Integrated Microfluidic-Based Platforms for On-Site Detection and Quantification of Infectious Pathogens: Towards On-Site Medical Translation of SARS-CoV-2 Diagnostic Platforms
Abstract
:1. Introduction
2. Integrated Microfluidic-Based Platforms (IMPs)
2.1. Classification of IMPs
2.2. Current Research Trends Guiding the Development of IMPs
2.3. Criteria for Assessing IMPs
2.3.1. WHO On-Site Diagnostic Device Standards
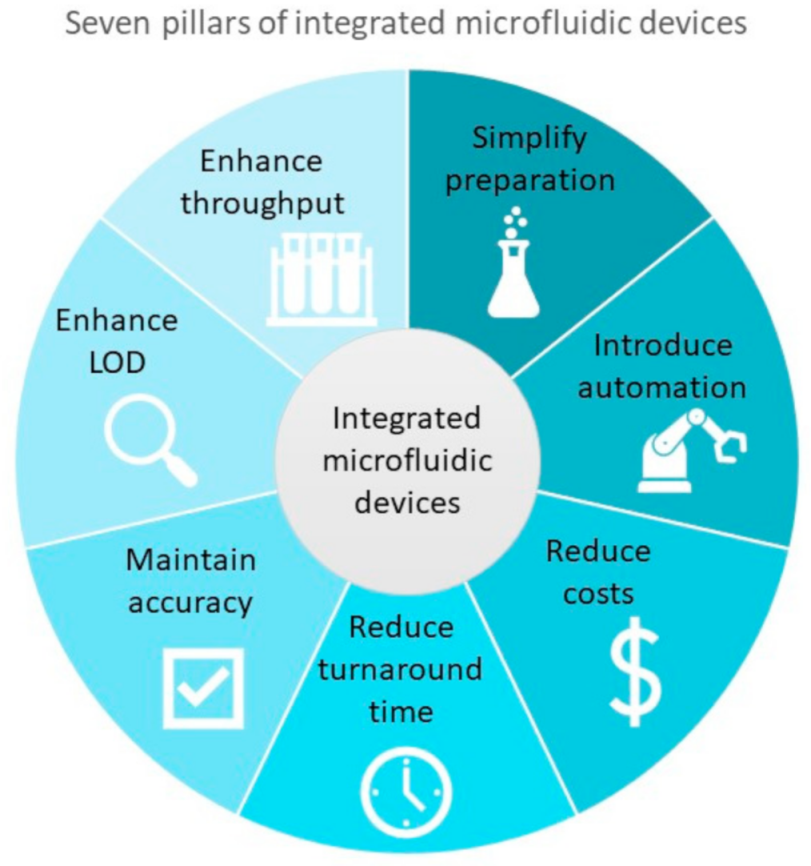
2.3.2. Pillars for Assessing Effective On-Site Diagnostic Tools
2.4. Advantages to Microfluidic Integration
2.4.1. On-Device Sample Preparation
2.4.2. Increased Automation
2.4.3. Cost Reduction
2.4.4. Shortened Turnaround Time
2.4.5. Maintaining the Level of Accuracy Seen in Conventional Counterparts
2.4.6. Improving the Limit of Detection (LOD) of Assays
2.4.7. Integrating High-Throughput Assays
3. Using IMPs in the On-Site Detection of Infectious Pathogens
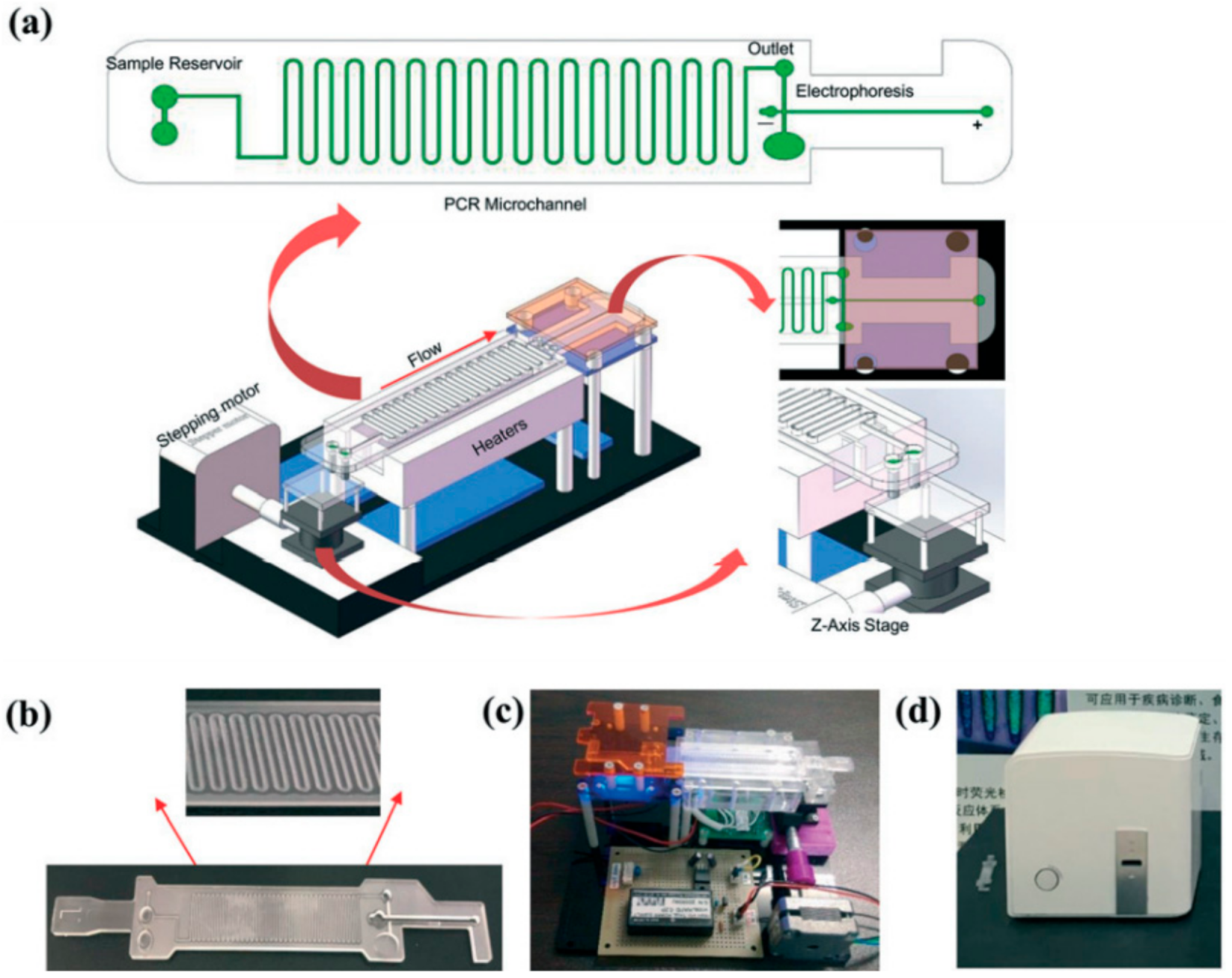
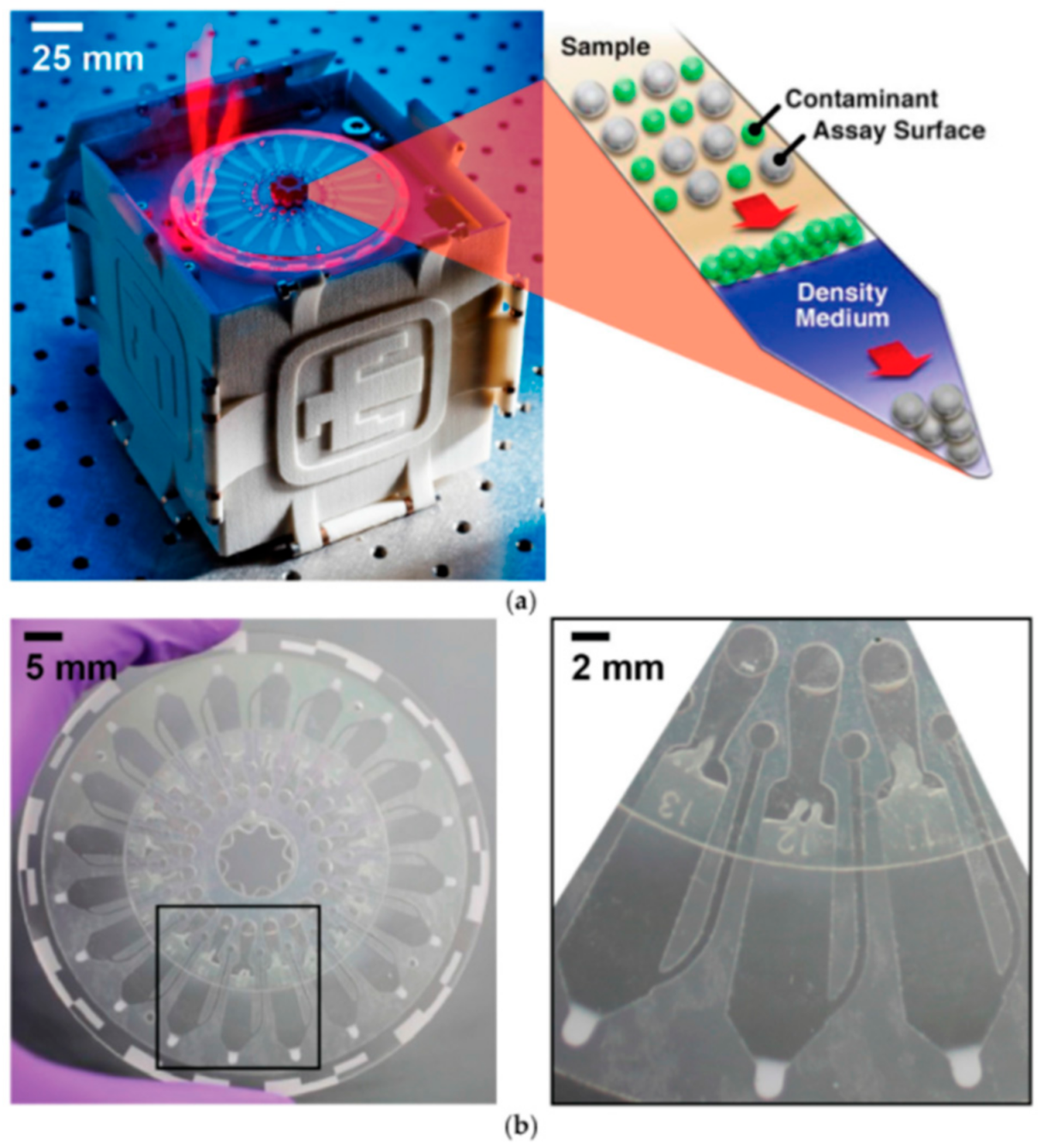
3.1. Partially Integrated IMPs in the On-Site Detection of Infectious Pathogens
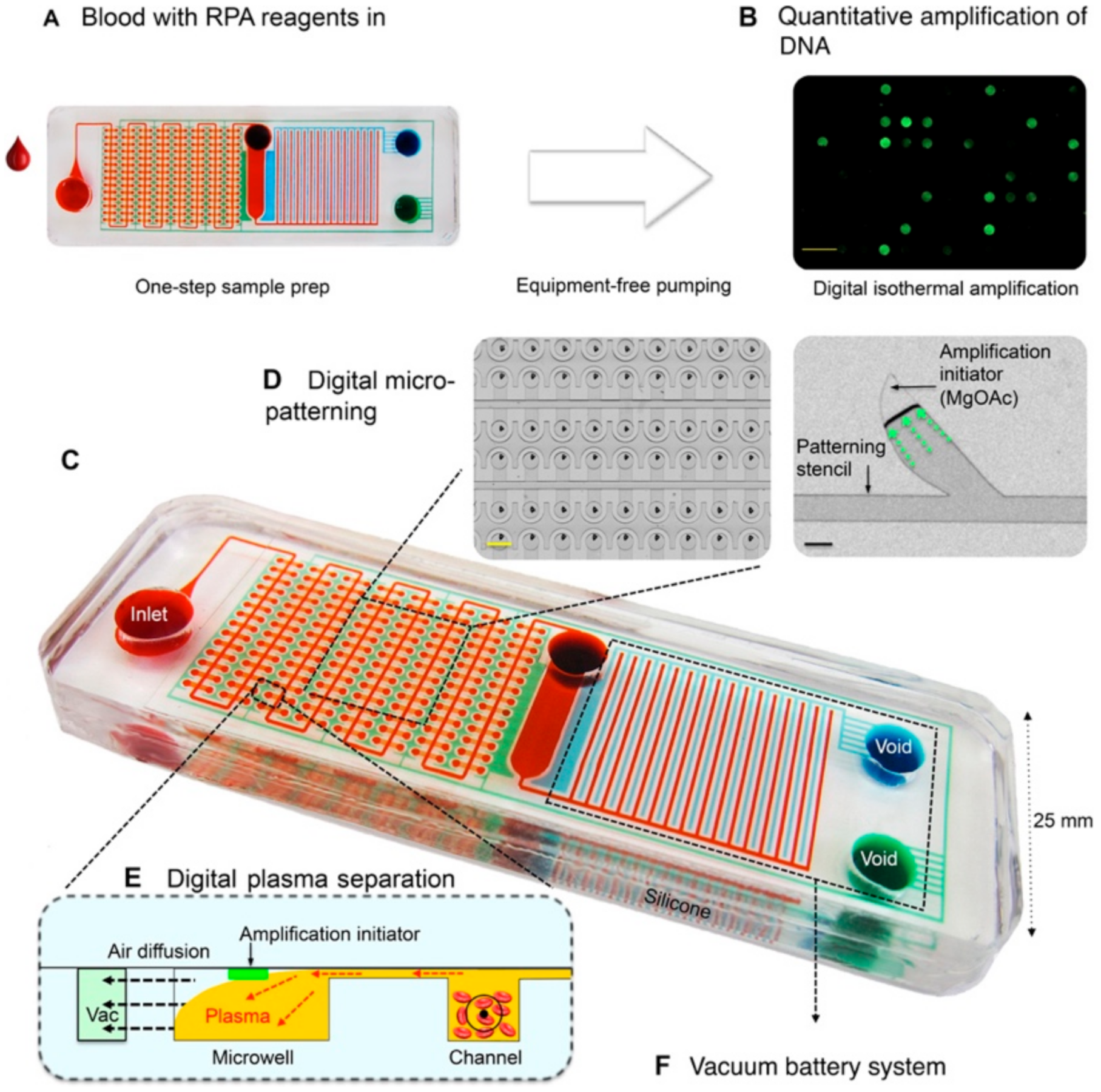
3.2. Fully Integrated IMPs Developed for the Detection of Infectious Pathogens
4. Clinical Translation of Integrated Microfluidic Devices for Detection and Quantification of SARS-CoV-2
Stage-Wise Implementation of Microfluidic SARS-CoV-2 Detection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Institute for Health Metrics and Evaluation (IHME). COVID-19 Results Briefing: Global; IHME, University of Washington: Seattle, WA, USA, 2021; Available online: https://www.healthdata.org/covid/updates (accessed on 23 August 2021).
- Meyerowitz-Katz, G.; Merone, L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int. J. Infect. Dis. 2020, 101, 138–148. [Google Scholar] [CrossRef] [PubMed]
- WHO. COVID-19 Weekly Epidemiological Update: 23 August 2021; World Health Organization: Geneva, Switzerland, 2021; Volume 41, pp. 1–31. [Google Scholar]
- Shpichka, A.; Bikmulina, P.; Peshkova, M.; Kosheleva, N.; Zurina, I.; Zahmatkesh, E.; Khoshdel-Rad, N.; Lipina, M.; Golubeva, E.; Butnaru, D.; et al. Engineering a Model to Study Viral Infections: Bioprinting, Microfluidics, and Organoids to Defeat Coronavirus Disease 2019 (COVID-19). Int. J. Bioprint. 2020, 6, 302. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Ranjan, P.; Sanghi, S.K.; Srivastava, A.K.; Khan, R. Point-of-Care Biosensor Based Diagnosis of COVID-19 Holds Promise to Combat Current and Future Pandemics. ACS Appl. Bio Mater. 2020, 3, 7326–7343. [Google Scholar] [CrossRef]
- Xie, X.; Gjorgjieva, T.; Attieh, Z.; Dieng, M.M.; Arnoux, M.; Khair, M.; Moussa, Y.; Al Jallaf, F.; Rahiman, N.; Jackson, C.A.; et al. Microfluidic Nano-Scale qPCR Enables Ultra-Sensitive and Quantitative Detection of SARS-CoV-2. Processes 2020, 8, 1425. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, H.; Xu, Y.; Lassakova, S.; Korabecna, M.; Neuzil, P. PCR, past, present and future. BioTechniques 2020, 69, 317–325. [Google Scholar] [CrossRef]
