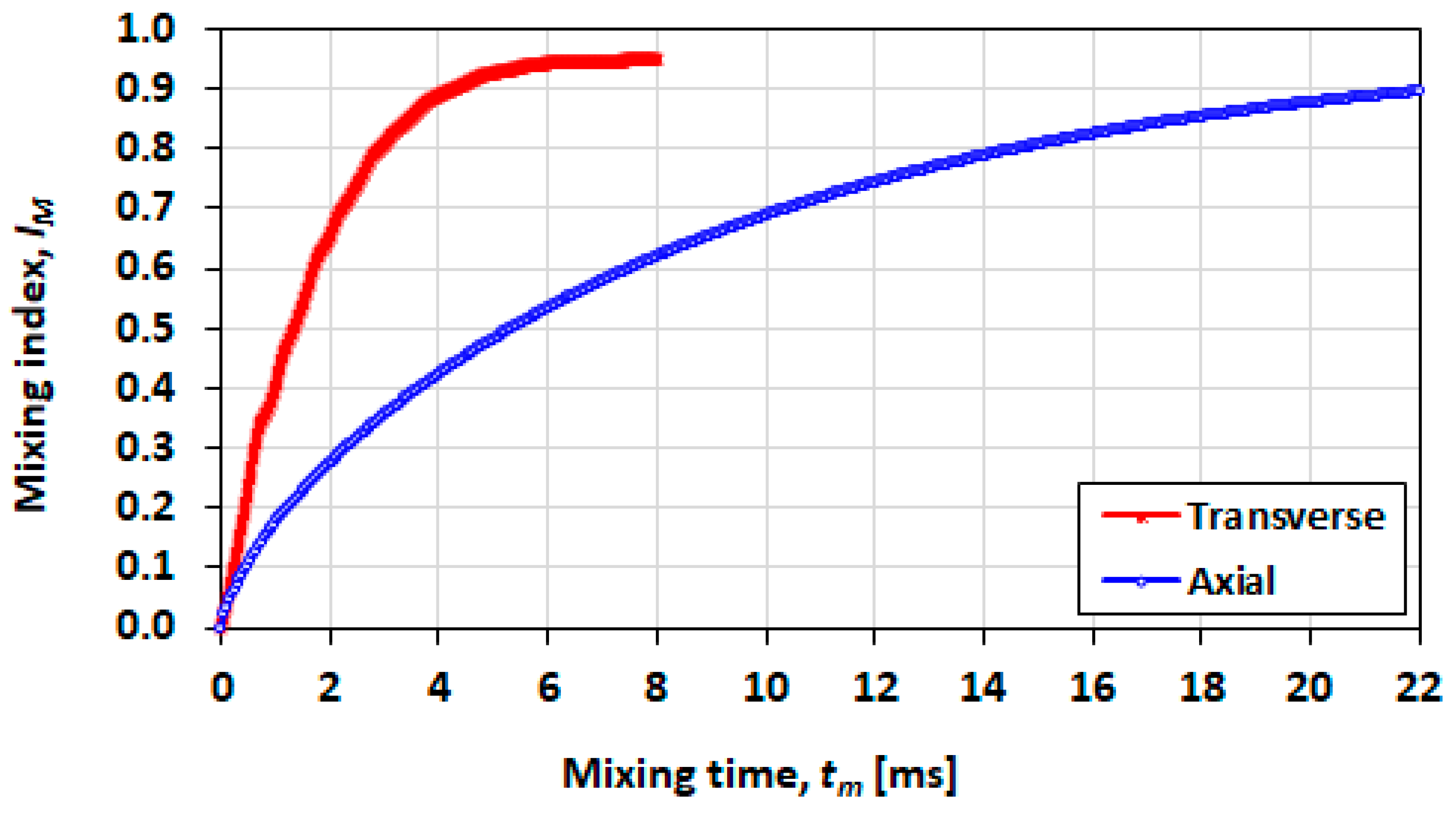
3.7. Effect of Initial Orientation of Solute Species
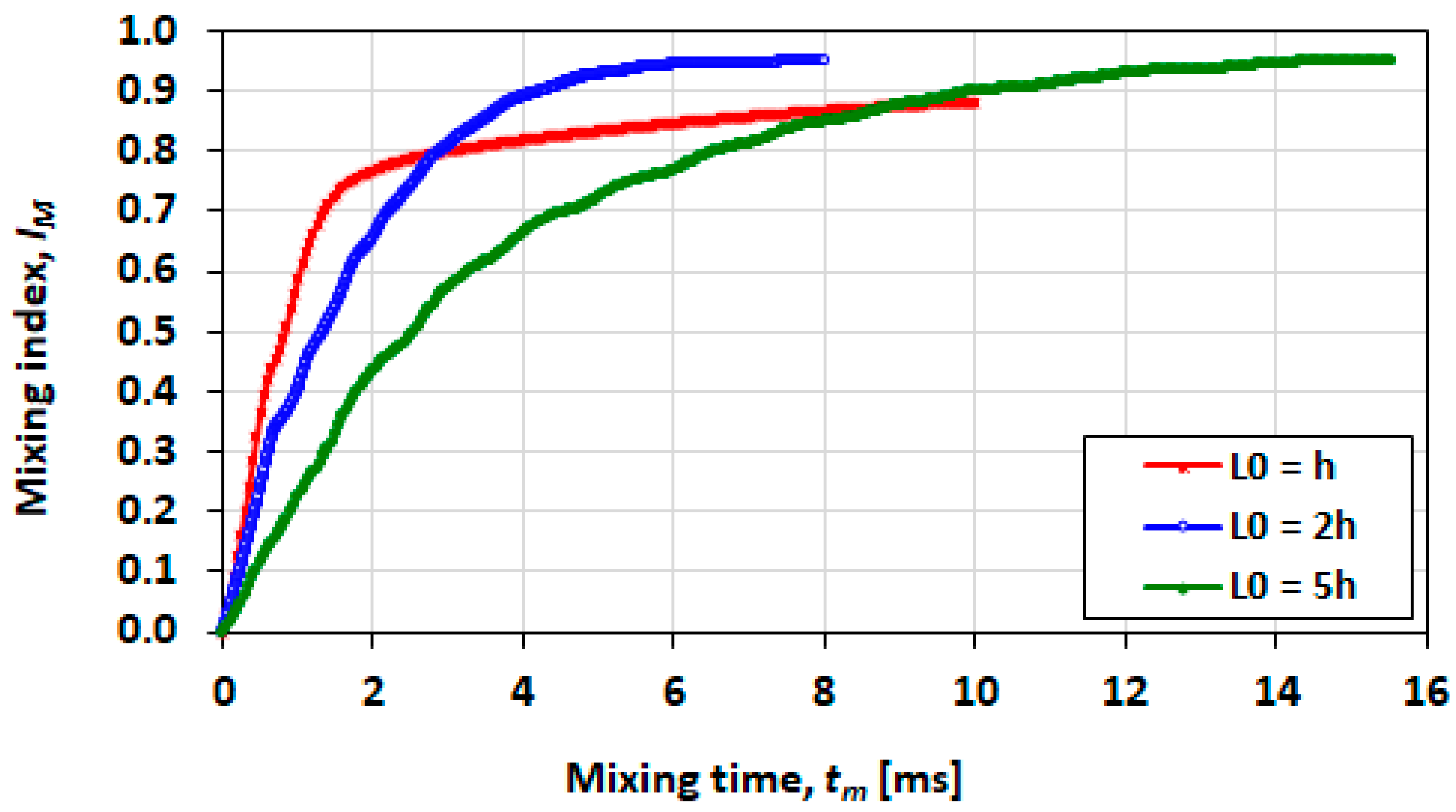
3.8. Effect of Droplet Size
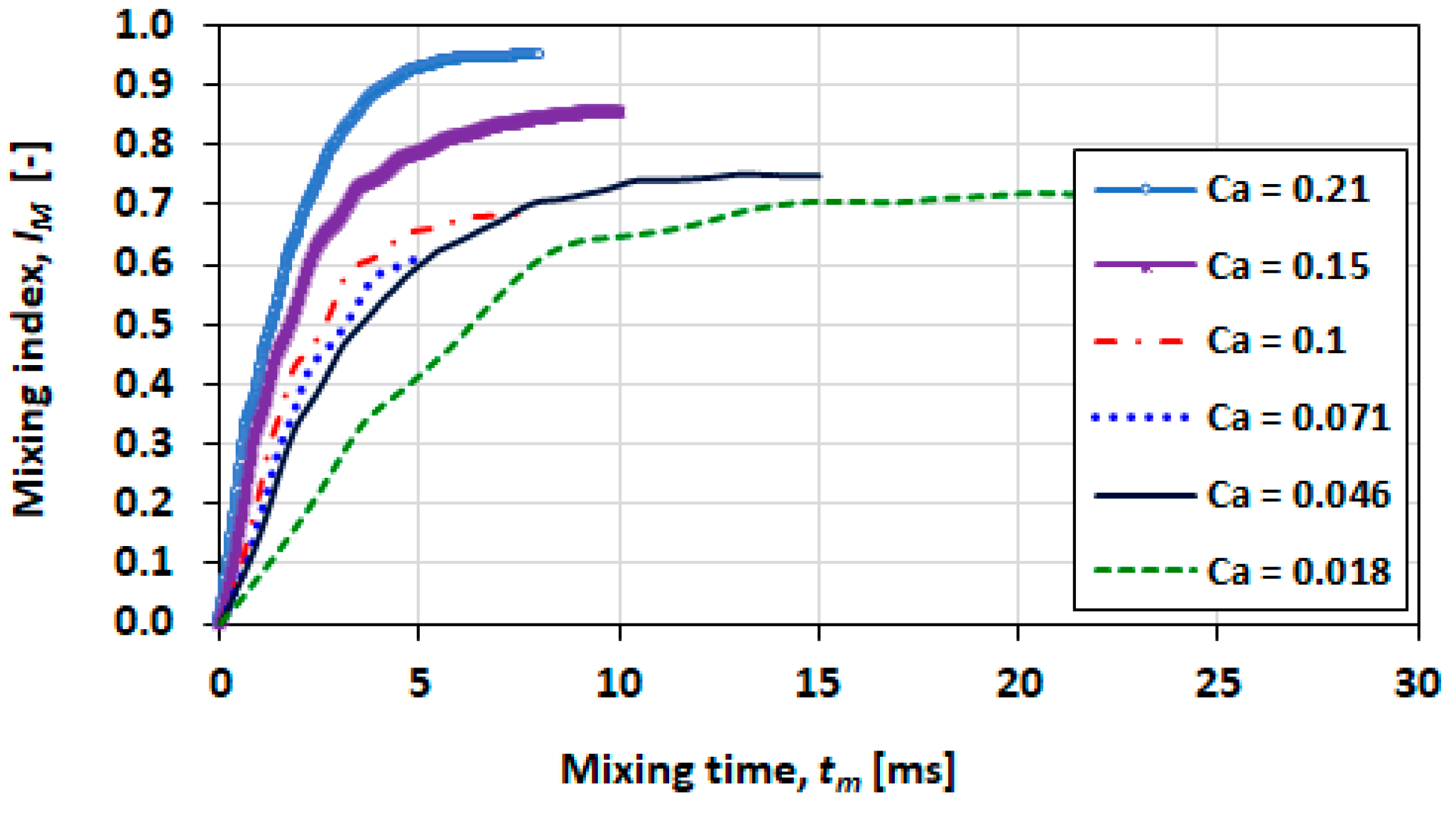
3.9. Effect of Capillary Number
3.10. Effect of Viscosity Ratio
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cedillo-Alcantar, D.F.; Han, Y.D.; Choi, J.; Garcia-Cordero, J.L.; Revzin, A. Automated Droplet-Based Microfluidic Platform for Multiplexed Analysis of Biochemical Markers in Small Volumes. Anal. Chem. 2019, 91, 5133–5141. [Google Scholar] [CrossRef] [PubMed]
- Hattori, S.; Tang, C.; Tanaka, D.; Yoon, D.H.; Nozaki, Y.; Fujita, H.; Akitsu, T.; Sekiguchi, T.; Shoji, S. Development of Microdroplet Generation Method for Organic Solvents Used in Chemical Synthesis. Molecules 2020, 25, 5360. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Z.; Xie, H.; Bao, J.; Wei, Y.; Lin, B.; Liu, Z. Facile precipitation microfluidic synthesis of Monodisperse and inorganic hollow microspheres for Photocatalysis. J. Chem. Technol. Biotechnol. 2021, 97, 1215–1223. [Google Scholar] [CrossRef]
- Yang, H.; Wei, Y.; Fan, B.; Liu, L.; Zhang, T.; Chen, D.; Wang, J.; Chen, J. A droplet-based microfluidic flow cytometry enabling absolute quantification of single-cell proteins leveraging constriction channel. Microfluid. Nanofluid. 2021, 25, 30. [Google Scholar] [CrossRef]
- Mbanjwa, M.B.; Land, K.J.; Windvoel, T.; Papala, P.M.; Fourie, L.; Korvink, J.G.; Visser, D.; Brady, D. Production of self-immobilised enzyme microspheres using microfluidics. Process Biochem. 2018, 69, 75–81. [Google Scholar] [CrossRef]
- Abalde-Cela, S.; Taladriz-Blanco, P.; De Oliveira, M.G.; Abell, C. Droplet microfluidics for the highly controlled synthesis of branched gold nanoparticles. Sci. Rep. 2018, 8, 2440. [Google Scholar] [CrossRef]
