Hemagglutination Detection with Paper–Plastic Hybrid Passive Microfluidic Chip
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Paper–Plastic Hybrid Microfluidic Chip
2.2. Reagents and Immobilized Reagent on Chip
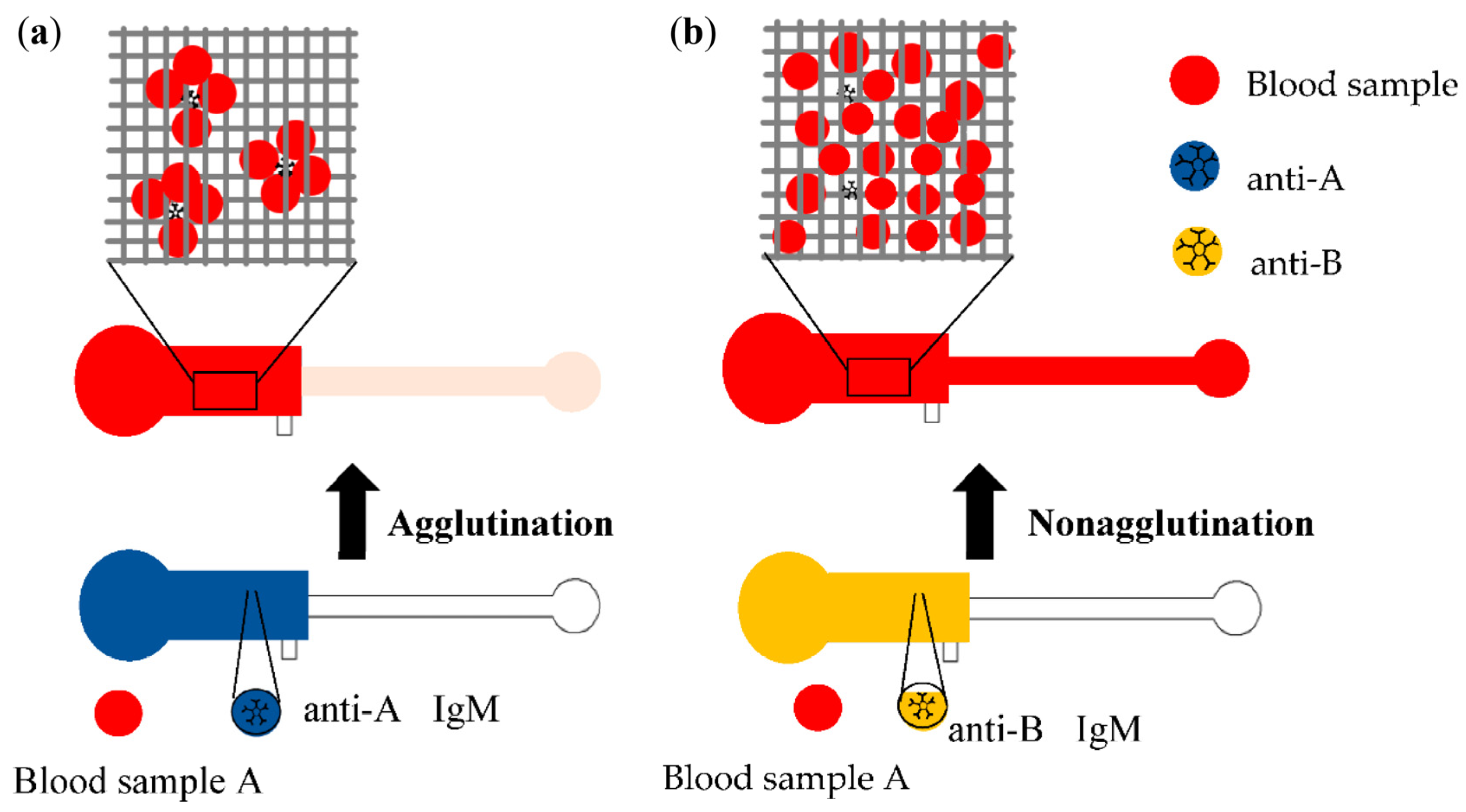
2.3. Red Blood Cell Agglutination Assays
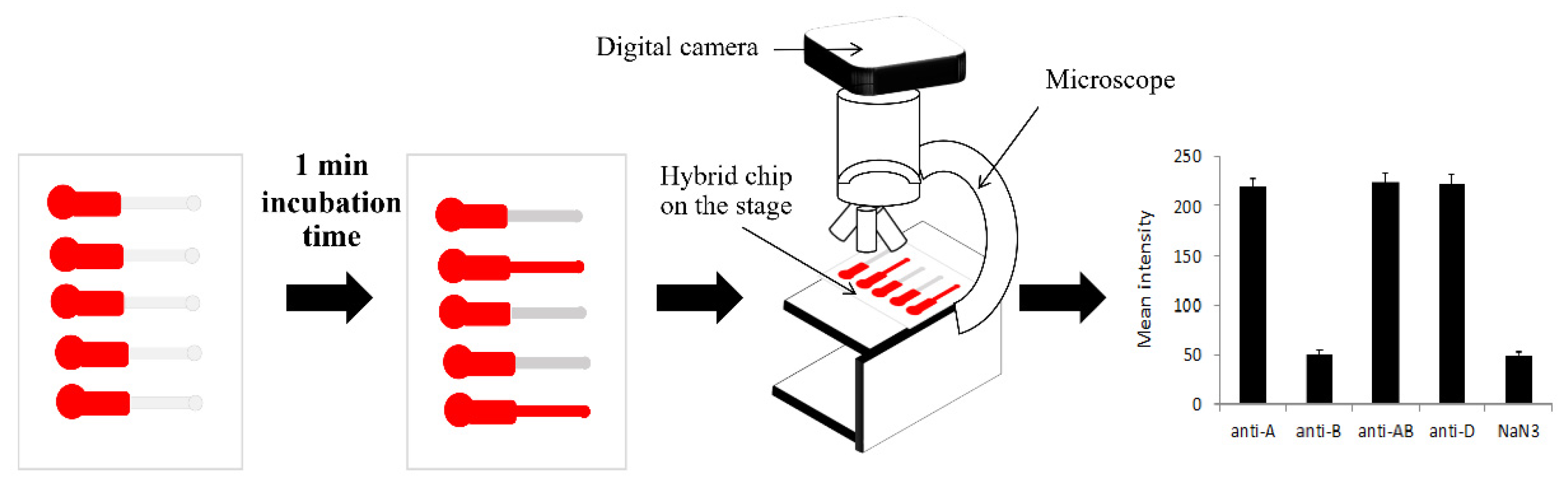
2.4. Quantification of Agglutination Assay
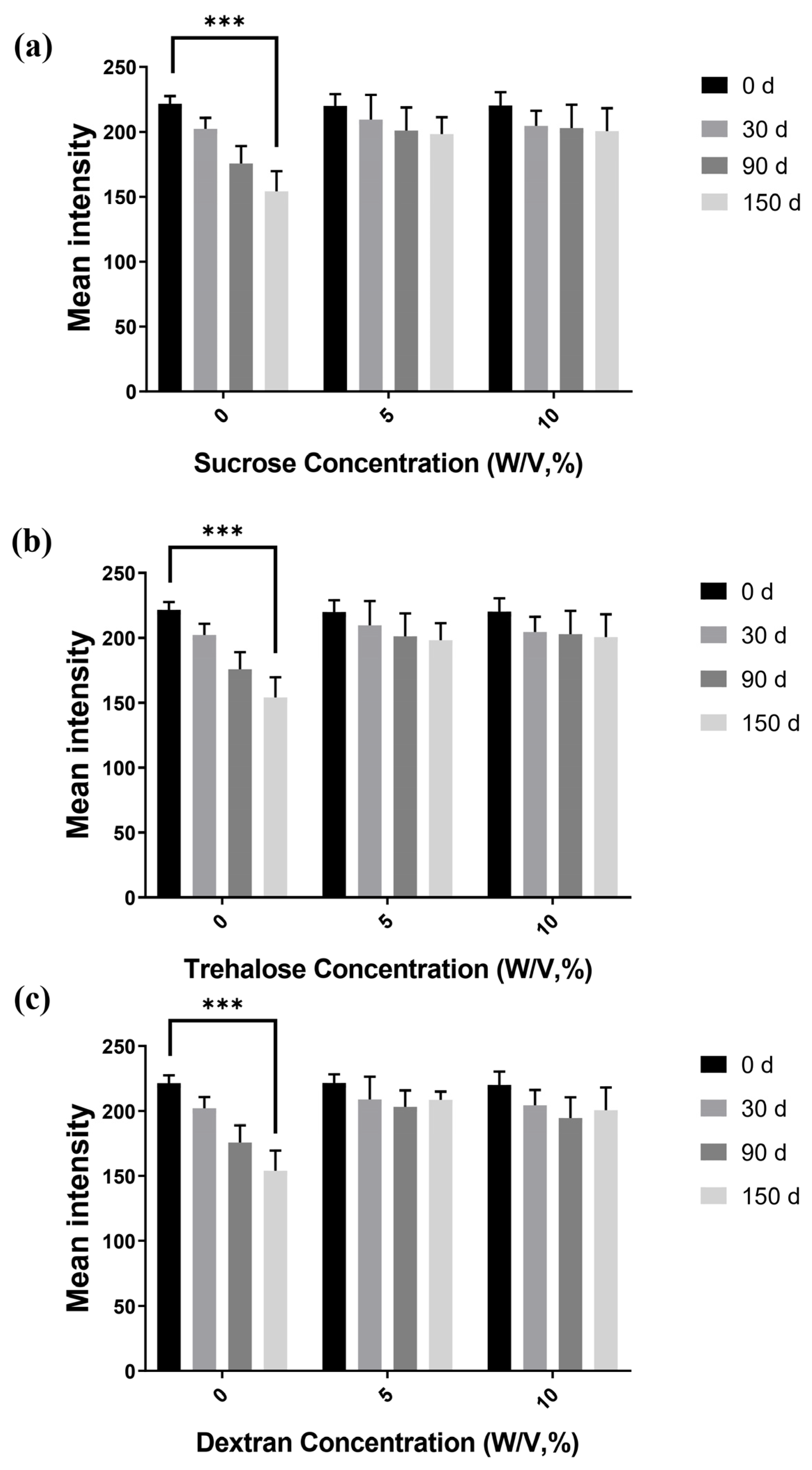
2.5. Storage Stability Studies with Embedded Antibodies
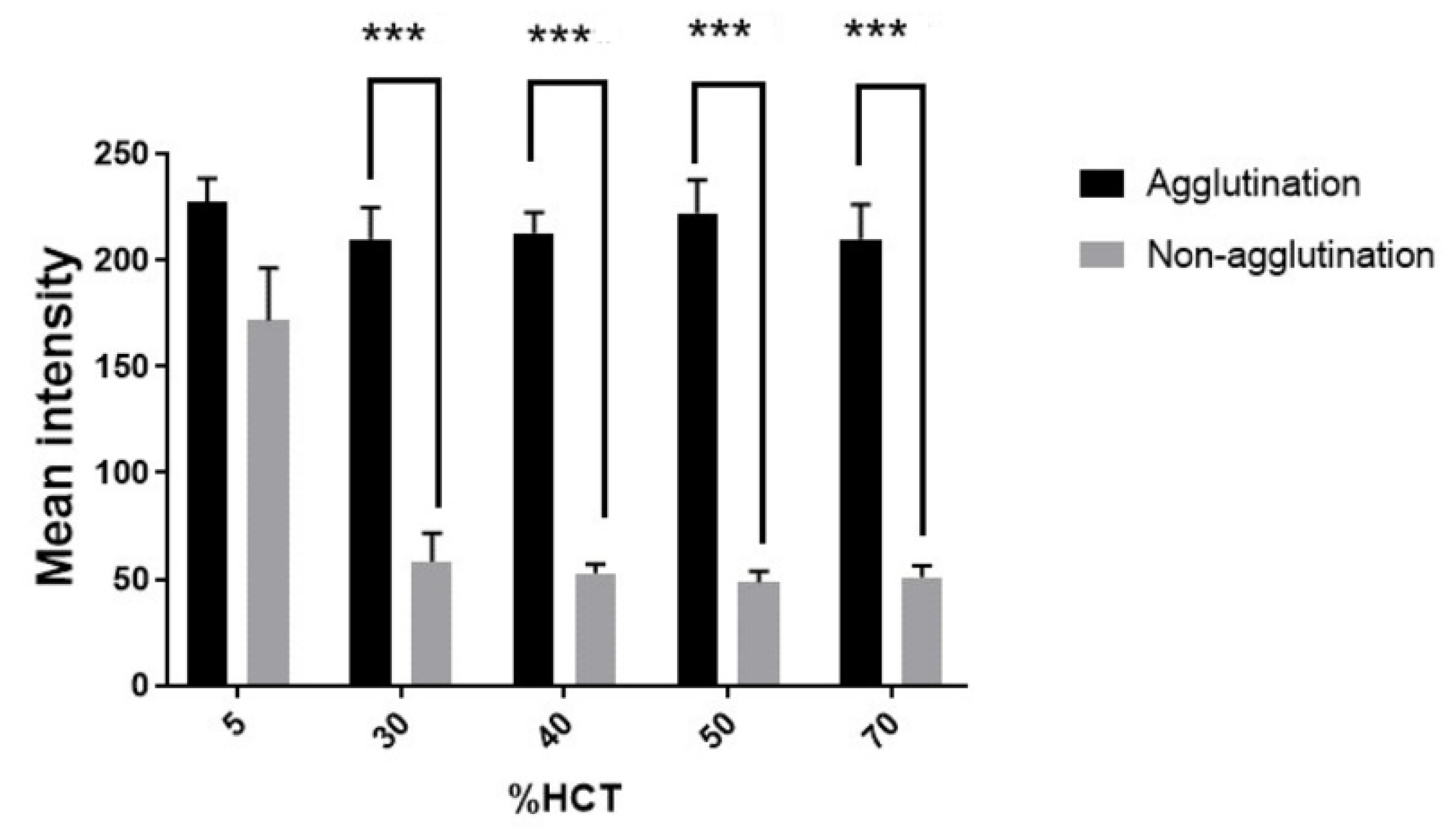
2.6. Hematocrit Variation and Incubation Time Study
2.7. Statistical Analysis
3. Results and Discussion
3.1. Image Observation of Blood Agglutination
3.2. Quantification of Blood Agglutination
3.3. Immobilized Antibody Stability and Storage Time
3.4. Effect of Hematocrit on Hemagglutination
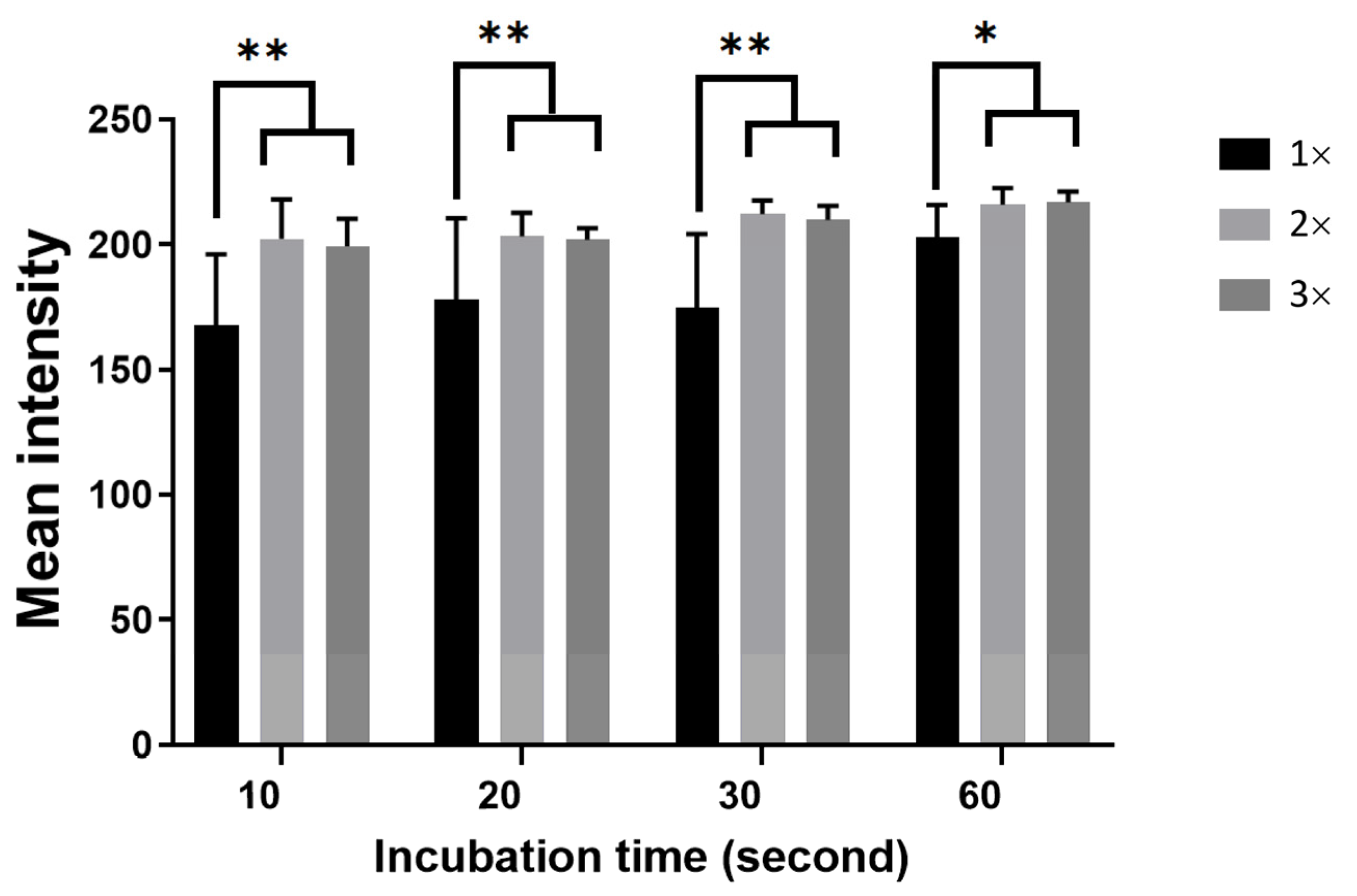
3.5. Effect of Incubation Time on Agglutination
3.6. Limitations of this Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jain, A.; Garg, S.; Marwaha, N.; Sharma, R.R. ABO Blood Grouping Discrepancies in the Donor Population. ISBT Sci. Ser. 2020, 15, 281–285. [Google Scholar] [CrossRef]
