Investigation on the Electrochemical Deposition of Nanocrystalline Zinc with Cationic Polyacrylamide (CPAM)-ZnSO4 Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
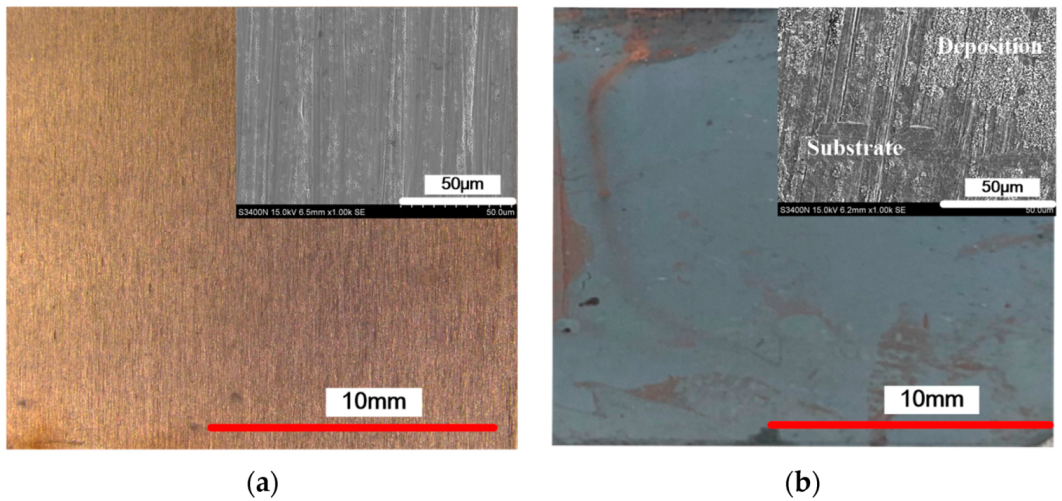