- Chittoor-Vinod, V. Molecular Biology of PCR Testing for COVID-19 Diagnostics; IntechOpen, Stanford University, Palo Alto: Santa Clara, CA, USA, 2021. [Google Scholar]
- Kulkarni, M.B.; Goel, S. Advances in continuous-flow based microfluidic PCR-devices—A review. Eng. Res. Express 2020, 2, 042001. [Google Scholar] [CrossRef]
- Tan, X.; Krel, M.; Dolgov, E.; Park, S.; Li, X.; Wu, W.; Sun, Y.; Zhang, J.; Khaing Oo, M.K.; Perlin, D.S.; et al. Rapid and quantitative detection of SARS-CoV-2 specific IgG for convalescent serum evaluation. Biosens. Bioelectron. 2020, 169, 112572. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lee, J.-S. Recent Developments in Affinity-Based Selection of Aptamers for Binding Disease-Related Protein Targets. Chem. Pap. 2019, 73, 2637–2653. [Google Scholar] [CrossRef]
- Swank, Z.; Michielin, G.; Yip, H.M.; Cohen, P.; Andrey, D.O.; Vuilleumier, N.; Kaiser, L.; Eckerle, I.; Meyer, B.; Maerkl, S.J. A High-Throughput Microfluidic Nanoimmunoassay for Detecting Anti–SARS-CoV-2 Antibodies in Serum or Ultralow-Volume Blood Samples. Proc. Natl. Acad. Sci. USA 2021, 118, e2025289118. [Google Scholar] [CrossRef] [PubMed]
- Tayyab, M.; Sami, A.S.; Raji, H.; Mushnoori, S.; Javanmard, M. Potential Microfluidic Devices for COVID-19 Antibody Detection at Point-of-Care (POC): A Review. IEEE Sens. J. 2021, 21, 4007–4017. [Google Scholar] [CrossRef]
- Basso, D.; Aita, A.; Padoan, A.; Cosma, C.; Navaglia, F.; Moz, S.; Contran, N.; Zambon, C.; Cattelan, A.M.; Plebani, M. Salivary SARS-CoV-2 antigen rapid detection: A prospective cohort study. Clin. Chim. Acta 2021, 517, 54–59. [Google Scholar] [CrossRef]
- Chang, K.; Li, J.; Yang, C.; Shiesh, S.; Lee, G. An integrated microfluidic system for measurement of glycated hemoglobin levels by using an aptamer-antibody assay on magnetic beads. Biosens. Bioelectron. 2015, 68, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.; Kumar Sen, R.; Dwivedi, N.; Khan, R.; Solanki, P.R.; Kumar Srivastava, A.; Dhand, C. 3D-Printed Microfluidics and Potential Biomedical Applications. Front. Nanotechnol. 2021, 3, 609355. [Google Scholar] [CrossRef]
- Saito, M.; Uchida, N.; Furutani, S.; Murahashi, M.; Espulgar, W.; Nagatani, N.; Nagai, H.; Inoue, Y.; Ikeuchi, T.; Kondo, S.; et al. Field-deployable rapid multiple biosensing system for detection of chemical and biological warfare agents. Microsyst. Nanoeng. 2018, 4, 17083. [Google Scholar] [CrossRef]
- Ramachandran, A.; Huyke, D.A.; Sharma, E.; Shaoo, M.K.; Huang, C.; Banaei, N.; Pinsky, B.; Santiago, J.G. Electric field-driven microfluidics for rapid CRISPR-based diagnostics and its application to detection of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 29518–29525. [Google Scholar] [CrossRef]
