Liquid Mixing Based on Electrokinetic Vortices Generated in a T-Type Microchannel
Abstract
1. Introduction
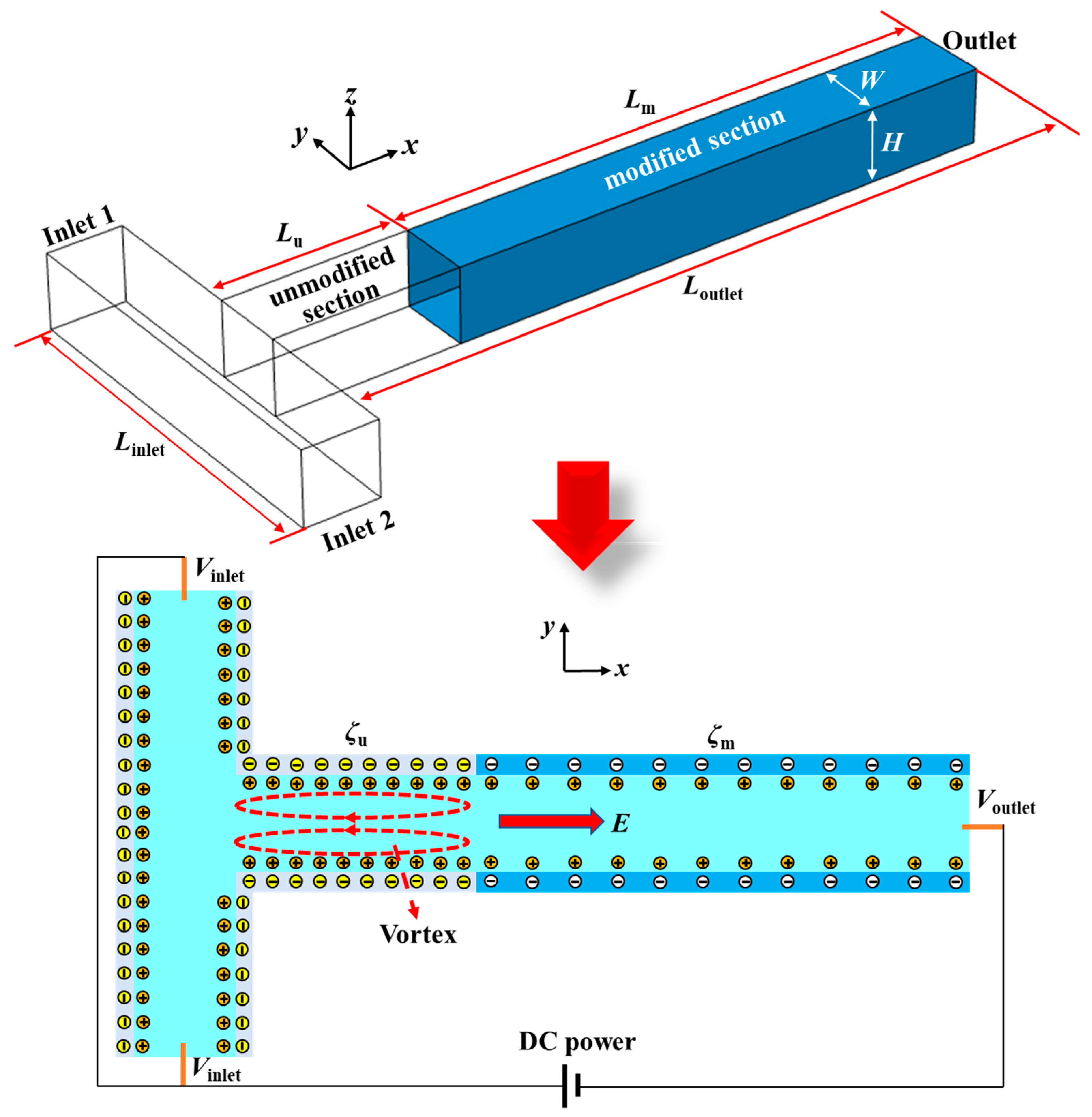
2. Structure and Working Principle of the Micromixer
3. Theoretical Model
3.1. Electric Field
3.2. Flow Field
3.3. Concentration Field
4. Results and Discussion
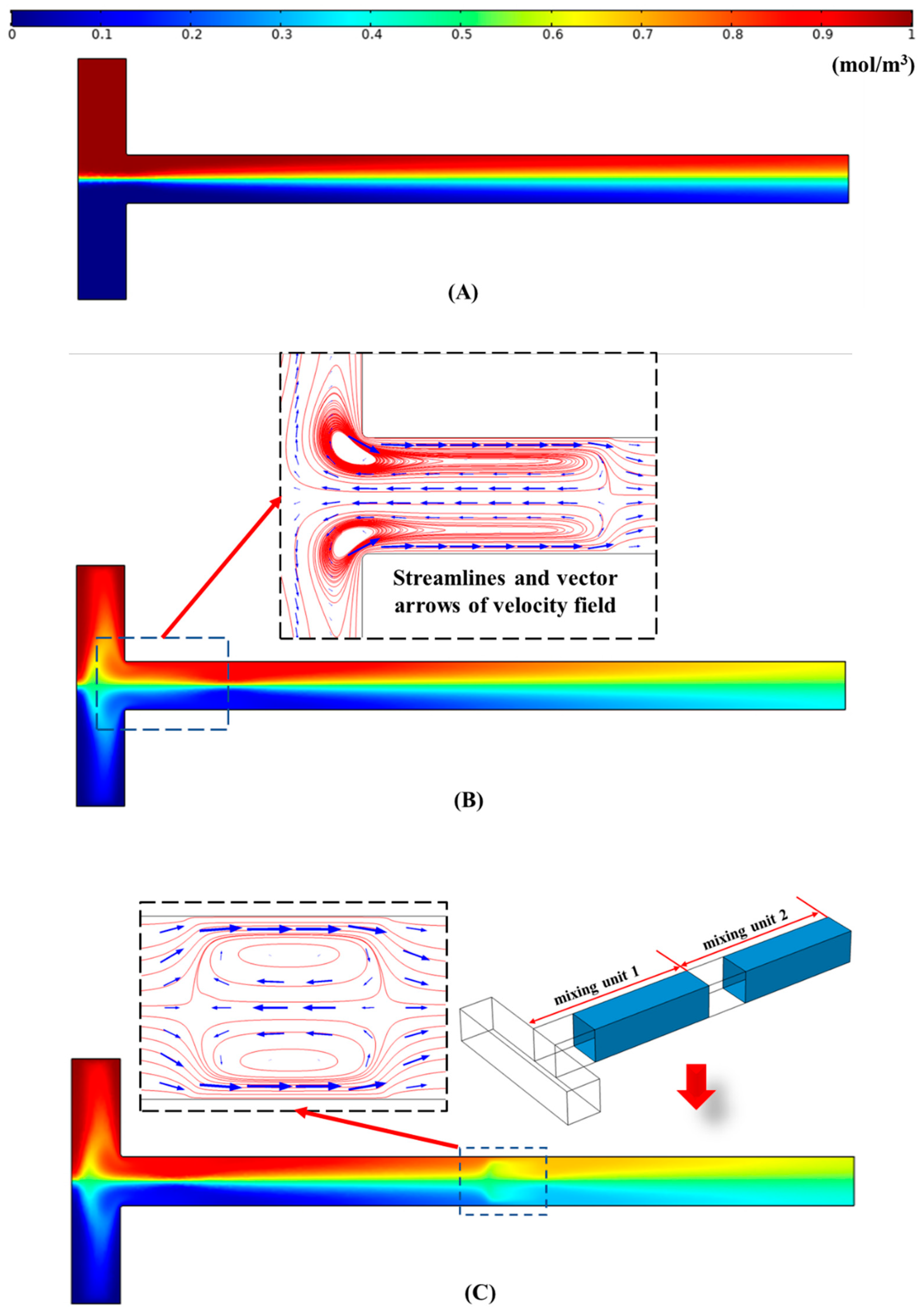
4.1. Distribution of Concentration Field in the Microchannels
4.2. Effect of Zeta Potential Ratio (α) on Mixing Index
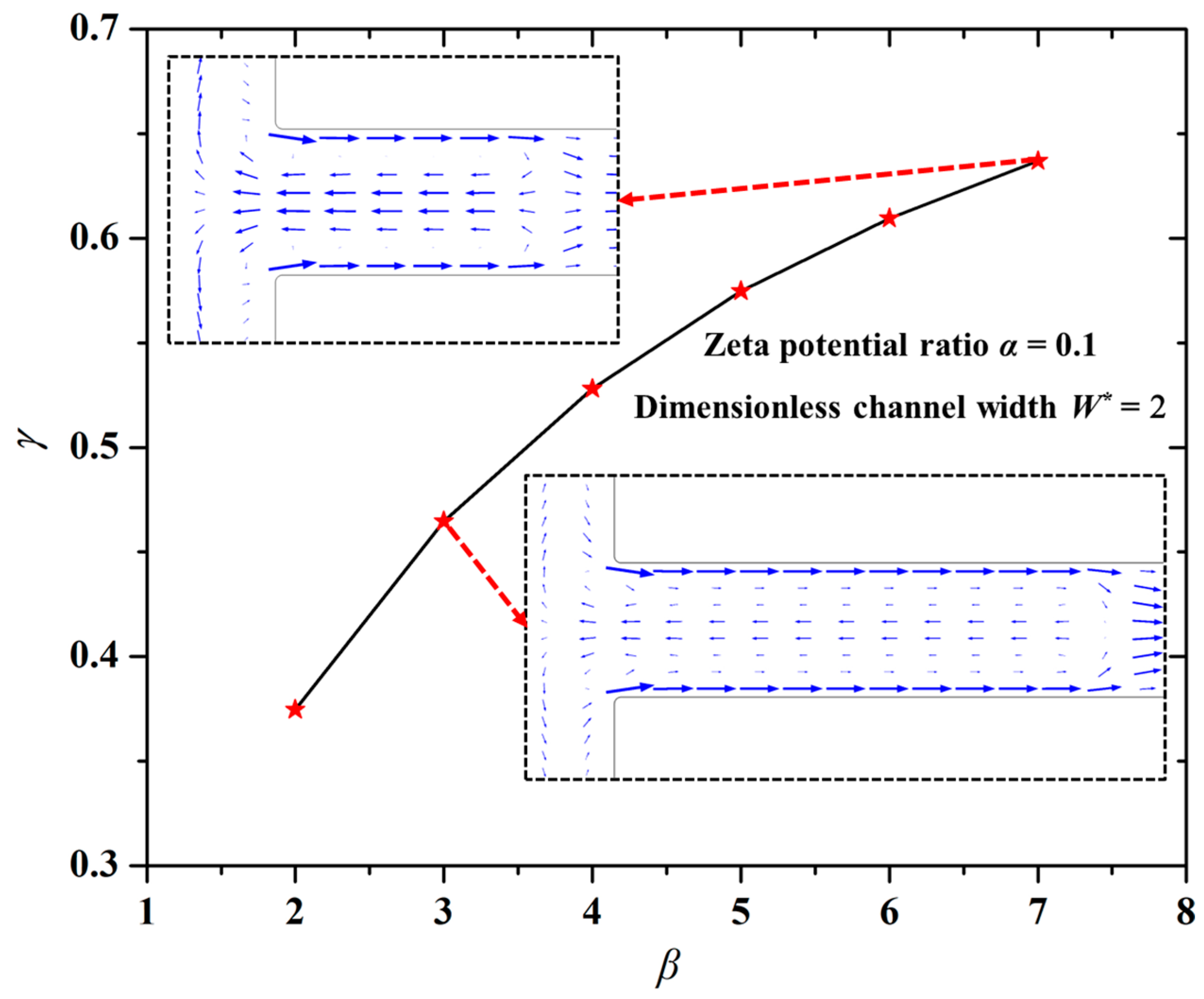
4.3. Effect of Length Ratio (β) on Mixing Index
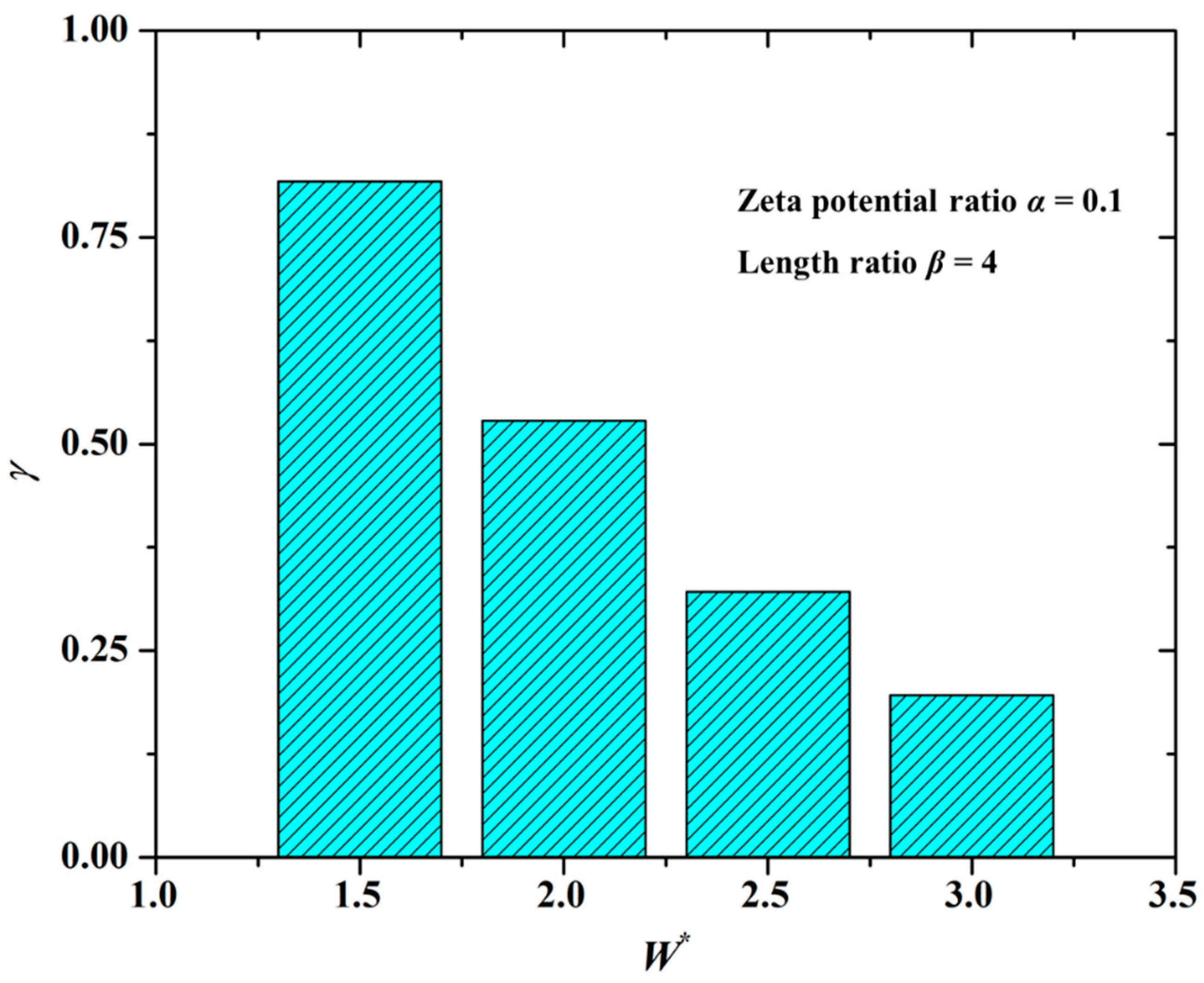
4.4. Effect of Channel Width (W) on Mixing Index
5. Conclusions
Funding
Conflicts of Interest
References
- Yang, Y.; Noviana, E.; Nguyen, M.P.; Geiss, B.J.; Dandy, D.S.; Henry, C.S. Paper-Based Microfluidic Devices: Emerging Themes and Applications. Anal. Chem. 2017, 89, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, S.; Bafekr, H.; Valipour, M.S.; Esfahani, J.A. A review on the application, simulation, and experiment of the electrokinetic mixers. Chem. Eng. Process. Process Intensif. 2018, 126, 108–122. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, T.; Liu, Z.; Wu, Y.; Qian, S.; Korvink, J.G. Topology optimization of electrode patterns for electroosmotic micromixer. Int. J. Heat Mass Transf. 2018, 126, 1299–1315. [Google Scholar] [CrossRef]
- Chen, L.; Deng, Y.; Zhou, T.; Pan, H.; Liu, Z. A novel electroosmotic micromixer with asymmetric lateral structures and DC electrode arrays. Micromachines 2017, 8, 105. [Google Scholar] [CrossRef]