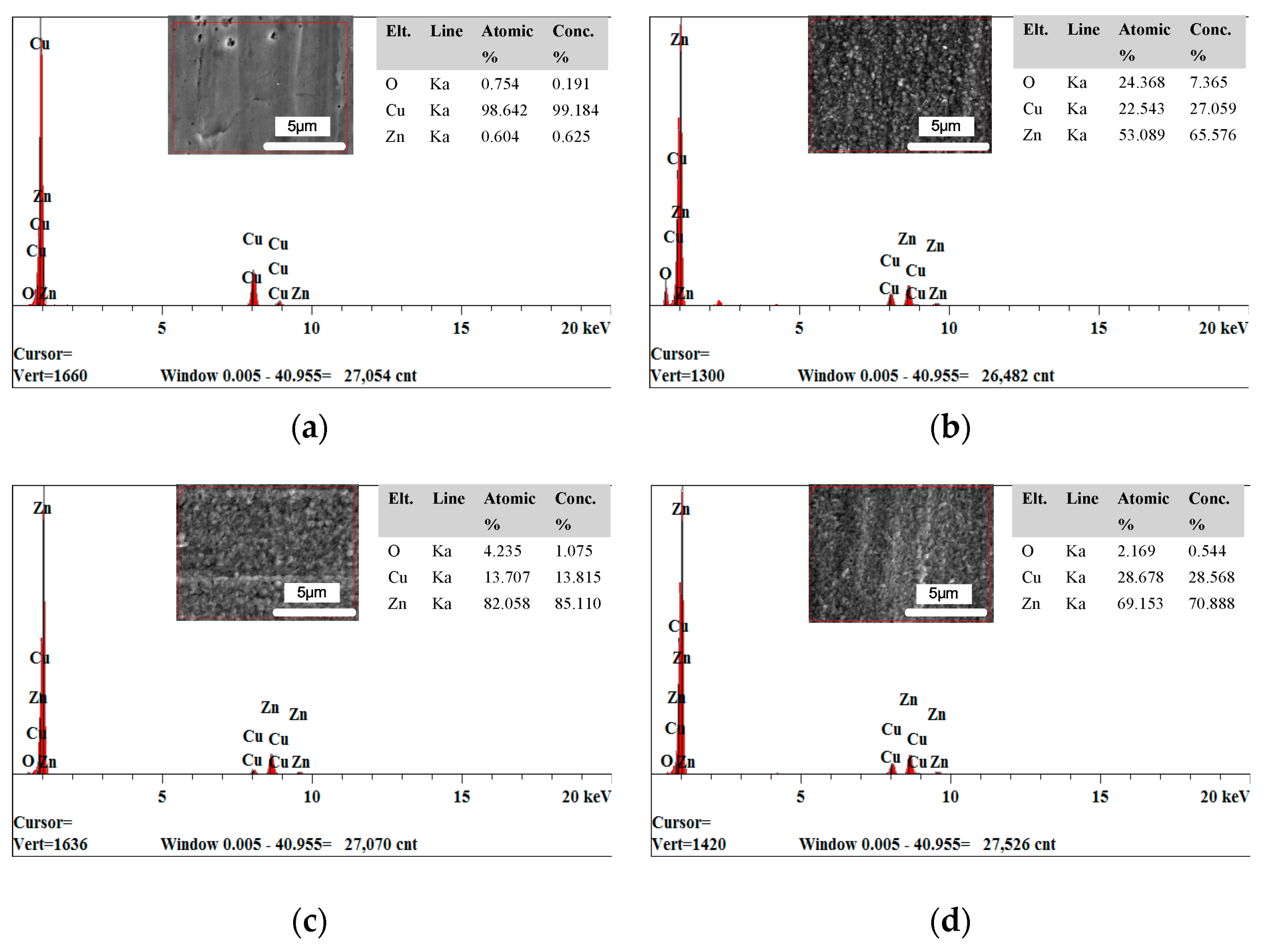
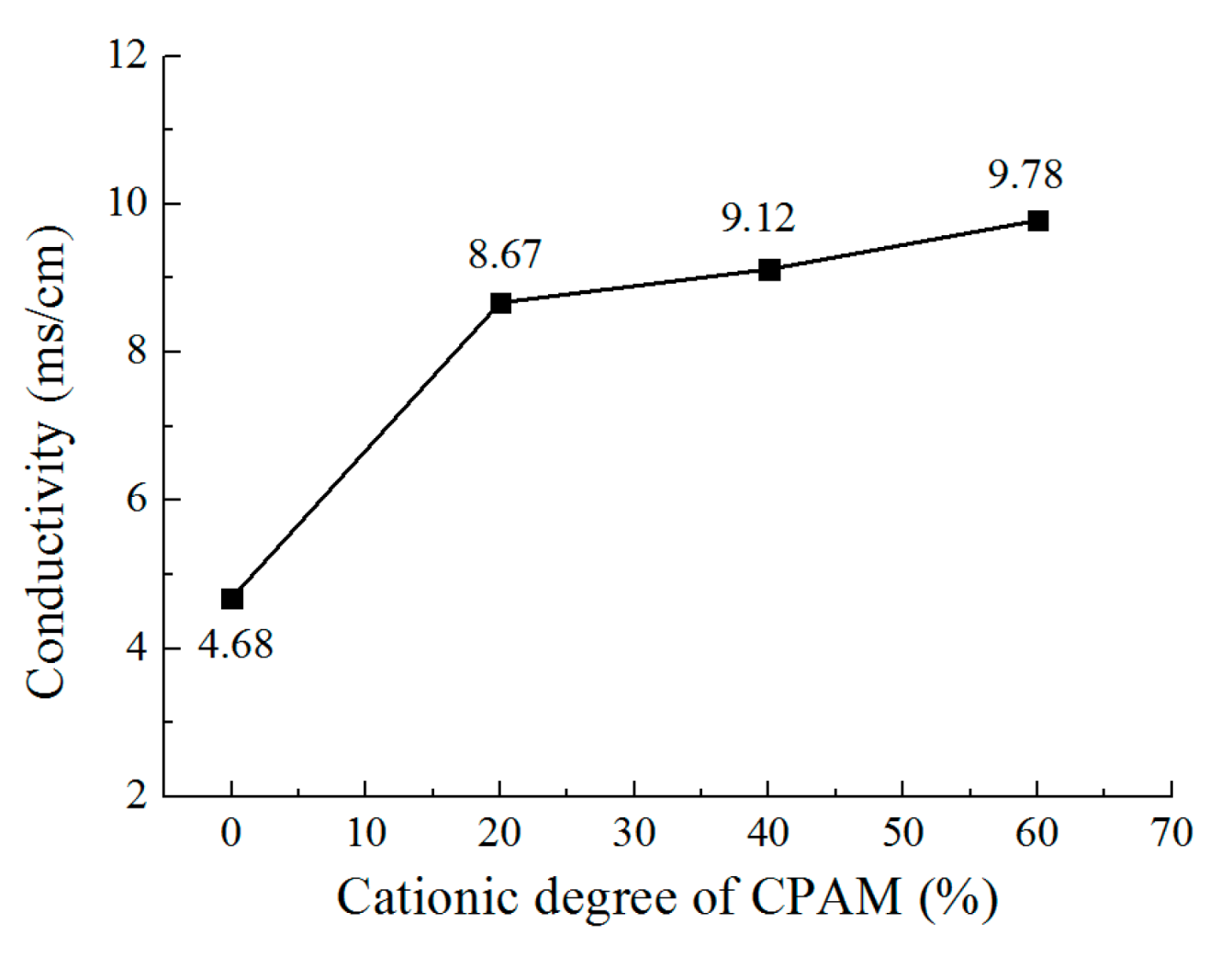
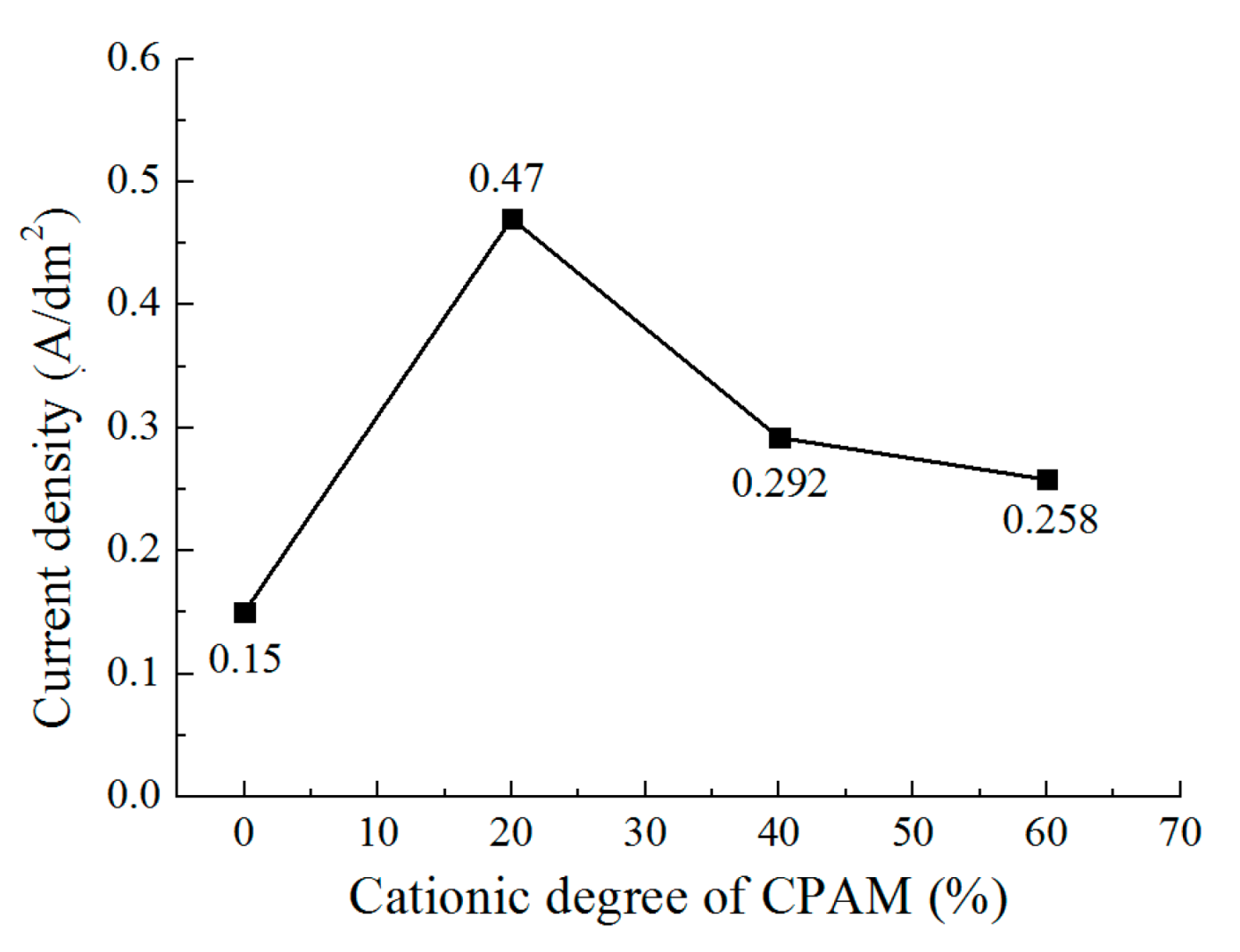
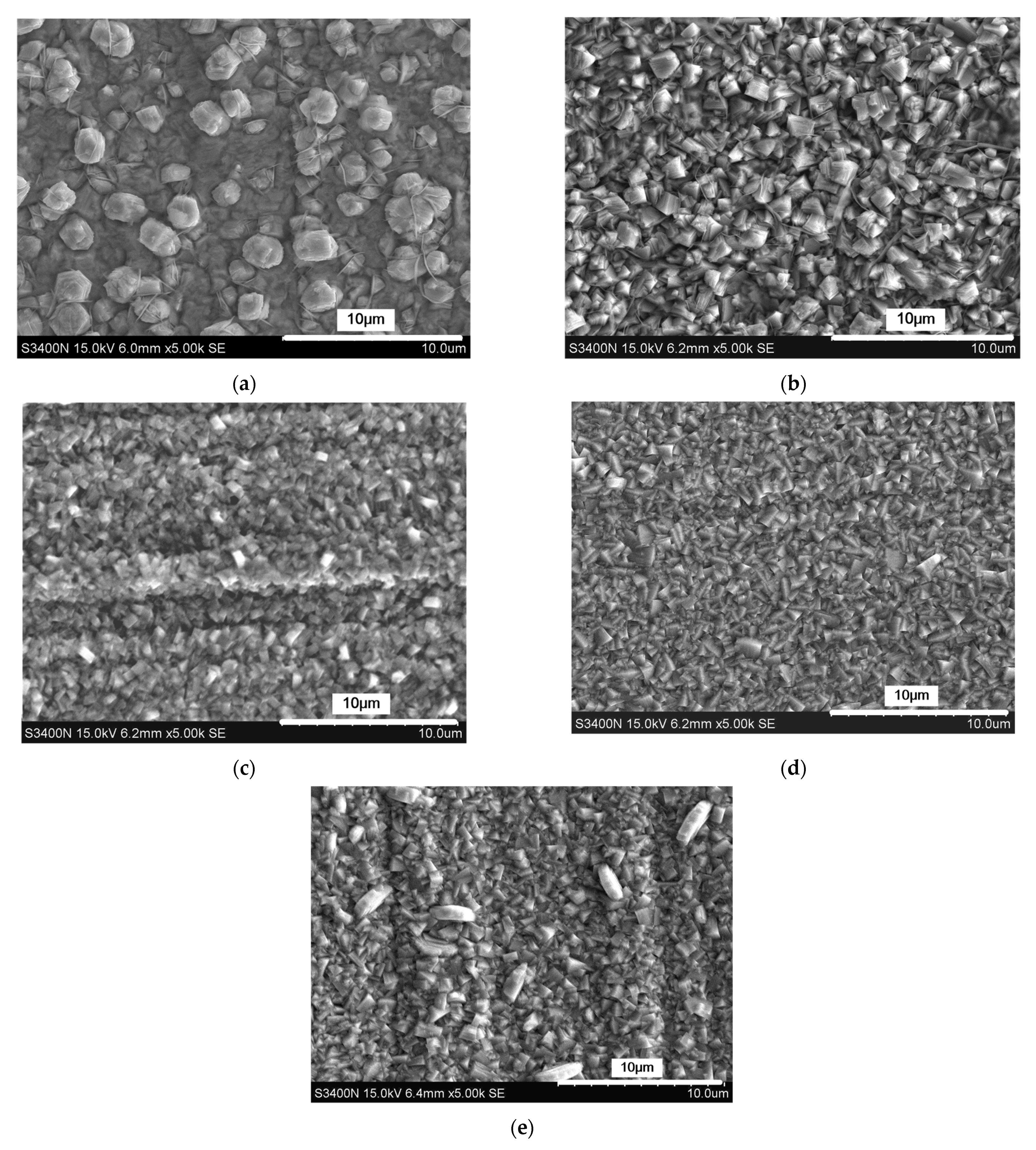
3.1. The Effect of Cationic Degree of CPAM on the Deposition Process