- Kim, H.; Abbas, N.; Shin, S. A rapid diagnosis of SARS-CoV-2 using DNA hydrogel formation on microfluidic pores. Biosens. Bioelectron. 2021, 177, 113005. [Google Scholar] [CrossRef]
- Berkenbrock, J.A.; Grecco-Machado, R.; Achenbach, S. Microfluidic devices for the detection of viruses: Aspects of emergency fabrication during the COVID-19 pandemic and other outbreaks. Proc. R. Soc. A Math. Phys. Eng. Sci. 2020, 476, 20200398. [Google Scholar] [CrossRef]
- Protti, M.; Mandrioli, R.; Mercolini, L. Quantitative microsampling for bioanalytical applications related to the SARS-CoV-2 pandemic: Usefulness, benefits and pitfalls. J. Pharm. Biomed. Anal. 2020, 191, 113597. [Google Scholar] [CrossRef]
- Tymm, C.; Zhou, J.; Tadimety, A.; Burklund, A.; Zhang, J.X.J. Scalable COVID-19 Detection Enabled by Lab-on-Chip Biosensors. Cell. Mol. Bioeng. 2020, 13, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.A.; Quandelacy, T.M.; Kada, S.; Prasad, P.V.; Steele, M.; Brooks, J.T.; Slayton, R.B.; Biggerstaff, M.; Butler, J.C. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Netw. Open 2021, 4, e2035057. [Google Scholar] [CrossRef] [PubMed]
- Oishee, M.J.; Ali, T.; Jahan, N.; Khandker, S.S.; Haq, M.A.; Khondoker, M.U.; Sil, B.K.; Lugova, H.; Krishnapillai, A.; Abubakar, A.R.; et al. COVID-19 Pandemic: Review of Contemporary and Forthcoming Detection Tools. Infect. Drug Resist. 2021, 14, 1049. [Google Scholar] [CrossRef]
- Sivaramakrishnan, M.; Kothandan, R.; Govindarajan, D.K.; Meganathan, Y.; Kandaswamy, K. Active microfluidic systems for cell sorting and separation. Curr. Opin. Biomed. Eng. 2019, 13, 60–68. [Google Scholar] [CrossRef]
- Sahore, V.; Sonker, M.; Nielsen, A.V.; Knob, R.; Kumar, S.; Woolley, A.T. Automated microfluidic devices integrating solid-phase extraction, fluorescent labeling, and microchip electrophoresis for preterm birth biomarker analysis. Anal. Bioanal. Chem. 2017, 410, 933–941. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Issadore, D.; Mitchell, M.J. Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials 2021, 274, 120826. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Suo, Y.; Zou, Z.; Sun, J.; Zhang, S.; Wang, B.; Xu, Y.; Darland, D.; Zhao, J.X.; Mu, Y. Integrated microfluidic systems with sample preparation and nucleic acid amplification. Lab Chip 2019, 19, 2769. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Liu, C.; Deng, J.; Han, Z.; Zhang, L.; Chen, Q.; Sun, J. A fully automated centrifugal microfluidic system for sample-to-answer viral nucleic acid testing. Sci. China Ser. B Chem. 2020, 63, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Jokhun, D.S.; Lim, C.T. Microfluidic detection of human diseases: From liquid biopsy to COVID-19 diagnosis. J. Biomech. 2021, 117, 110235. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, T.S.; Garstecki, P. Controlled droplet microfluidic systems for multistep chemical and biological assays. Chem. Soc. Rev. 2017, 46, 6210–6226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarim, E.A.; Karakuzu, B.; Oksuz, C.; Sarigil, O.; Kizilkaya, M.; Al-Ruweidi, M.K.A.A.; Yalcin, H.C.; Ozcivici, E.; Tekin, H.C. Microfluidic-based virus detection methods for respiratory diseases. Emergent Mater. 2021, 4, 143–168. [Google Scholar] [CrossRef]