- Chen, Y.; Kim, C.N. Numerical analysis of the mixing of two electrolyte solutions in an electromagnetic rectangular micromixer. J. Ind. Eng. Chem. 2018, 60, 377–389. [Google Scholar] [CrossRef]
- Wang, Y.; Zhe, J.; Chung, B.T.F.; Dutta, P. A rapid magnetic particle driven micromixer. Microfluid. Nanofluidics 2008, 4, 375–389. [Google Scholar] [CrossRef]
- Dallakehnejad, M.; Mirbozorgi, S.A.; Niazmand, H. A numerical investigation of magnetic mixing in electroosmotic flows. J. Electrostat. 2019, 100, 103354. [Google Scholar] [CrossRef]
- Rasouli, M.R.; Tabrizian, M. An ultra-rapid acoustic micromixer for synthesis of organic nanoparticles. Lab Chip 2019, 19, 3316–3325. [Google Scholar] [CrossRef]
- Shang, X.; Huang, X.; Yang, C. Vortex generation and control in a microfluidic chamber with actuations. Phys. Fluids 2016, 28, 122001. [Google Scholar] [CrossRef]
- Ahmed, D.; Mao, X.; Juluri, B.K.; Huang, T.J. A fast microfluidic mixer based on acoustically driven sidewall-trapped microbubbles. Microfluid. Nanofluidics 2009, 5, 727–731. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2012, 295, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Mehta, S.K.; Patowari, P.K.; Pati, S. Numerical study of mixing in wavy micromixers: Comparison between raccoon and serpentine mixer. Chem. Eng. Process.-Process Intensif. 2019, 136, 44–61. [Google Scholar] [CrossRef]
- Raza, W.; Kim, K.-Y. Unbalanced Split and Recombine Micromixer with Three-Dimensional Steps. Ind. Eng. Chem. Res. 2019. [Google Scholar] [CrossRef]
- Bazaz, S.R.; Mehrizi, A.A.; Ghorbani, S.; Vasilescu, S.; Asadnia, M.; Warkiani, M.E. A hybrid micromixer with planar mixing units. RSC Adv. 2018, 8, 33103–33120. [Google Scholar] [CrossRef]