- Heo, W.Y.; Chung, Y.N.; Kim, T.Y.; Yu, H.; Bae, J.C.; Kim, H.; Cho, D. Analysis of ABO Grouping Discrepancies among Patients from a Tertiary Hospital in Korea. Transfus. Apher. Sci. 2021, 2021, 103230. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-P.; Wen, Y.; Lu, J.-J.; Tseng, C.-P.; Wang, W.-T. Rapid Rare ABO Blood Typing Using a Single PCR Based on a Multiplex SNaPshot Reaction. J. Formos. Med. Assoc. 2019, 118, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Fürst, D.; Tsamadou, C.; Neuchel, C.; Schrezenmeier, H.; Mytilineos, J.; Weinstock, C. Next-Generation Sequencing Technologies in Blood Group Typing. Transfus. Med. Hemother. 2020, 47, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Lane, W.J.; Westhoff, C.M.; Gleadall, N.S.; Aguad, M.; Smeland-Wagman, R.; Vege, S.; Simmons, D.P.; Mah, H.H.; Lebo, M.S.; Walter, K.; et al. Automated Typing of Red Blood Cell and Platelet Antigens: A Whole-Genome Sequencing Study. Lancet Haematol. 2018, 5, e241–e251. [Google Scholar] [CrossRef] [Green Version]
- Michalopoulos, G.; Pitot, H.C. Primary Culture of Parenchymal Liver Cells on Collagen Membranes: Morphological and Biochemical Observations. Exp. Cell Res. 1975, 94, 70–78. [Google Scholar] [CrossRef]
- Pandey, C.M.; Augustine, S.; Kumar, S.; Kumar, S.; Nara, S.; Srivastava, S.; Malhotra, B.D. Microfluidics Based Point-of-Care Diagnostics. Biotechnol. J. 2018, 13, 1700047. [Google Scholar] [CrossRef]