- Garneret, P.; Coz, E.; Martin, E.; Manuguerra, J.; Brient-Litzler, E.; Enouf, V.; Obando, D.F.G.; Olivo-Marin, J.; Monti, F.; van der Werf, S.; et al. Performing point-of-care molecular testing for SARS-CoV-2 with RNA extraction and isothermal amplification. PLoS ONE 2021, 16, e0243712. [Google Scholar] [CrossRef]
- Li, Z.; Ju, R.; Sekine, S.; Zhang, D.; Zhuang, S.; Yamaguchi, Y. All-in-one microfluidic device for on-site diagnosis of pathogens based on an integrated continuous flow PCR and electrophoresis biochip. Lab Chip 2019, 19, 2663–2668. [Google Scholar] [CrossRef]
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A Review of Current Methods in Microfluidic Device Fabrication and Future Commercialization Prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef] [Green Version]
- Ren, K.; Zhou, J.; Wu, H. Materials for Microfluidic Chip Fabrication. Accounts Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
- Matuła, K.; Rivello, F.; Huck, W.T.S. Single-Cell Analysis Using Droplet Microfluidics. Adv. Biosyst. 2019, 4, e1900188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labarre, P.; Boyle, D.S.; Hawkins, K.; Weigl, B.H. Instrument-free nucleic acid amplification for global settings. Proc. SPIE—Int. Soc. Opt. Eng. 2011, 8029, 802902. [Google Scholar]
- Peeling, R.W.; Holmes, K.K.; Mabey, D.; Ronald, A. Rapid Tests for Sexually Transmitted Infections (STIs): The Way Forward. Sex. Transm. Infect. 2006, 82, v1–v6. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, M.; Wang, Z.; Li, S.; Deng, Y.; He, N. Point-of-care diagnostics for infectious diseases: From methods to devices. Nano Today 2021, 37, 101092. [Google Scholar] [CrossRef] [PubMed]
- Manmana, Y.; Kubo, T.; Otsuka, K. Recent developments of point-of-care (POC) testing platform for biomolecules. TrAC Trends Anal. Chem. 2020, 135, 116160. [Google Scholar] [CrossRef]
- Liu, T.-H.; You, H.-L.; Lee, M.S.; Lee, G.-B. An Integrated Microfluidic System for Bacteria Identification from Human Joint Fluids. In Proceedings of the 2018 IEEE 13th Annual International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Singapore, 22–26 April 2018; pp. 40–43. [Google Scholar]
- Ganesh, I.; Tran, B.M.; Kim, Y.; Kim, J.; Cheng, H.; Lee, N.Y.; Park, S. An Integrated Microfluidic PCR System with Immunomagnetic Nanoparticles for the Detection of Bacterial Pathogens. Biomed. Microdevices 2016, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Li, Y.; Fang, X.; Kong, J. Integrated Microfluidic Sample-to-Answer System for Direct Nucleic Acid-Based Detection of Group B Streptococci in Clinical Vaginal/Anal Swab Samples. ACS Sens. 2020, 5, 1132–1139. [Google Scholar] [CrossRef]