- Wang, H.; Shi, L.; Zhou, T.; Xu, C.; Deng, Y. A novel passive micromixer with modified asymmetric lateral wall structures. Asia-Pacific J. Chem. Eng. 2018, 13, e2202. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, Y.; Liu, Z.; Joo, S.W. An enhanced one-layer passive microfluidic mixer with an optimized lateral structure with the dean effect. J. Fluids Eng. 2015, 137, 091102. [Google Scholar] [CrossRef]
- Chen, X.; Li, T. A novel passive micromixer designed by applying an optimization algorithm to the zigzag microchannel. Chem. Eng. J. 2017, 313, 1406–1414. [Google Scholar] [CrossRef]
- Afzal, A.; Kim, K. Convergent–divergent micromixer coupled with pulsatile flow. Sensors Actuators B Chem. 2015, 211, 198–205. [Google Scholar] [CrossRef]
- Khaydarov, V.; Borovinskaya, E.S.; Reschetilowski, W. Numerical and experimental investigations of a micromixer with chicane mixing geometry. Appl. Sci. 2018, 8, 2458. [Google Scholar] [CrossRef]
- Raza, W.; Kim, K. Asymmetrical Split-and-Recombine Micromixer with Baffles. Micromachines 2019, 10, 844. [Google Scholar] [CrossRef]
- Wang, L.; Ma, S.; Wang, X.; Bi, H.; Han, X. Mixing enhancement of a passive microfluidic mixer containing triangle baffles. Asia-Pacific J. Chem. Eng. 2014, 9, 877–885. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Z. Numerical investigation on layout optimization of obstacles in a three-dimensional passive micromixer. Anal. Chim. Acta 2017, 964, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.-T.; Wu, C.-Y. An efficient micromixer based on multidirectional vortices due to baffles and channel curvature. Biomicrofluidics 2011, 5, 014103. [Google Scholar] [CrossRef]