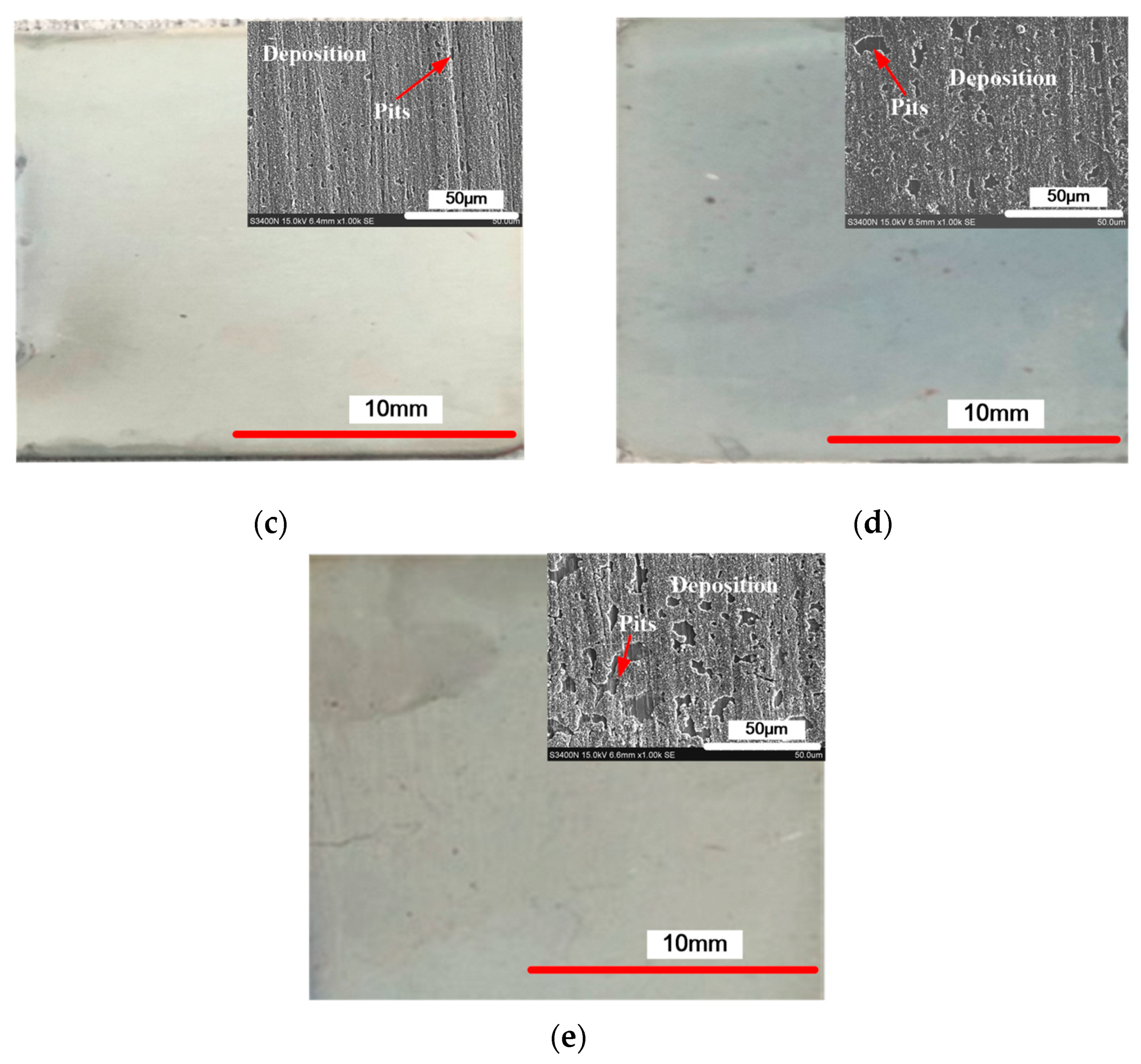
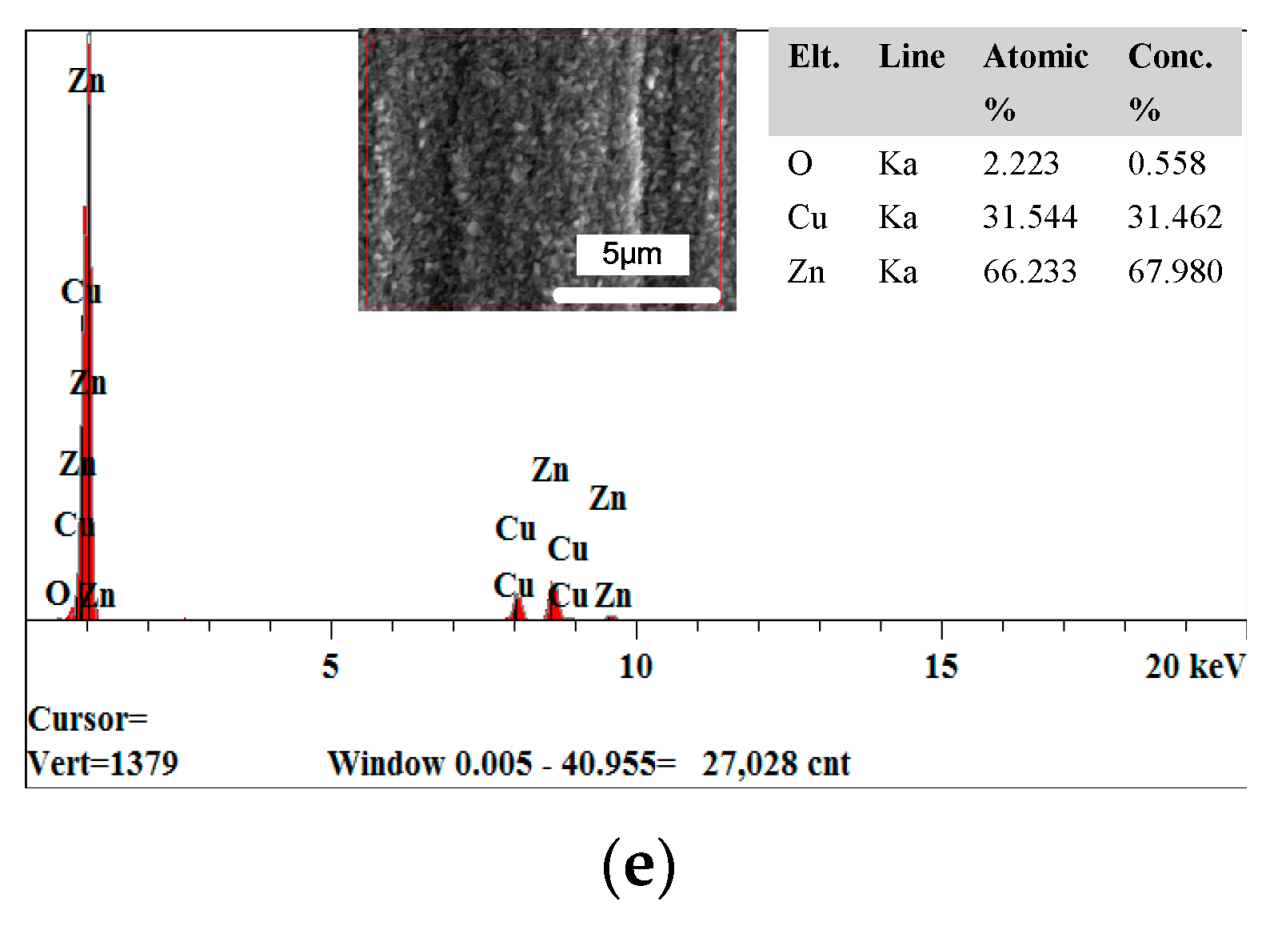
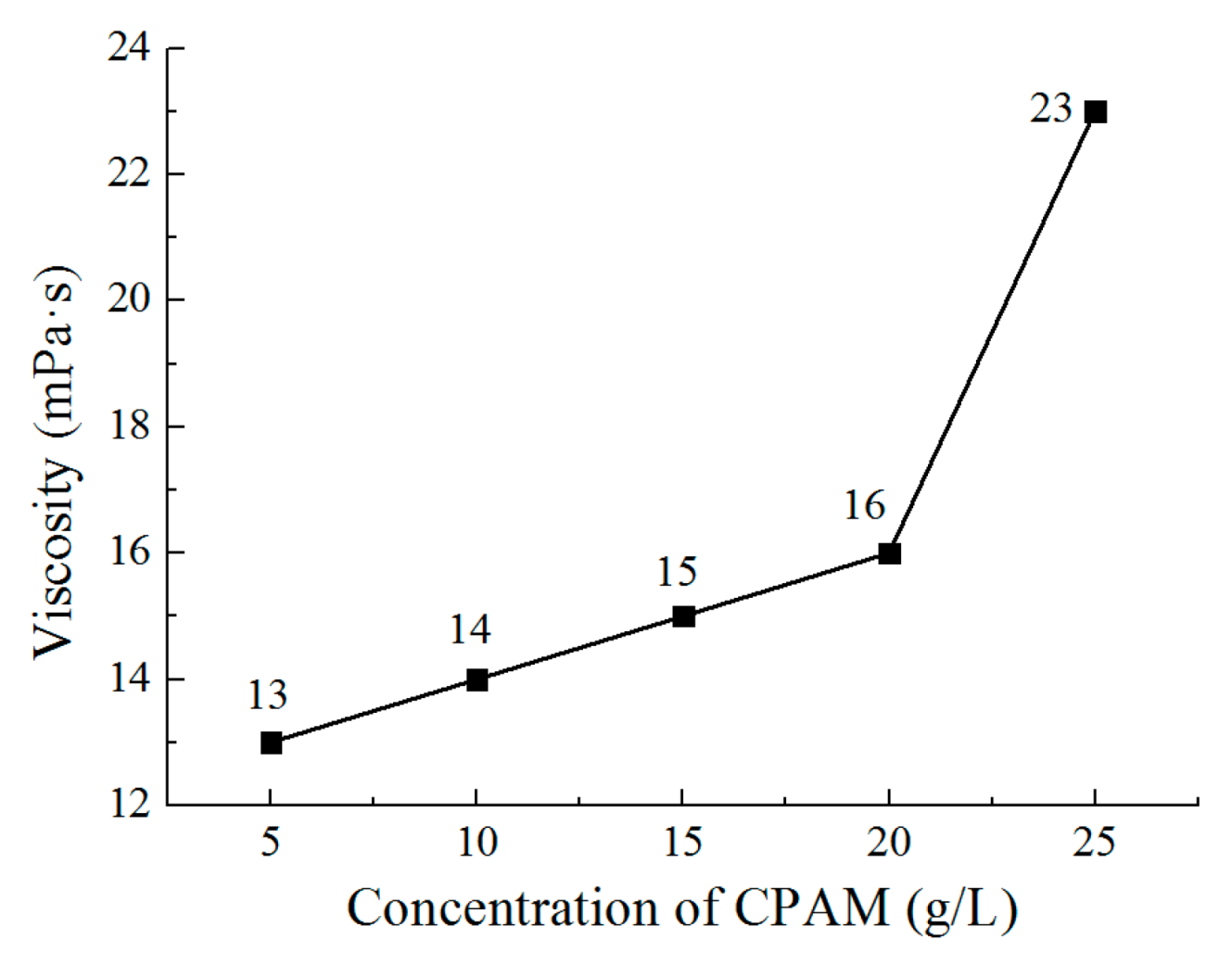
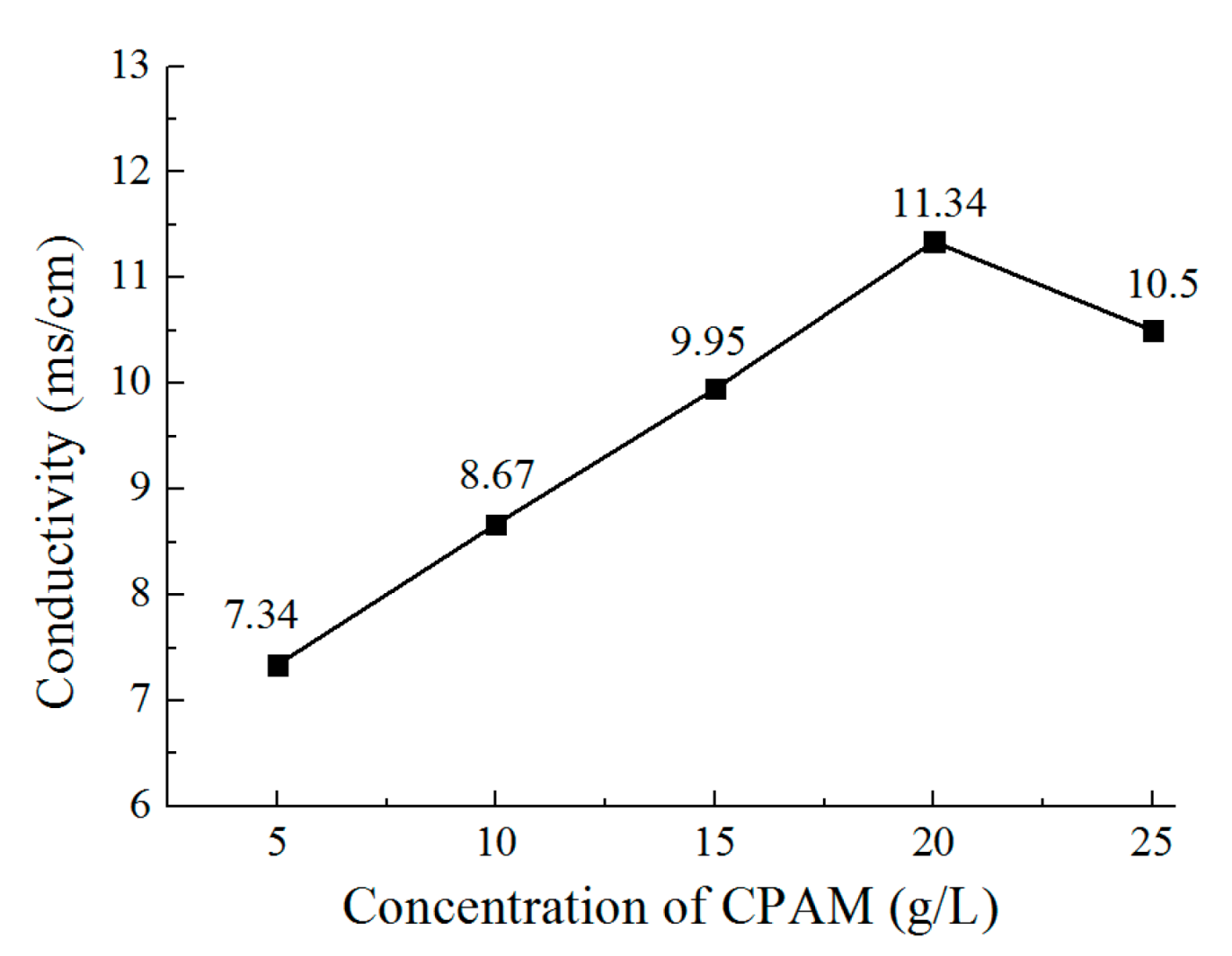
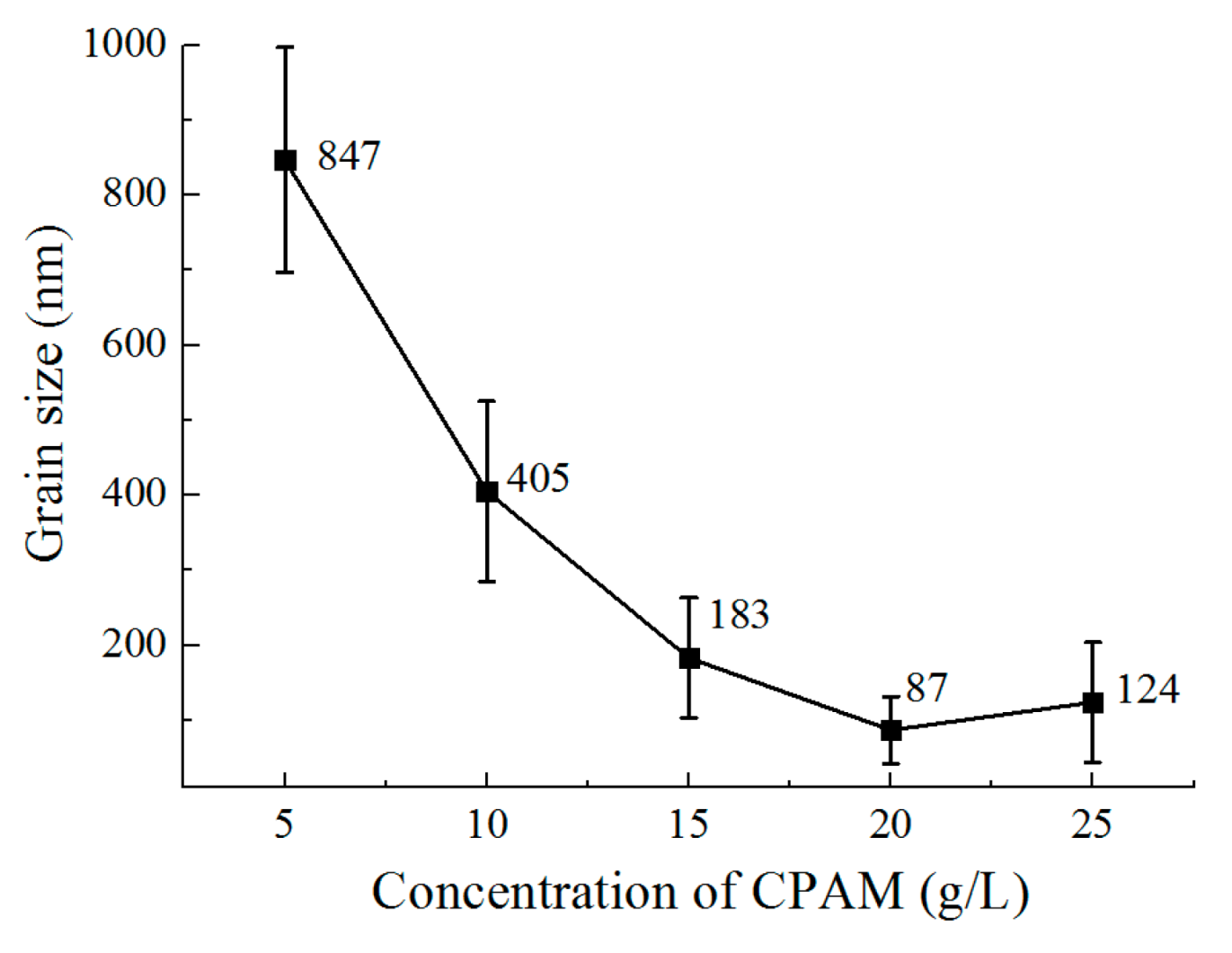