- Payne, E.M.; Holland-Moritz, D.A.; Sun, S.; Kennedy, R.T. High-throughput screening by droplet microfluidics: Perspective into key challenges and future prospects. Lab Chip 2020, 20, 2247–2262. [Google Scholar] [CrossRef]
- Bringer, M.R.; Gerdts, C.J.; Song, H.; Tice, J.D.; Ismagilov, R.F. Microfluidic systems for chemical kinetics that rely on chaotic mixing in droplets. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 362, 1087–1104. [Google Scholar] [CrossRef]
- Song, H.; Chen, D.L.; Ismagilov, R.F. Reactions in Droplets in Microfluidic Channels. Angew. Chem. Int. Ed. 2006, 45, 7336–7356. [Google Scholar] [CrossRef]
- Tice, J.D.; Song, H.; Lyon, A.D.; Ismagilov, R.F. Formation of Droplets and Mixing in Multiphase Microfluidics at Low Values of the Reynolds and the Capillary Numbers. Langmuir 2003, 19, 9127–9133. [Google Scholar] [CrossRef]
- Sarrazin, F.; Loubière, K.; Prat, L.; Gourdon, C.; Bonometti, T.; Magnaudet, J. Experimental and numerical study of droplets hydrodynamics in microchannels. AIChE J. 2006, 52, 4061–4070. [Google Scholar] [CrossRef]
- Sattari-Najafabadi, M.; Esfahany, M.N.; Wu, Z.; Sundén, B. Hydrodynamics and mass transfer in liquid-liquid non-circular microchannels: Comparison of two aspect ratios and three junction structures. Chem. Eng. J. 2017, 322, 328–338. [Google Scholar] [CrossRef]
- Bruneau, C.H.; Colin, T.; Galusinski, C.; Tancogne, S.; Vigneaux, P. Simulations of 3D dynamics of microdroplets: A comparison of rectangular and cylindrical channels. In Numerical Mathematics and Advanced Applications; Kunisch, K., Of, G., Steinbach, O., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 449–456. [Google Scholar] [CrossRef]
- Chao, X.; Xu, F.; Yao, C.; Liu, T.; Chen, G. CFD Simulation of Internal Flow and Mixing within Droplets in a T-Junction Microchannel. Ind. Eng. Chem. Res. 2021, 60, 6038–6047. [Google Scholar] [CrossRef]