- Bayareh, M.; Ashani, M.N.; Usefian, A. Active and passive micromixers: A comprehensive review. Chem. Eng. Process.-Process Intensif. 2020, 147, 107771. [Google Scholar] [CrossRef]
- Cai, G.; Li, X.; Zhang, H.; Lin, J. A review on micromixers. Micromachines 2017, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: London, UK, 1981; ISBN 1483214087. [Google Scholar]
- Squires, T.M.; Bazant, M.Z. Induced-charge electro-osmosis. J. Fluid Mech. 2004, 509, 217–252. [Google Scholar] [CrossRef]
- Daghighi, Y.; Sinn, I.; Kopelman, R.; Li, D. Experimental validation of induced-charge electrokinetic motion of electrically conducting particles. Electrochim. Acta 2013, 87, 270–276. [Google Scholar] [CrossRef]
- Wang, C.; Song, Y.; Pan, X.; Li, D. A novel microfluidic valve controlled by induced charge electro-osmotic flow. J. Micromech. Microeng. 2016, 26, 075002. [Google Scholar] [CrossRef]
- Song, Y.; Wang, C.; Li, M.; Pan, X.; Li, D. Focusing particles by induced charge electrokinetic flow in a microchannel. Electrophoresis 2016, 37, 666–675. [Google Scholar] [CrossRef]
- Wu, Z.; Li, D. Micromixing using induced-charge electrokinetic flow. Electrochim. Acta 2008, 53, 5827–5835. [Google Scholar] [CrossRef]
- Wu, Z.; Li, D. Mixing and flow regulating by induced-charge electrokinetic flow in a microchannel with a pair of conducting triangle hurdles. Microfluid. Nanofluidics 2008, 5, 65–76. [Google Scholar] [CrossRef]
- Fosdick, S.E.; Knust, K.N.; Scida, K.; Crooks, R.M. Bipolar electrochemistry. Angew. Chemie Int. Ed. 2013, 52, 10438–10456. [Google Scholar] [CrossRef] [PubMed]