- Tsao, C.-W. Polymer Microfluidics: Simple, Low-Cost Fabrication Process Bridging Academic Lab Research to Commercialized Production. Micromachines 2016, 7, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, M.K.; Chakraborty, S. PDMS Microfluidics: A Mini Review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Tong, X.; Ga, L.; Zhao, R.; Ai, J. Research Progress on the Applications of Paper Chips. RSC Adv. 2021, 11, 8793–8820. [Google Scholar] [CrossRef]
- Parween, S.; Bhatnagar, I.; Bhosale, S.; Paradkar, S.; Michael, I.J.; Rao, C.M.; Asthana, A. Cross-Linked Chitosan Biofunctionalized Paper-Based Microfluidic Device towards Long Term Stabilization of Blood Typing Antibodies. Int. J. Biol. Macromol. 2020, 163, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Xiaopei, Q.; Yurui, Z.; Yanyao, Y.; Chao, Q.; Lingyun, Z.; Xiang, Y.; Ke, Y.; Yuanfeng, Z.; Yongjun, Y.; et al. A Dye-Assisted Paper-Based Point-of-Care Assay for Fast and Reliable Blood Grouping. Sci. Transl. Med. 2017, 9, eaaf9209. [Google Scholar] [CrossRef]
- Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Paper-Based Microfluidics: Simplified Fabrication and Assay Methods. Sens. Actuators B Chem. 2021, 336, 129681. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Dai, J.; Zhang, W.; Jiang, Y.; Zhou, A. A Low-Cost Mobile Platform for Whole Blood Glucose Monitoring Using Colorimetric Method. Microchem. J. 2021, 162, 105814. [Google Scholar] [CrossRef]
- Bartholomeusz, D.A.; Boutte, R.W.; Andrade, J.D. Xurography: Rapid Prototyping of Microstructures Using a Cutting Plotter. Microelectromech. Syst. J. 2005, 14, 1364–1374. [Google Scholar] [CrossRef]
- Samae, M.; Ritmetee, P.; Chirasatitsin, S.; Kojić, S.; Kojić, T.; Jevremov, J.; Stojanović, G.; Al Salami, H. Precise Manufacturing and Performance Validation of Paper-Based Passive Microfluidic Micromixers. Int. J. Precis. Eng. Manuf. 2020, 21, 499–508. [Google Scholar] [CrossRef]
- Samae, M.; Chirasatitsin, S. Improvement of Hemagglutination on Plastic Microfluidic Chip. In Proceedings of the 2018 11th Biomedical Engineering International Conference (BMEiCON), Chiang Mai, Thailand, 21 November 2018; pp. 1–5. [Google Scholar]
- Park, J.; Park, J.-K. Finger-Actuated Microfluidic Display for Smart Blood Typing. Anal. Chem. 2019, 91, 11636–11642. [Google Scholar] [CrossRef]