- Dore, V.; Tsaoulidis, D.; Angeli, P. Mixing patterns in water plugs during water/ionic liquid segmented flow in microchannels. Chem. Eng. Sci. 2012, 80, 334–341. [Google Scholar] [CrossRef]
- Tice, J.D.; Lyon, A.D.; Ismagilov, R.F. Effects of viscosity on droplet formation and mixing in microfluidic channels. Anal. Chim. Acta 2004, 507, 73–77. [Google Scholar] [CrossRef]
- Kovalev, A.; Yagodnitsyna, A.; Bilsky, A. Plug flow of immiscible liquids with low viscosity ratio in serpentine microchannels. Chem. Eng. J. 2020, 417, 127933. [Google Scholar] [CrossRef]
- Liu, R.H.; Stremler, M.A.; Sharp, K.V.; Olsen, M.G.; Santiago, J.G.; Adrian, R.J.; Aref, H.; Beebe, D.J. Passive mixing in a three-dimensional serpentine microchannel. J. Microelectromech. Syst. 2000, 9, 190–197. [Google Scholar] [CrossRef]
- He, B.; Burke, B.J.; Zhang, X.; Zhang, R.; Regnier, F.E. A Picoliter-Volume Mixer for Microfluidic Analytical Systems. Anal. Chem. 2001, 73, 1942–1947. [Google Scholar] [CrossRef]
- Wang, H.; Lovenitti, P.; Harvey, E.; Masood, S. Optimizing layout of obstacles for enhanced mixing in microchannels. Smart Mater. Struct. 2002, 11, 662–667. [Google Scholar] [CrossRef]
- Wang, H.; Iovenitti, P.; Harvey, E.; Masood, S. Numerical investigation of mixing in microchannels with patterned grooves. J. Micromech. Microeng. 2003, 13, 801–808. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Chaotic Mixer for Microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Khodaparast, S.; Borhani, N.; Thome, J. Application of micro particle shadow velocimetry μPSV to two-phase flows in microchannels. Int. J. Multiph. Flow 2014, 62, 123–133. [Google Scholar] [CrossRef]