- Hau, W.L.W.; Lee, L.M.; Lee, Y.-K.; Wong, M.; Zohar, Y. Experimental investigation of electrokinetically generated in-plane vorticity in a microchannel. In Proceedings of the TRANSDUCERS’03. 12th International Conference on Solid-State Sensors, Actuators and Microsystems, IEEE. Boston, MA, USA, 9–12 June 2003; Volume 1, pp. 651–654. [Google Scholar]
- Stroock, A.D.; Weck, M.; Chiu, D.T.; Huck, W.T.S.; Kenis, P.J.A.; Ismagilov, R.F.; Whitesides, G.M. Patterning electro-osmotic flow with patterned surface charge. Phys. Rev. Lett. 2000, 84, 3314–3317. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.; Li, D. Three-dimensional structure of electroosmotic flow over heterogeneous surfaces. J. Phys. Chem. B 2003, 107, 12212–12220. [Google Scholar] [CrossRef]
- Erickson, D.; Li, D. Influence of surface heterogeneity on electrokinetically driven microfluidic mixing. Langmuir 2002, 18, 1883–1892. [Google Scholar] [CrossRef]
- Erickson, D.; Li, D. Microchannel flow with patchwise and periodic surface heterogeneity. Langmuir 2002, 18, 8949–8959. [Google Scholar] [CrossRef]
- Kirby, B.J.; Hasselbrink, E.F., Jr. Zeta potential of microfluidic substrates: 1.Theory, experimental techniques, and effects on separations. Electrophoresis 2004, 25, 187–202. [Google Scholar] [CrossRef]
- Wang, C.; Song, Y.; Pan, X. Electrokinetic-vortex formation near a two-part cylinder with same-sign zeta potentials in a straight microchannel. Electrophoresis 2020, 41, 793–801. [Google Scholar] [CrossRef]