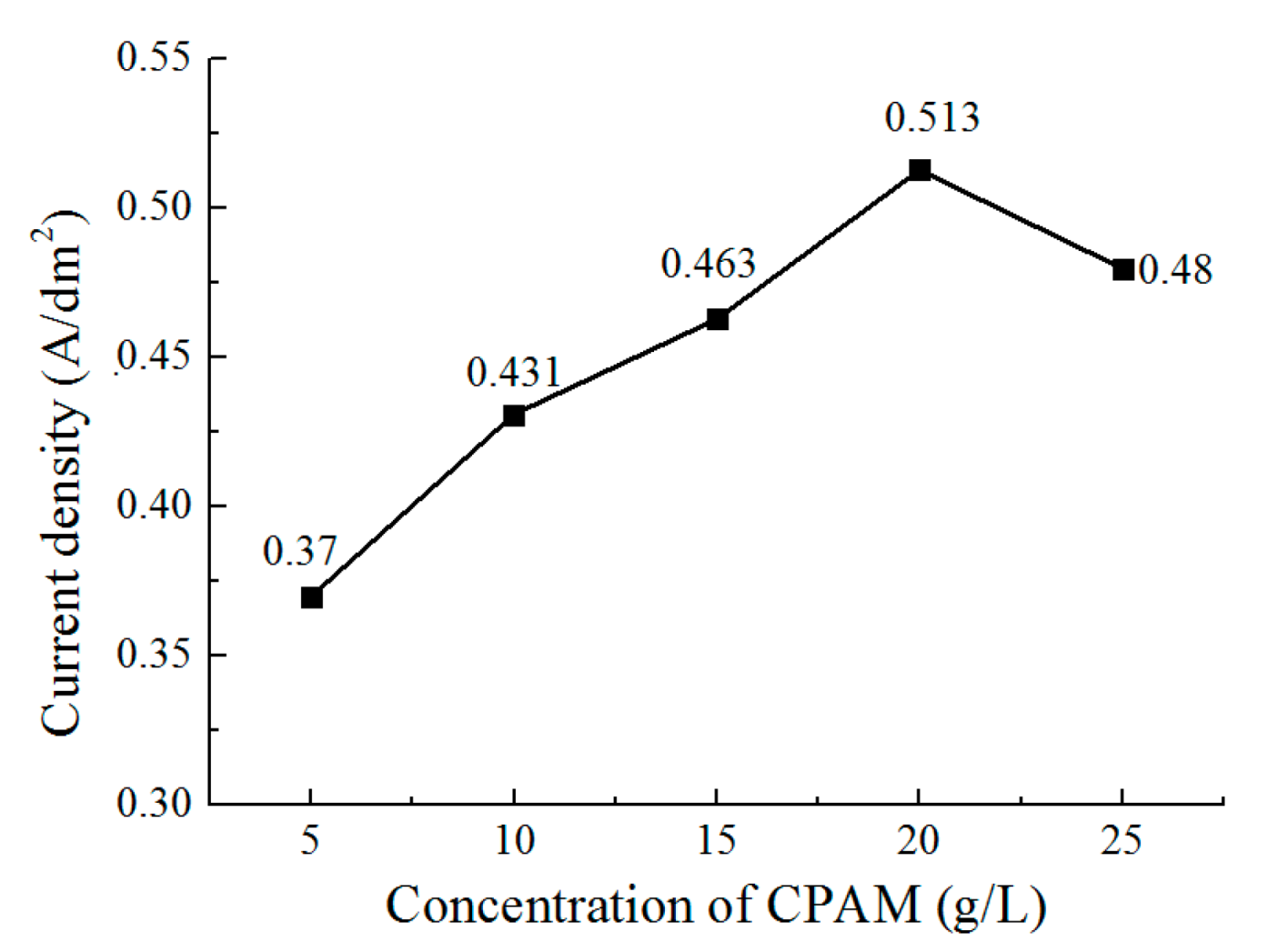
3.2. The Effect of the Concentration of CPAM on the Deposition Quality
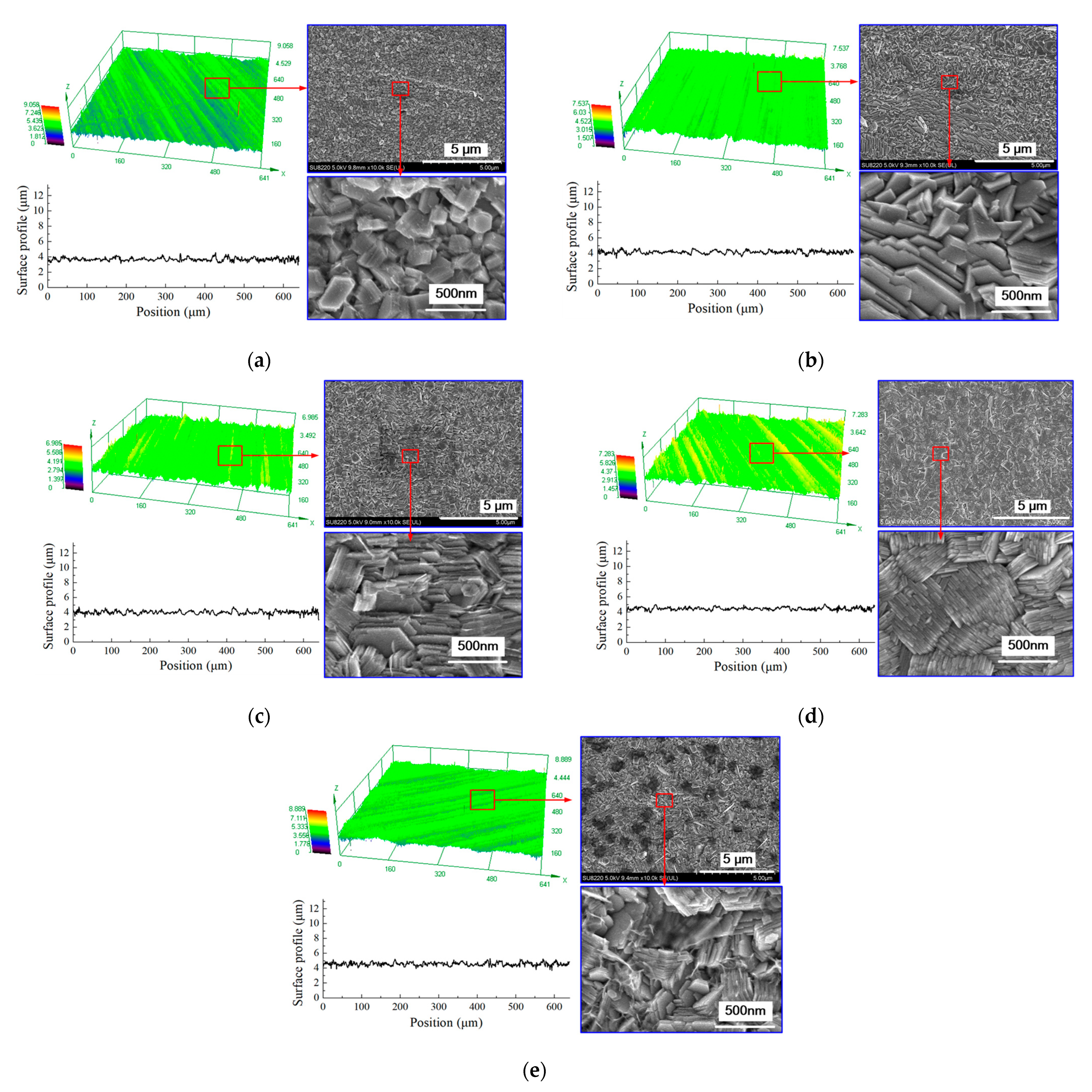
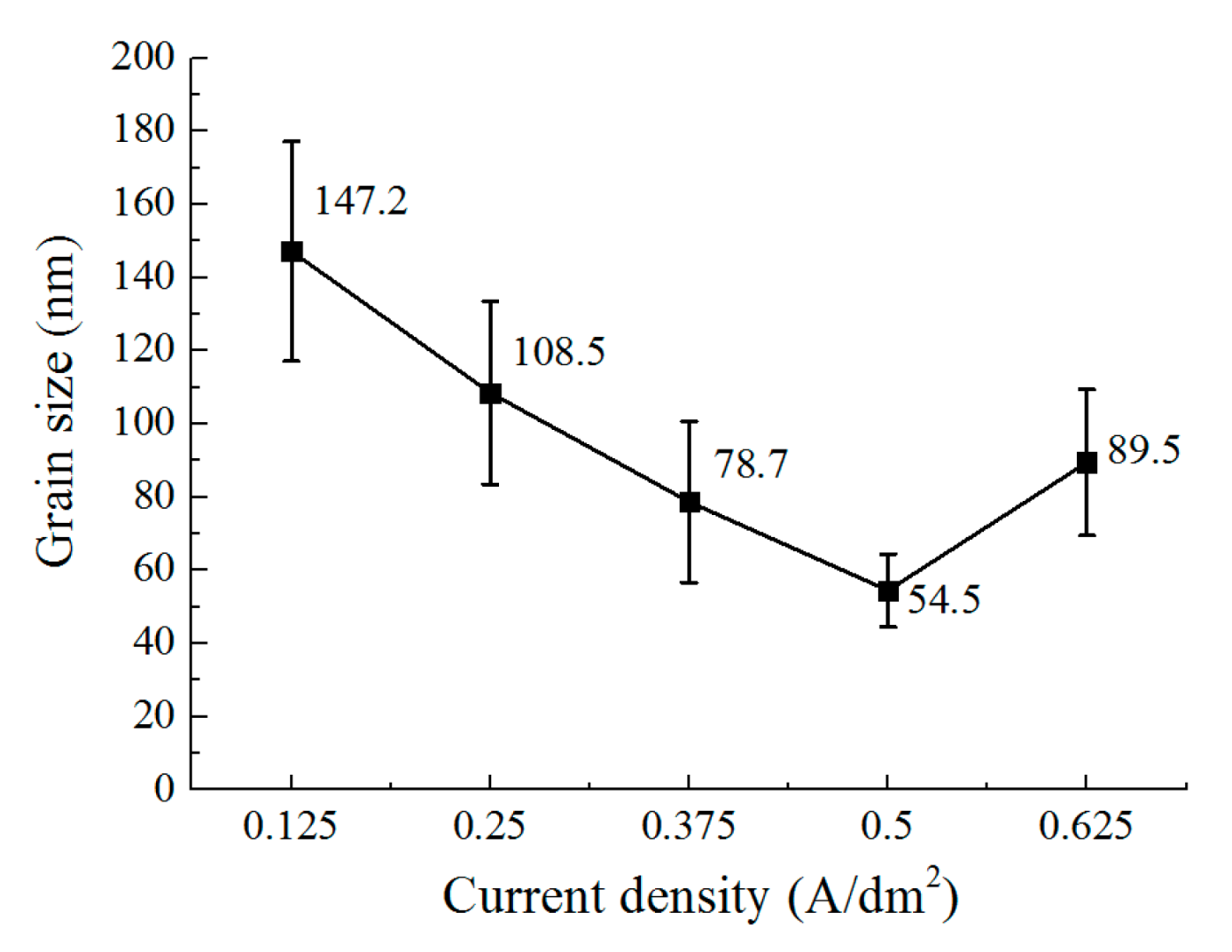