- Solvas, X.C.I.; Demello, A. Droplet microfluidics: Recent developments and future applications. Chem. Commun. 2010, 47, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-L.; Hsiao, T.-H. Quantitative analysis of microfluidic mixing using microscale schlieren technique. Microfluid. Nanofluid. 2013, 15, 253–265. [Google Scholar] [CrossRef]
- Gleichmann, N.; Malsch, D.; Horbert, P.; Henkel, T. Toward microfluidic design automation: A new system simulation toolkit for the in silico evaluation of droplet-based lab-on-a-chip systems. Microfluid. Nanofluid. 2014, 18, 1095–1105. [Google Scholar] [CrossRef]
- Rossinelli, D.; Tang, Y.H.; Lykov, K.; Alexeev, D.; Bernaschi, M.; Hadjidoukas, P.; Bisson, M.; Joubert, W.; Conti, C.; Karniadakis, G.; et al. The in-silico lab-on-a-chip: Petascale and high-throughput simulations of microfluidics at cell resolution. In Proceedings of the International Conference for High Performance Computing, Networking, Storage and Analysis, Association for Computing Machinery (ACM) Digital Library, Austin, TX, USA, 15–20 November 2015. [Google Scholar]
- Díaz-Zuccarini, V.; Lawford, P.V. An in silico future for the engineering of functional tissues and organs. Organogenesis 2010, 6, 245–251. [Google Scholar] [CrossRef][Green Version]
- Bordbar, A.; Taassob, A.; Zarnaghsh, A.; Kamali, R. Slug flow in microchannels: Numerical simulation and applications. J. Ind. Eng. Chem. 2018, 62, 26–39. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Li, X.-J.; Wu, Z.; Jin, Z.-J.; Sunden, B. A comprehensive review on liquid–liquid two-phase flow in microchannel: Flow pattern and mass transfer. Microfluid. Nanofluid. 2019, 23, 116. [Google Scholar] [CrossRef]
- Wörner, M. Numerical modeling of multiphase flows in microfluidics and micro process engineering: A review of methods and applications. Microfluid. Nanofluid. 2012, 12, 841–886. [Google Scholar] [CrossRef]