- Huh, Y.S.; Park, T.J.; Lee, E.Z.; Hong, W.H.; Lee, S.Y. Development of a fully integrated microfluidic system for sensing infectious viral disease. Electrophoresis 2008, 29, 2960–2969. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, R.M.; Svobodova, Z.; Bilkova, Z.; Otto, M.; Taverna, M.; Descroix, S.; Viovy, J. An integrated microfluidic chip for immunocapture, preconcentration and separation of β-amyloid peptides. Biomicrofluidics 2015, 9, 054117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Bi, H.; Liu, B.; Qiao, L. Detection of Pathogenic Microorganisms by Microfluidics Based Analytical Methods. Anal. Chem. 2018, 90, 5512–5520. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Zou, F.; Wang, Y.; Zhang, Y.; Xu, X.; Lin, X.; Tian, T.; Zhang, H.; Zhou, L.; Zhu, Z.; et al. Sensitive, Rapid, and Automated Detection of DNA Methylation Based on Digital Microfluidics. ACS Appl. Mater. Interfaces 2021, 13, 8042–8048. [Google Scholar] [CrossRef]
- Luo, L.; He, Y. Magnetically driven microfluidics for isolation of circulating tumor cells. Cancer Med. 2020, 9, 4207–4231. [Google Scholar] [CrossRef] [Green Version]
- Esmaeilsabzali, H.; Beischlag, T.V.; Cox, M.E.; Dechev, N.; Parameswaran, A.M.; Park, E.J. An integrated microfluidic chip for immunomagnetic detection and isolation of rare prostate cancer cells from blood. Biomed. Microdevices 2016, 18, 22. [Google Scholar] [CrossRef]
- Lee, T.Y.; Hyun, K.-A.; Kim, S.-I.; Jung, H.-I. An integrated microfluidic chip for one-step isolation of circulating tumor cells. Sens. Actuators B Chem. 2017, 238, 1144–1150. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, H.; Shu, W.; Tian, J.; Huang, Y.; Song, Y.; Wang, R.; Li, E.; Slamon, D.; Hou, D.; et al. An integrated microfluidic device for rapid and high-sensitivity analysis of circulating tumor cells. Sci. Rep. 2017, 7, srep42612. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.-C.; Lin, C.-S.; Lin, C.-N.; Hsu, K.-F.; Lee, G.-B. Screening aptamers targeting the cell membranes of clinical cancer tissues on an integrated microfluidic system. Sens. Actuators B Chem. 2020, 330, 129334. [Google Scholar] [CrossRef]
- Sierra, J.; Marrugo-Ramirez, J.; Rodriguez-Trujillo, R.; Mir, M.; Samitier, J. Sensor-Integrated Microfluidic Approaches for Liquid Biopsies Applications in Early Detection of Cancer. Sensors 2020, 20, 1317. [Google Scholar] [CrossRef] [Green Version]
- Kurani, N.; Pollitz, K.; Cotliar, D.; Ramirez, G.; Cox, C. COVID-19 Test Prices and Payment Policy. In Peterson-KFF Health System Tracker; New York, NY, USA; Available online: https://www.healthsystemtracker.org/brief/covid-19-test-prices-and-payment-policy/ (accessed on 23 August 2021).