- Sklavounos, A.A.; Lamanna, J.; Modi, D.; Gupta, S.; Mariakakis, A.; Callum, J.; Wheeler, A.R. Digital Microfluidic Hemagglutination Assays for Blood Typing, Donor Compatibility Testing, and Hematocrit Analysis. Clin. Chem. 2021, 67, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, S.H.; Ahn, C.H.; Lee, J.Y.; Kwon, T.H. Disposable Integrated Microfluidic Biochip for Blood Typing by Plastic Microinjection Moulding. Lab Chip 2006, 6, 794–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Tian, J.; Al-Tamimi, M.; Shen, W. Paper-Based Blood Typing Device That Reports Patient’s Blood Type “in Writing”. Angew. Chem. 2012, 51, 5497–5501. [Google Scholar] [CrossRef]
- Jarujamrus, P.; Tian, J.; Li, X.; Siripinyanond, A.; Shiowatana, J.; Shen, W. Mechanisms of Red Blood Cells Agglutination in Antibody-Treated Paper. Analyst 2012, 137, 2205–2210. [Google Scholar] [CrossRef]
- Huet, M.; Cubizolles, M.; Buhot, A. Red Blood Cell Agglutination for Blood Typing Within Passive Microfluidic Biochips. High-Throughput 2018, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Sakurai, R.; Motosuke, M. Fully-Automatic Blood-Typing Chip Exploiting Bubbles for Quick Dilution and Detection. Biomicrofluidics 2020, 14, 024111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.-P.; Chen, C.; Wu, P.-Y.; Lin, Y.-H.; Lin, W.-T.; Yan, Y.-L. Micro-Droplet Platform for Exploring the Mechanism of Mixed Field Agglutination in B3 Subtype. Biosensors 2021, 11, 276. [Google Scholar] [CrossRef]