- Abdollahi, A.; Sharma, R.N.; Vatani, A. Fluid flow and heat transfer of liquid-liquid two phase flow in microchannels: A review. Int. Commun. Heat Mass Transf. 2017, 84, 66–74. [Google Scholar] [CrossRef]
- Bandara, T.; Nguyen, N.-T.; Rosengarten, G. Slug flow heat transfer without phase change in microchannels: A review. Chem. Eng. Sci. 2014, 126, 283–295. [Google Scholar] [CrossRef]
- Talimi, V.; Muzychka, Y.; Kocabiyik, S. A review on numerical studies of slug flow hydrodynamics and heat transfer in microtubes and microchannels. Int. J. Multiph. Flow 2012, 39, 88–104. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Feng, L.; Lin, T. Fluid mixing in droplet-based microfluidics with a serpentine microchannel. RSC Adv. 2015, 5, 104138–104144. [Google Scholar] [CrossRef]
- Tanthapanichakoon, W.; Aoki, N.; Matsuyama, K.; Mae, K. Design of mixing in microfluidic liquid slugs based on a new dimensionless number for precise reaction and mixing operations. Chem. Eng. Sci. 2006, 61, 4220–4232. [Google Scholar] [CrossRef]
- Cherlo, S.K.R.; Kariveti, S.; Pushpavanam, S. Experimental and Numerical Investigations of Two-Phase (Liquid−Liquid) Flow Behavior in Rectangular Microchannels. Ind. Eng. Chem. Res. 2009, 49, 893–899. [Google Scholar] [CrossRef]
- Mießner, U.; Helmers, T.; Lindken, R.; Westerweel, J. µPIV measurement of the 3D velocity distribution of Taylor droplets moving in a square horizontal channel. Exp. Fluids 2020, 61, 1–17. [Google Scholar] [CrossRef]
- Ma, S.; Sherwood, J.M.; Huck, W.T.S.; Balabani, S. On the flow topology inside droplets moving in rectangular microchannels. Lab Chip 2014, 14, 3611–3620. [Google Scholar] [CrossRef]
- Taha, T.; Cui, Z. CFD modelling of slug flow inside square capillaries. Chem. Eng. Sci. 2006, 61, 665–675. [Google Scholar] [CrossRef]
- Taha, T.; Cui, Z. CFD modelling of slug flow in vertical tubes. Chem. Eng. Sci. 2006, 61, 676–687. [Google Scholar] [CrossRef]