- Martinez, A.W.; Phillips, S.T.; Butte, M.; Whitesides, G.M. Patterned Paper as a Platform for Inexpensive, Low-Volume, Portable Bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [Green Version]
- Scott, S.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef]
- Tenda, K.; Ota, R.; Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. High-Resolution Microfluidic Paper-Based Analytical Devices for Sub-Microliter Sample Analysis. Micromachines 2016, 7, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding Wax Printing: A Simple Micropatterning Process for Paper-Based Microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef]
- Chen, C.-S.; Breslauer, D.N.; Luna, J.I.; Grimes, A.; Chin, W.-C.; Lee, L.P.; Khine, M. Shrinky-Dink microfluidics: 3D polystyrene chips. Lab Chip 2008, 8, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.; Veigas, B.; Fernandes, A.R.; Águas, H.; Martins, R.; Fortunato, E.; Baptista, P.V. Fast Prototyping Microfluidics: Integrating Droplet Digital Lamp for Absolute Quantification of Cancer Biomarkers. Sensors 2020, 20, 1624. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, T.C. Polymerase Chain Reaction: Basic Protocol Plus Troubleshooting and Optimization Strategies. J. Vis. Exp. 2012, e3998. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, L.; Jing, W.; Liu, S.; Tachibana, H.; Cheng, X.; Sui, G. An integrated microfluidic device for rapid serodiagnosis of amebiasis. Biomicrofluidics 2013, 7, 011101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Ma, H.; Xu, F.; Wang, X.; Sun, W. Microfluidics in cardiovascular disease research: State of the art and future outlook. Microsyst. Nanoeng. 2021, 7, 1–19. [Google Scholar] [CrossRef]
- Sinha, A.; Gopinathan, P.; Chung, Y.; Shiesh, S.; Lee, G. Simultaneous detection of multiple NT-proBNP clinical samples utilizing an aptamer-based sandwich assay on an integrated microfluidic system. Lab Chip 2019, 19, 1676. [Google Scholar] [CrossRef]
- Shim, J.-U.; Ranasinghe, R.T.; Smith, C.A.; Ibrahim, S.M.; Hollfelder, F.; Huck, W.T.S.; Klenerman, D.; Abell, C. Ultrarapid Generation of Femtoliter Microfluidic Droplets for Single-Molecule-Counting Immunoassays. ACS Nano 2013, 7, 5955–5964. [Google Scholar] [CrossRef]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.C.; DeRosa, M.C.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics Integrated Biosensors: A Leading Technology towards Lab-on-a-Chip and Sensing Applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [Green Version]
- Tian, T.; Bi, Y.; Xu, X.; Zhu, Z.; Yang, C.J. Integrated paper-based microfluidic devices for point-of-care testing. Anal. Methods 2018, 10, 3567–3581. [Google Scholar] [CrossRef]
- Zhu, C.; Hu, A.; Cui, J.; Yang, K.; Zhu, X.; Liu, Y.; Deng, G.; Zhu, L. A Lab-on-a-Chip Device Integrated DNA Extraction and Solid Phase PCR Array for the Genotyping of High-Risk HPV in Clinical Samples. Micromachines 2019, 10, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Li, W.; Dai, B.; Zheng, L.; Zhang, Z.; Qi, D.; Cheng, X.; Zhang, D.; Zhuang, S. Diagnosis of mixed infections with swine viruses using and integrated microfluidic platform. Sens. Actuators B Chem. 2020, 312, 128005. [Google Scholar] [CrossRef]
- Lu, C.-Y.; Chang, L.-Y.; Chen, P.-J.; Xia, N.-S.; Shao, P.-L.; Huang, L.-M. A Highly Specific ELISA for Diagnosis of 2009 Influenza A (H1N1) Virus Infections. J. Formos. Med. Assoc. 2012, 111, 693–697. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Qu, H.; Alonso, D.G.; Yu, Z.; Yu, Y.; Shi, Y.; Hu, C.; Zhu, T.; Wu, N.; Shen, F. Portable integrated digital PCR system for the point-of-care quantification of BK virus from urine samples. Biosens. Bioelectron. 2020, 175, 112908. [Google Scholar] [CrossRef]
- Hajji, I.; Serra, M.; Geremie, L.; Ferrante, I.; Renault, R.; Viovy, J.-L.; Descroix, S.; Ferraro, D. Droplet Microfluidic Platform for Fast and Continuous-Flow RT-QPCR Analysis Devoted to Cancer Diagnosis Application. Sens. Actuators B Chem. 2020, 303, 127171. [Google Scholar] [CrossRef]
- Li, Z.; Bai, Y.; You, M.; Hu, J.; Yao, C.; Cao, L.; Xu, F. Fully integrated microfluidic devices for qualitative, quantitative and digital nucleic acids testing at point of care. Biosens. Bioelectron. 2021, 177, 112952. [Google Scholar] [CrossRef] [PubMed]
- Wippold, J.A.; Wang, H.; Tingling, J.; Leibowitz, J.L.; de Figueiredo, P.; Han, A. PRESCIENT: Platform for the rapid evaluation of antibody success using integrated microfluidics enabled technology. Lab Chip 2020, 20, 1628–1638. [Google Scholar] [CrossRef]
- WHO Zika Virus. Available online: https://www.who.int/news-room/fact-sheets/detail/zika-virus (accessed on 22 January 2021).