- Song, Y.; Wang, C.; Li, J.; Li, D. Vortex generation in electroosmotic flow in a straight polydimethylsiloxane microchannel with different polybrene modified-to-unmodified section length ratios. Microfluid. Nanofluidics 2019, 23, 24. [Google Scholar] [CrossRef]
- Biddiss, E.; Erickson, D.; Li, D. Heterogeneous surface charge enhanced micromixing for electrokinetic flows. Anal. Chem. 2004, 76, 3208–3213. [Google Scholar] [CrossRef]
- Wang, C.; Li, M.; Song, Y.; Pan, X.; Li, D. Electrokinetic motion of a spherical micro particle at an oil−water interface in microchannel. Electrophoresis 2018, 39, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, J.; Li, D. Zeta potentials of polydimethylsiloxane surfaces modified by polybrene of different. Electrophoresis 2016, 37, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, H.; Shi, L.; Liu, Z.; Joo, S.W. An enhanced electroosmotic micromixer with an efficient asymmetric lateral structure. Micromachines 2016, 7, 218. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C. Liquid Mixing Based on Electrokinetic Vortices Generated in a T-Type Microchannel. Micromachines 2021, 12, 130. https://doi.org/10.3390/mi12020130
Wang C. Liquid Mixing Based on Electrokinetic Vortices Generated in a T-Type Microchannel. Micromachines. 2021; 12(2):130. https://doi.org/10.3390/mi12020130
Chicago/Turabian StyleWang, Chengfa. 2021. "Liquid Mixing Based on Electrokinetic Vortices Generated in a T-Type Microchannel" Micromachines 12, no. 2: 130. https://doi.org/10.3390/mi12020130
APA StyleWang, C. (2021). Liquid Mixing Based on Electrokinetic Vortices Generated in a T-Type Microchannel. Micromachines, 12(2), 130. https://doi.org/10.3390/mi12020130