- Handique, K.; Burns, M.A. Mathematical modeling of drop mixing in a slit-type microchannel. J. Micromech. Microeng. 2001, 11, 548–554. [Google Scholar] [CrossRef]
- Hysing, S. Mixed element FEM level set method for numerical simulation of immiscible fluids. J. Comput. Phys. 2011, 231, 2449–2465. [Google Scholar] [CrossRef]
- Chowdhury, I.U.; Mahapatra, P.S.; Sen, A.K. Self-driven droplet transport: Effect of wettability gradient and confinement. Phys. Fluids 2019, 31, 042111. [Google Scholar] [CrossRef]
- Yuan, S.; Zhou, M.; Peng, T.; Li, Q.; Jiang, F. An investigation of chaotic mixing behavior in a planar microfluidic mixer. Phys. Fluids 2022, 34, 032007. [Google Scholar] [CrossRef]
- Sussman, M.; Smereka, P.; Osher, S. A Level Set Approach for Computing Solutions to Incompressible Two-Phase Flow. J. Comput. Phys. 1994, 114, 146–159. [Google Scholar] [CrossRef]
- Olsson, E.; Kreiss, G. A conservative level set method for two phase flow. J. Comput. Phys. 2005, 210, 225–246. [Google Scholar] [CrossRef]
- Olsson, E.; Kreiss, G.; Zahedi, S. A conservative level set method for two phase flow II. J. Comput. Phys. 2007, 225, 785–807. [Google Scholar] [CrossRef]
- Mbanjwa, M.B. Numerical simulation of multiphase dynamics in droplet microfluidics. In School of Chemical and Metallurgical Engineering; University of the Witwatersrand: Johannesburg, South Africa, 2019. [Google Scholar]
- Zahedi, S.; Kronbichler, M.; Kreiss, G. Spurious currents in finite element based level set methods for two-phase flow. Int. J. Numer. Methods Fluids 2011, 69, 1433–1456. [Google Scholar] [CrossRef]
- Brackbill, J.U.; Kothe, D.B.; Zemach, C. A continuum method for modeling surface tension. J. Comput. Phys. 1992, 100, 335–354. [Google Scholar] [CrossRef]