- Kaarj, K.; Akarapipad, P.; Yoon, J.-Y. Simpler, Faster, and Sensitive Zika Virus Assay Using Smartphone Detection of Loop-mediated Isothermal Amplification on Paper Microfluidic Chips. Sci. Rep. 2018, 8, 12438. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-Mediated Isothermal Amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [Green Version]
- Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Paper-based microfluidics: Simplified fabrication and assay methods. Sens. Actuators B Chem. 2021, 336, 129681. [Google Scholar] [CrossRef]
- Payne, S. Viruses: From Understanding to Investigation; Academic Press: Cambridge, MA, USA, 2017; Chapter 15: Family Flaviviridae; pp. 129–139. [Google Scholar]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stadler, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Thi, V.L.D.; Herbst, K.; Boerner, K.; Meurer, M.; Kremer, L.P.; Kirrmaier, D.; Freistaedter, A.; Papagiannidis, D.; Galmozzi, C.; Stanifer, M.L.; et al. A Colorimetric RT-LAMP Assay and LAMP-Sequencing for Detecting SARS-CoV-2 RNA in Clinical Samples. Sci. Transl. Med. 2020, 12, 556. [Google Scholar]
- Childhood Diseases. Available online: https://www.unicef.org/health/childhood-diseases (accessed on 24 August 2021).
- Phaneuf, C.R.; Mangadu, B.; Piccini, M.E.; Singh, A.K.; Koh, C.-Y. Rapid, Portable, Multiplexed Detection of Bacterial Pathogens Directly from Clinical Sample Matrices. Biosensors 2016, 6, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainsworth, M.; Andersson, M.; Auckland, K.; Baillie, J.K.; Barnes, E.; Beer, S.; Beveridge, A.; Bibi, S.; Blackwell, L.; Borak, M.; et al. Performance Characteristics of Five Immunoassays for SARS-CoV-2: A Head-to-Head Benchmark Comparison. Lancet Infect. Dis. 2020, 20, 1390–1400. [Google Scholar] [CrossRef]
- Yeh, E.-C.; Fu, C.-C.; Hu, L.; Thakur, R.; Feng, J.; Lee, L.P. Self-powered integrated microfluidic point-of-care low-cost enabling (SIMPLE) chip. Sci. Adv. 2017, 3, e1501645. [Google Scholar] [CrossRef] [Green Version]
- Cruceanu, M.; Urbaneja, M.A.; Hixson, C.V.; Johnson, D.G.; Datta, S.A.; Fivash, M.J.; Stephen, A.G.; Fisher, R.J.; Gorelick, R.J.; Casas-Finet, J.R.; et al. Nucleic Acid Binding and Chaperone Properties of HIV-1 Gag and Nucleocapsid Proteins. Nucleic Acids Res. 2006, 34, 593–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Yu, L.; Liu, C.; Ye, S.; Chen, W.; Li, D.; Huang, W. One-Tube SARS-CoV-2 Detection Platform Based on RT-RPA and CRISPR/Cas12a. J. Transl. Med. 2021, 19, 74. [Google Scholar] [CrossRef]
- Lau, Y.L.; Ismail, I.B.; Mustapa, N.I.B.; Lai, M.Y.; Soh, T.S.T.; Hassan, A.H.; Peariasamy, K.M.; Lee, Y.L.; Kahar, M.K.B.A.; Chong, J.; et al. Development of a Reverse Transcription Recombinase Polymerase Amplification Assay for Rapid and Direct Visual Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). PLoS ONE 2021, 16, e0245164. [Google Scholar]
- Chang, L.; Zhao, L.; Gong, H.; Wang, L.; Wang, L. Severe Acute Respiratory Syndrome Coronavirus 2 RNA Detected in Blood Donations. Emerg. Infect. Dis. 2020, 26, 1631–1633. [Google Scholar] [CrossRef] [PubMed]
- Nikolai, L.A.; Meyer, C.G.; Kremsner, P.G.; Velavan, T.P. Asymptomatic SARS Coronavirus 2 Infection: Invisible yet Invincible. Int. J. Infect. Dis. 2020, 100, 112–116. [Google Scholar] [CrossRef]
- Chin, C.D.; Linder, V.; Sia, S.K. Lab-on-a-chip devices for global health: Past studies and future opportunities. Lab Chip 2007, 7, 41–57. [Google Scholar] [CrossRef]
- Mejia-Salazar, J.R.; Rodrigues Cruz, K.; Materon Vasquez, E.M.; de Oliveira, O.N., Jr. Microfluidic Point-of-Care Devices: New Trends and Future Prospects for eHealth Diagnostics. Sensors 2020, 20, 1951. [Google Scholar] [CrossRef] [Green Version]
- Xing, W.; Liu, Y.; Wang, H.; Li, S.; Lin, Y.; Chen, L.; Zhao, Y.; Chao, S.; Huang, X.; Ge, S.; et al. A High-Throughput, Multi-Index Isothermal Amplification Platform for Rapid Detection of 19 Types of Common Respiratory Viruses Including SARS-CoV-2. Engineering 2020, 6, 1130–1140. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.; Yao, Y.; Jing, W.; Liu, S.; Sui, G. A novel microfluidic module for rapid detection of airborne and waterborne pathogens. Sens. Actuators B Chem. 2017, 258, 1138–1145. [Google Scholar] [CrossRef]
- Ranjan, P.; Singhal, A.; Yadav, S.; Kumar, N.; Murali, S.; Sanghi, S.K.; Khan, R. Rapid diagnosis of SARS-CoV-2 using potential point-of-care electrochemical immunosensor: Toward the future prospects. Int. Rev. Immunol. 2021, 40, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Tariq, M.O.; Nawaz, M.; Ahmed, J. MEMS Sensors for Diagnostics and Treatment in the Fight Against COVID-19 and Other Pandemics. IEEE Access 2021, 9, 61123–61149. [Google Scholar] [CrossRef]
- Funari, R.; Chu, K.; Shen, A.Q. Detection of antibodies against SARS-CoV-2 spike protein by gold nanospikes in an opto-microfluidic chip. Biosens. Bioelectron. 2020, 169, 112578. [Google Scholar] [CrossRef]
- Qu, J.; Chenier, M.; Zhang, Y.; Xu, C. A Microflow Cytometry-Based Agglutination Immunoassay for Point-of-Care Quantitative Detection of SARS-CoV-2 IgM and IgG. Micromachines 2021, 12, 443. [Google Scholar]
- Antiochia, R. Paper-Based Biosensors: Frontiers in Point-of-Care Detection of COVID-19 Disease. Biosensors 2021, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel 2021; Division of Viral Diseases CDC-006-00019; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021.
