Theoretical and Experimental Research on Multi-Layer Vessel-like Structure Printing Based on 3D Bio-Printing Technology
Abstract
:1. Introduction
2. Theory, Fabrication Methods, and Materials
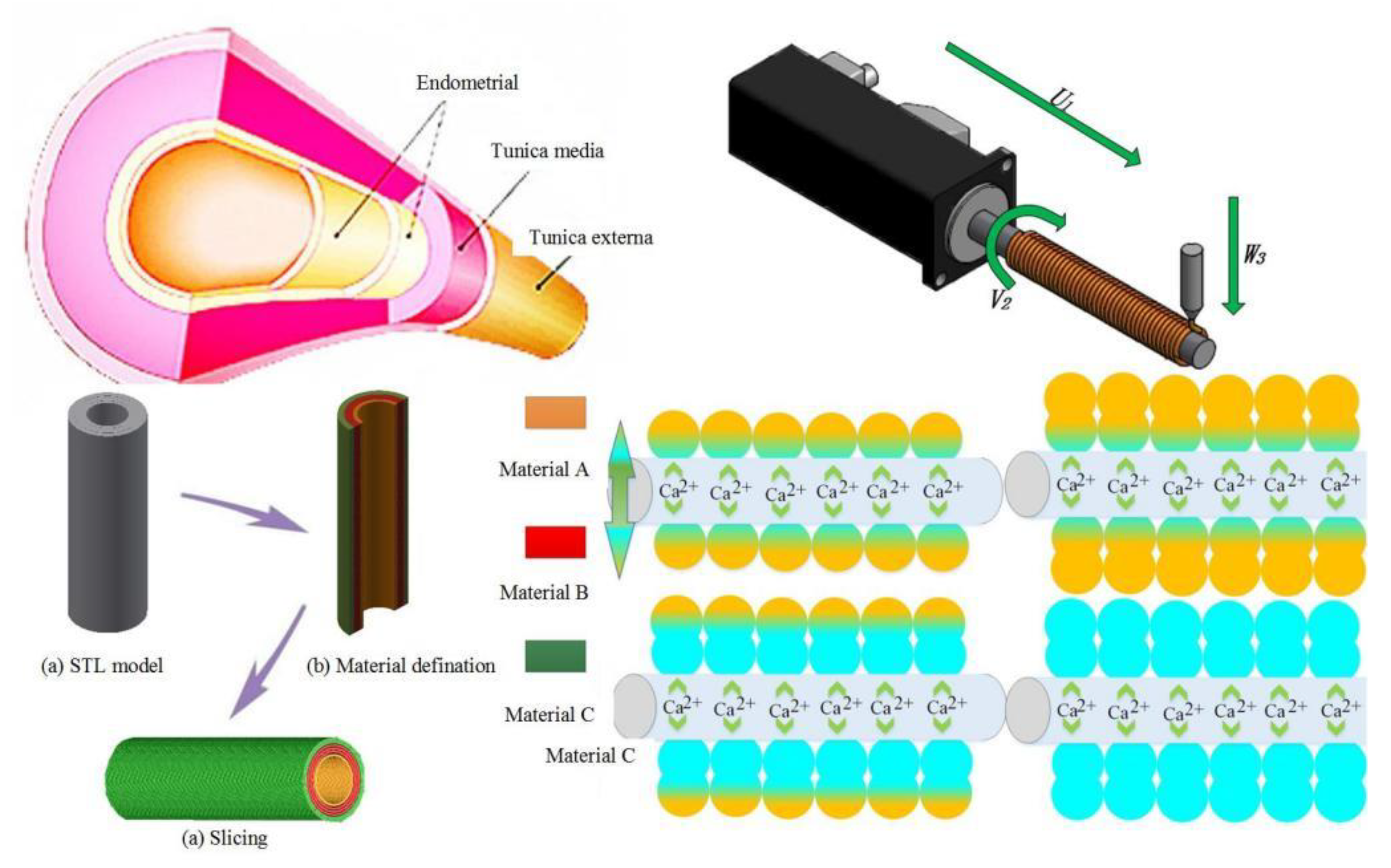