- Kirby, B.J. Micro and Nanoscale Fluid Mechanics: Transport in Microfluidic Devices; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Walker, E.D. Numerical Studies of Liquid-Liquid Segmented Flows in Square Microchannels Using a Front-Tracking Algorithm; Louisiana State University: Baton Rouge, LA, USA, 2016. [Google Scholar]
- Boudreaux, J.M. Exploration of Segmented Flow in Microchannels Using the Volume of Fluid Method in ANSYS Fluent; Louisiana State University: Baton Rouge, LA, USA, 2015. [Google Scholar]
- Kreutzer, M.T.; Kapteijn, F.; Moulijn, J.A.; Kleijn, C.R.; Heiszwolf, J.J. Inertial and interfacial effects on pressure drop of Taylor flow in capillaries. AIChE J. 2005, 51, 2428–2440. [Google Scholar] [CrossRef]
- Tung, K.-Y.; Li, C.-C.; Yang, J.-T. Mixing and hydrodynamic analysis of a droplet in a planar serpentine micromixer. Microfluid. Nanofluid. 2009, 7, 545–557. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbanjwa, M.B.; Harding, K.; Gledhill, I.M.A. Numerical Modelling of Mixing in a Microfluidic Droplet Using a Two-Phase Moving Frame of Reference Approach. Micromachines 2022, 13, 708. https://doi.org/10.3390/mi13050708
Mbanjwa MB, Harding K, Gledhill IMA. Numerical Modelling of Mixing in a Microfluidic Droplet Using a Two-Phase Moving Frame of Reference Approach. Micromachines. 2022; 13(5):708. https://doi.org/10.3390/mi13050708
Chicago/Turabian StyleMbanjwa, Mesuli B., Kevin Harding, and Irvy M. A. Gledhill. 2022. "Numerical Modelling of Mixing in a Microfluidic Droplet Using a Two-Phase Moving Frame of Reference Approach" Micromachines 13, no. 5: 708. https://doi.org/10.3390/mi13050708
APA StyleMbanjwa, M. B., Harding, K., & Gledhill, I. M. A. (2022). Numerical Modelling of Mixing in a Microfluidic Droplet Using a Two-Phase Moving Frame of Reference Approach. Micromachines, 13(5), 708. https://doi.org/10.3390/mi13050708