- Samae, M.; Chirasatitsin, S. The Degree of Hemagglutination on a Plastic Microfluidic Chip Using Impedimetric Detection. In Proceedings of the 2019 12th Biomedical Engineering International Conference (BMEiCON), Ubon Ratchathani, Thailand, 19–22 November 2019; pp. 1–5. [Google Scholar]
- Cao, R.; Tian, W.; Shen, W. Polysaccharides as Protectants for Paper-Based Analytical Devices with Antibody. Talanta 2017, 165, 357–363. [Google Scholar] [CrossRef]
- Carpenter, J.F.; Crowe, J.H. Modes of Stabilization of a Protein by Organic Solutes during Desiccation. Cryobiology 1988, 25, 459–470. [Google Scholar] [CrossRef]
- Lutz, B.; Liang, T.; Fu, E.; Ramachandran, S.; Kauffman, P.; Yager, P. Dissolvable Fluidic Time Delays for Programming Multi-Step Assays in Instrument-Free Paper Diagnostics. Lab Chip 2013, 13, 2840–2847. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N.; Lis, H. Lectins: Cell-Agglutinating and Sugar-Specific Proteins. Science 1972, 177, 949–959. [Google Scholar] [CrossRef]
- Cohen, E.; Kramer, M.; Shochat, T.; Goldberg, E.; Krause, I. Relationship between Hematocrit Levels and Intraocular Pressure in Men and Women. Medicine 2017, 96, e8290. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B. Antigen–Antibody Reactions. ISBT Sci. Ser. 2008, 3, 21–32. [Google Scholar] [CrossRef]