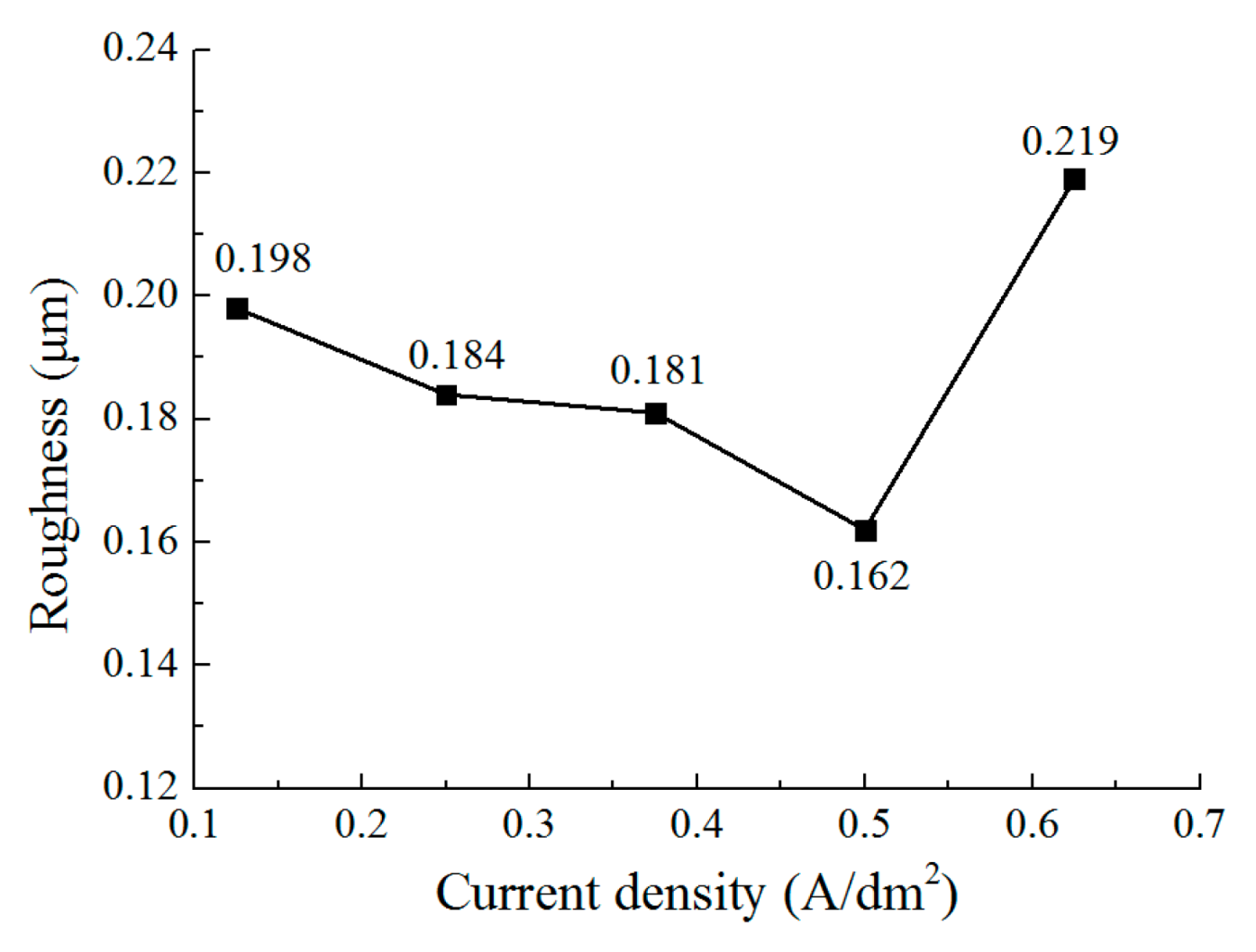
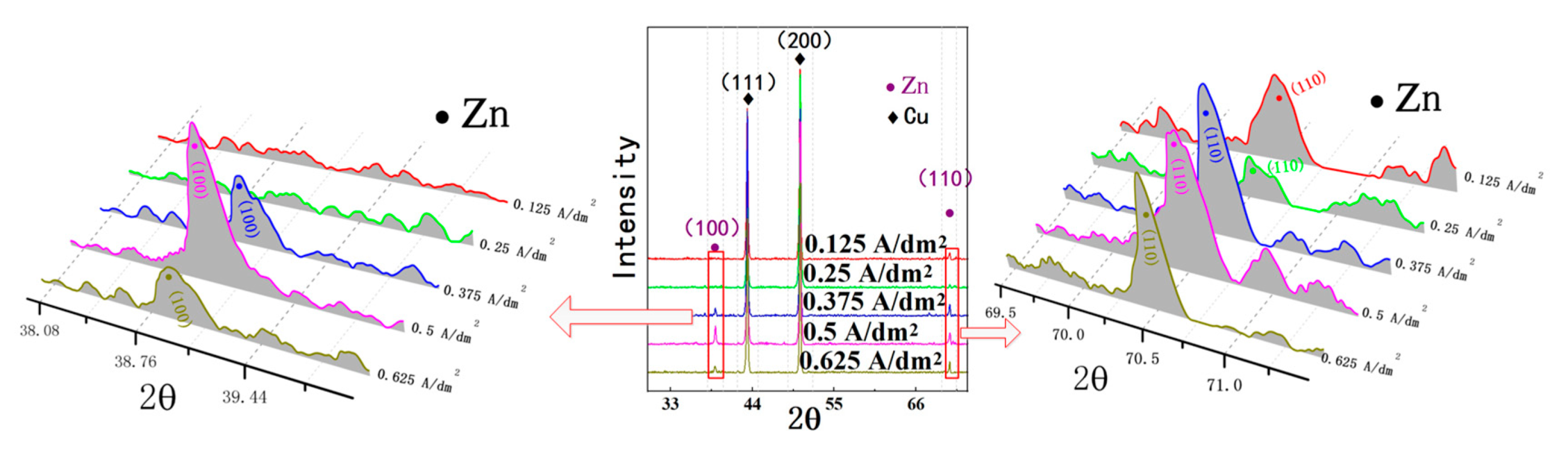
3.3. The Effect of Current Density on the Deposition Quality
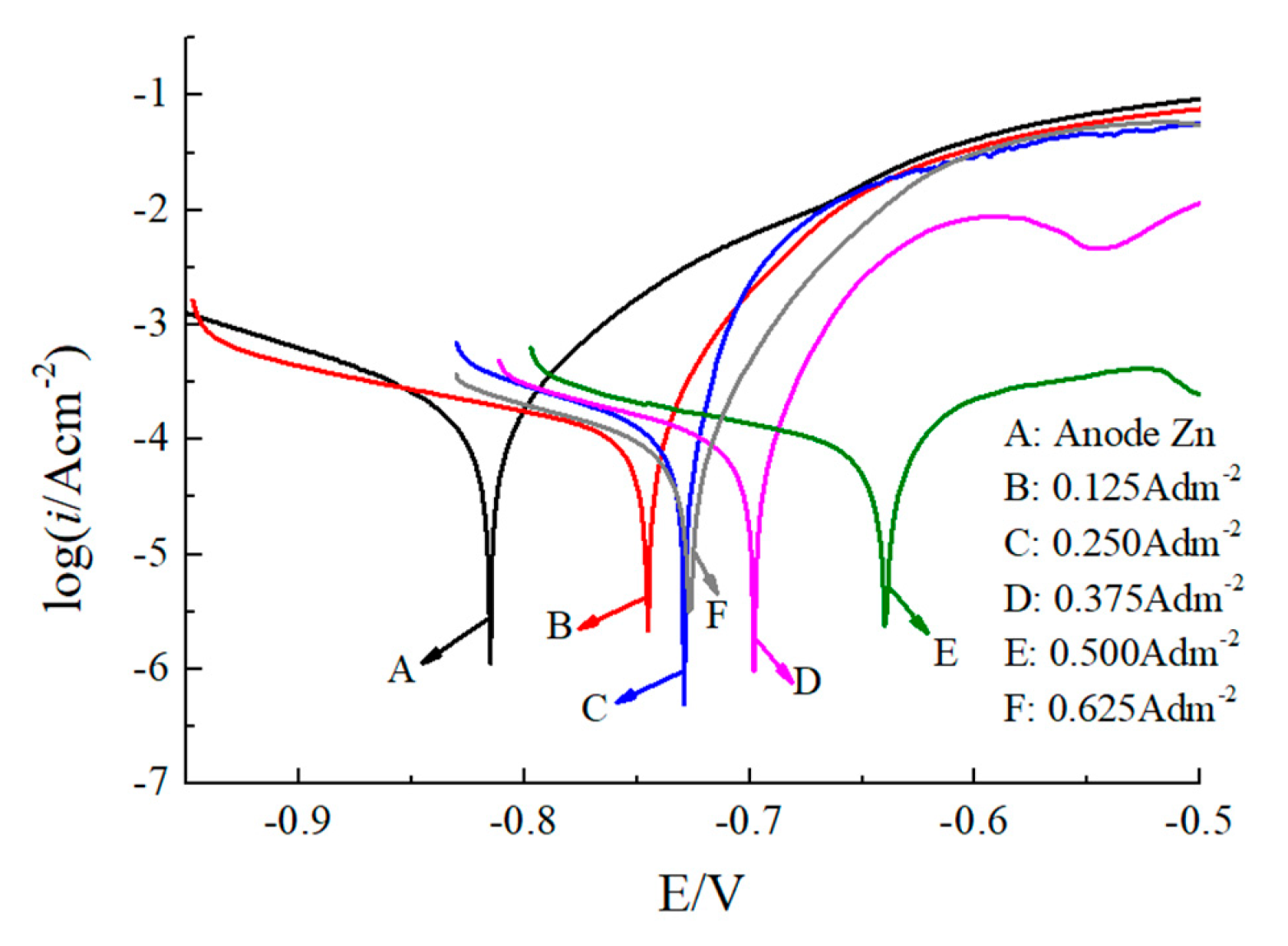
3.4. Tafel Polarization Curves
4. Conclusions
- Compared to the electrolyte without CPAM, the CPAM–ZnSO4 electrolyte enables the electrochemical deposition of zinc at the low voltage of 0.3 V. The cationic degree of CPAM has a significant influence on the deposition process, and compared to other cationic degrees of CPAM, the cationic degree of 20% enhances the electrolyte conductivity as well as the current density, which improves the deposition density.