- Ritchie, H.; Mathieu, E.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus (COVID-19) Testing. In Our World Data; Oxford Martin School, Oxford University: Oxford, UK, 2021; Available online: https://ourworldindata.org/coronavirus-testing (accessed on 23 August 2021).
- Abbott Receives CE Mark for Its PanbioTM. Rapid Antigen Self-Test, Opening Access Throughout Europe to Fast, Reliable COVID-19 Testing. Available online: https://abbott.mediaroom.com/2021-06-28-Abbott-Receives-CE-Mark-for-its-Panbio-TM-Rapid-Antigen-Self-Test,-Opening-Access-Throughout-Europe-to-Fast,-Reliable-COVID-19-Testing (accessed on 21 August 2021).
- Kawasuji, H.; Takegoshi, Y.; Kaneda, M.; Ueno, A.; Miyajima, Y.; Kawago, K.; Fukui, Y.; Yoshida, Y.; Kimura, M.; Yamada, H.; et al. Transmissibility of COVID-19 depends on the viral load around onset in adult and symptomatic patients. PLoS ONE 2020, 15, e0243597. [Google Scholar] [CrossRef]
- Fajnzylber, J.; Regan, J.; Coxen, K.; Corry, H.; Wong, C.; Rosenthal, A.; Worrall, D.; Giguel, F.; Piechocka-Trocha, A.; Atyeo, C.; et al. SARS-CoV-2 Viral Load Is Associated with Increased Disease Severity and Mortality. Nat. Commun. 2020, 11, 5493. [Google Scholar] [CrossRef] [PubMed]
- FDA. Antibody (Serology) Testing for COVID-19: Information for Patients and Consumers; FDA: Silver Spring, MD, USA, 2021. [Google Scholar]


| Glass | Silicon | Polymer | Paper | |
|---|---|---|---|---|
| Fabrication Techniques | Photolithography Etching | Bulk or surface micromachining Nano-imprint lithography Electron beam irradiation | Soft lithography Injection molding 3D printing | Wax and inkjet printing Photolithography |
| Advantages | Transparent Inert and stable Solvent compatible Hydrophilic | Mechanically strong Thermostable Chemical resistance | Transparent Easy fabrication Low cost | Flexible and lightweight Low cost No need for external pumps or valves Biocompatible Recyclable |
| Limitations | Brittle Not flexible High cost | High cost Biocompatibility | Hydrophobic Short shelf life | Humidity and temperature sensitive Difficult to design and integrate into single chip |
| Immunoassay | RT-PCR | Nanoparticle | Microflow Cytometry | |
|---|---|---|---|---|
| Reagent Consumption | 10 µg (in tube) | 20 µL (in tube) | Negligible | 50 µL (in tube) |
| Target of Detection | IgG, IgA, IgM | N gene, E gene | Gold-spiked | IgM, IgG |
| Limit of Detection | 0.15 mg/L | 1-10 copy per µL | 0.08 mg/L | 0.06-0.10 mg/L |
| Total Assay Time | 1 h | 2 h | 2–5 h | 30 min |
| Sample Volume | 20 µL | 120 µL | 1 µL | 10 µL |
| Assay Control | Automated | Manual | Manual | Automated |
| Cost per Test | ~ 6 (USD) | ~ 4 (USD) | ~ 10 (USD) | ~ 5 (USD) |
| Quantitative | No | Yes | Yes | Yes |
| Mobile | Yes | Yes | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar, A.; Chiu, P.; Qu, J.; Zhang, Y.; Xu, C.-q. Integrated Microfluidic-Based Platforms for On-Site Detection and Quantification of Infectious Pathogens: Towards On-Site Medical Translation of SARS-CoV-2 Diagnostic Platforms. Micromachines 2021, 12, 1079. https://doi.org/10.3390/mi12091079
Escobar A, Chiu P, Qu J, Zhang Y, Xu C-q. Integrated Microfluidic-Based Platforms for On-Site Detection and Quantification of Infectious Pathogens: Towards On-Site Medical Translation of SARS-CoV-2 Diagnostic Platforms. Micromachines. 2021; 12(9):1079. https://doi.org/10.3390/mi12091079
Chicago/Turabian StyleEscobar, Andres, Phyllis Chiu, Jianxi Qu, Yushan Zhang, and Chang-qing Xu. 2021. "Integrated Microfluidic-Based Platforms for On-Site Detection and Quantification of Infectious Pathogens: Towards On-Site Medical Translation of SARS-CoV-2 Diagnostic Platforms" Micromachines 12, no. 9: 1079. https://doi.org/10.3390/mi12091079
APA StyleEscobar, A., Chiu, P., Qu, J., Zhang, Y., & Xu, C.-q. (2021). Integrated Microfluidic-Based Platforms for On-Site Detection and Quantification of Infectious Pathogens: Towards On-Site Medical Translation of SARS-CoV-2 Diagnostic Platforms. Micromachines, 12(9), 1079. https://doi.org/10.3390/mi12091079