- Khan, M.S.; Thouas, G.; Shen, W.; Whyte, G.; Garnier, G. Paper Diagnostic for Instantaneous Blood Typing. Anal. Chem. 2010, 82, 4158–4164. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Steckl, A.J. Paper Microfluidics for Point-of-Care Blood-Based Analysis and Diagnostics. Anal. Chem. 2019, 91, 352–371. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samae, M.; Chatpun, S.; Chirasatitsin, S. Hemagglutination Detection with Paper–Plastic Hybrid Passive Microfluidic Chip. Micromachines 2021, 12, 1533. https://doi.org/10.3390/mi12121533
Samae M, Chatpun S, Chirasatitsin S. Hemagglutination Detection with Paper–Plastic Hybrid Passive Microfluidic Chip. Micromachines. 2021; 12(12):1533. https://doi.org/10.3390/mi12121533
Chicago/Turabian StyleSamae, Mahdee, Surapong Chatpun, and Somyot Chirasatitsin. 2021. "Hemagglutination Detection with Paper–Plastic Hybrid Passive Microfluidic Chip" Micromachines 12, no. 12: 1533. https://doi.org/10.3390/mi12121533
APA StyleSamae, M., Chatpun, S., & Chirasatitsin, S. (2021). Hemagglutination Detection with Paper–Plastic Hybrid Passive Microfluidic Chip. Micromachines, 12(12), 1533. https://doi.org/10.3390/mi12121533