- The concentration of CPAM affects the electrolyte viscosity and conductivity. Compared to other concentrations, the concentration of 20 g/L was observed to improve the electrolyte conductivity and to maintain the viscosity at a low value at the same time. A bright deposited coating with a grain size of 87 nm could be obtained at this concentration, which is better than the grain size observed with other concentrations.
- The current density affects the grain structure of the deposited coating and the surface roughness. When the current density is increased, the grain structure changes from a blocky grain shape to a lamellar grain shape, and the grain size is also refined at the same time. With the current density of 0.5 A/dm2, a dense coating was deposited, the size of the lamellar grain was 54.5 nm, and the surface roughness was reduced to 0.162 μm.
- The Tafel polarization curves show that with a current density of 0.5 A/dm2, the Ecorr increased to −0.64 V, and the icorr reduced to 21.18 μA/cm2. The corrosion resistant property of the deposited coating also improved.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naik, Y.A.; Venkatesha, T.V. A new condensation product for zinc plating from non-cyanide alkaline bath. Bull. Mater. Sci. 2005, 28, 495–501. [Google Scholar] [CrossRef]
- Muralidhara, H.B.; Naik, Y.A.; Venkatesha, T.V. Effect of condensation product of glycyl-glycine and furfural on electrodeposition of zinc from sulphate bath. Bull. Mater. Sci. 2006, 29, 497–503. [Google Scholar] [CrossRef]
- Chung, P.P.; Wang, J.; Durandet, Y. Deposition processes and properties of coatings on steel fasteners—A review. Friction 2019, 7, 389–416. [Google Scholar] [CrossRef] [Green Version]
- Su, H.Y.; Lin, C.S. Effect of additives on the properties of phosphate conversion coating on electrogalvanized steel sheet. Corros. Sci. 2014, 83, 137–146. [Google Scholar] [CrossRef]
- Li, Q.Y.; Zhong, B.; Liu, L.H.; Sun, J.; Qu, Y.T.; Li, F.H.; An, M.Z. Research on the tribological behavior of a nanocrystalline zinc coating prepared by pulse reverse electrodeposition. RSC Adv. 2015, 5, 12025–12033. [Google Scholar] [CrossRef]
- Youssef, K.M.S.; Koch, C.C.; Fedkiw, P.S. Improved corrosion behavior of nanocrystalline zinc produced by pulse-current electrodeposition. Corros. Sci. 2004, 46, 51–64. [Google Scholar] [CrossRef]
- Zhu, Z.M.; Wang, Z.X.; Chu, R.B. Common problems and bath maintenance of potassium chloride zinc plating. Electroplat. Finish. 2017, 36, 62–64. [Google Scholar]
- Zhan, Z.W.; Zhang, Q.; Wang, S.X.; Liu, X.; Sun, Z.H.; Zhang, K.; Du, N.; Shu, W.F. Effect of Additives on Electrodeposition of Zinc from Alkaline Zincate Solution and Their Synergy Mechanism. Int. J. Electrochem. Sci. 2021, 16, 210334. [Google Scholar] [CrossRef]
- Polyakov, N.A.; Botryakova, I.G.; Glukhov, V.G.; Red’kina, G.V.; Kuznetsov, Y.I. Formation and anticorrosion properties of superhydrophobic zinc coatings on steel. Chem. Eng. J. 2021, 421, 127775. [Google Scholar] [CrossRef]
- Ramanauskas, R.; Gudaviciute, L.; Juskenas, R.; Scit, O. Tructural and corrosion characterization of pulse plated nanocrystalline zinc coatings. Electrochim. Acta 2007, 53, 1801–1810. [Google Scholar] [CrossRef]
- Kenta, F.; Satoshi, O.; Yoshiharu, K.; Shinya, A.; Tomio, T.; Hiroaki, N. Effect of Organic Additives on the Electrodeposition Behavior of Zn from an Alkaline Zincate Solution and Its Microstructure. Mater. Trans. 2020, 61, 497–505. [Google Scholar]
- Saber, K.; Koch, C.C.; Fedkiw, P.S. Pulse current electrodeposition of nanocrystalline zinc. Mater. Sci. Eng. A 2003, 341, 174–181. [Google Scholar] [CrossRef]
- Muralidhara, H.B.; Naik, Y.A. Electrochemical deposition of nanocrystalline zinc on steel substrate from acid zincate bath. Surf. Coat. Technol. 2008, 202, 3403–3412. [Google Scholar] [CrossRef]
- Meng, G.Z.; Zhang, L.; Shao, Y.W.; Zhang, T.; Wang, F.H. Study of the electrochemical behaviour of nanocrystalline zinc by statistical methods. Corros. Sci. 2009, 51, 1685–1689. [Google Scholar] [CrossRef]
- Nayana, K.O.; Venkatesha, T.V. Bright zinc electrodeposition and study of influence of synergistic interaction of additives on coating properties. J. Ind. Eng. Chem. 2015, 26, 107–115. [Google Scholar] [CrossRef]
- Li, Q.Y.; Ge, W.; Yang, P.X.; Zhang, J.Q.; An, M.Z. Insight into the Role and Its Mechanism of Polyacrylamide as an Additive in Sulfate Electrolytes for Nanocrystalline Zinc Electrodeposition. J. Electrochem. Soc. 2016, 163, D127–D132. [Google Scholar] [CrossRef] [Green Version]
- Onkarappa, N.K.; Satyanarayana, J.; Suresh, H.; Malingappa, P. Influence of additives on morphology, orientation and anti-corrosion property of bright zinc electrodeposit. Surf. Coat. Technol. 2020, 397, 126062. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| Applied voltage | 0.3 V |
| Current density | 0.125, 0.25, 0.375, 0.5, 0.625 A/dm2 |
| Cationic degree of CPAM | 0, 20%, 40%, 60% |
| Concentration of CPAM | 5, 10, 15, 20, 25 g/L |
| Concentration of ZnSO4·7H2O | 40 g/L |
| Deposition time, | 10 min |
| Temperature | 25 °C |
| Stirring rate | 200 r/min |
| No. | Current Density (A/dm2) | Ecorr (V) | icorr (μA/cm2) | v (g/(m2·h)) |
|---|---|---|---|---|
| A | Anode Zn | −0.82 | 39.46 | 0.48 |
| B | 0.125 | −0.74 | 34.46 | 0.42 |
| C | 0.25 | −0.725 | 29.22 | 0.36 |
| D | 0.375 | −0.69 | 24.34 | 0.30 |
| E | 0.5 | −0.64 | 21.18 | 0.26 |
| F | 0.625 | −0.72 | 28.25 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Chen, J.; Zhu, J.; Cai, T.; Guo, Z. Investigation on the Electrochemical Deposition of Nanocrystalline Zinc with Cationic Polyacrylamide (CPAM)-ZnSO4 Electrolyte. Micromachines 2021, 12, 1120. https://doi.org/10.3390/mi12091120
Chen X, Chen J, Zhu J, Cai T, Guo Z. Investigation on the Electrochemical Deposition of Nanocrystalline Zinc with Cationic Polyacrylamide (CPAM)-ZnSO4 Electrolyte. Micromachines. 2021; 12(9):1120. https://doi.org/10.3390/mi12091120
Chicago/Turabian StyleChen, Xiaolei, Jiasen Chen, Jiajun Zhu, Tianyu Cai, and Zhongning Guo. 2021. "Investigation on the Electrochemical Deposition of Nanocrystalline Zinc with Cationic Polyacrylamide (CPAM)-ZnSO4 Electrolyte" Micromachines 12, no. 9: 1120. https://doi.org/10.3390/mi12091120
APA StyleChen, X., Chen, J., Zhu, J., Cai, T., & Guo, Z. (2021). Investigation on the Electrochemical Deposition of Nanocrystalline Zinc with Cationic Polyacrylamide (CPAM)-ZnSO4 Electrolyte. Micromachines, 12(9), 1120. https://doi.org/10.3390/mi12091120