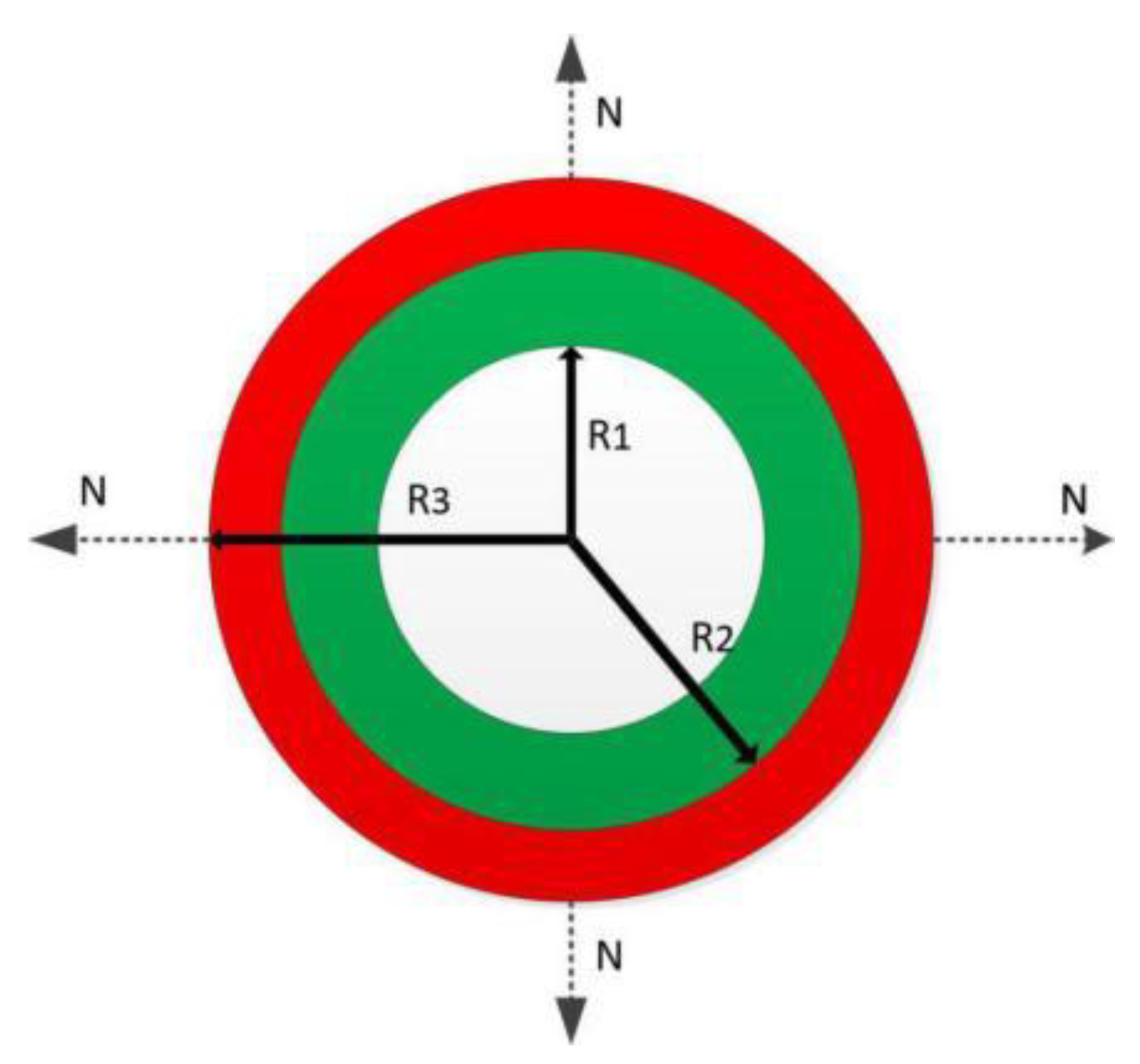
2.1. Theory
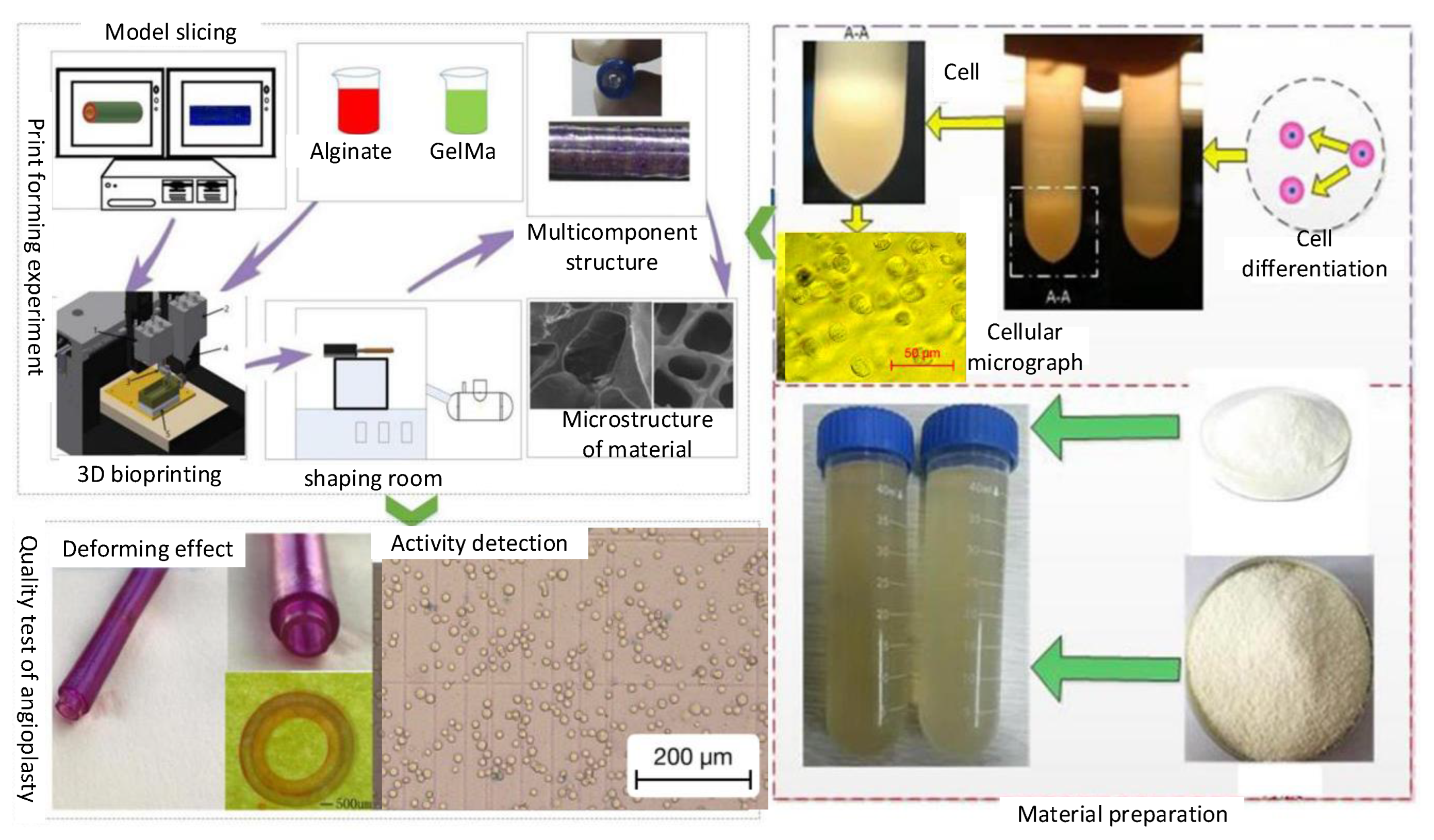
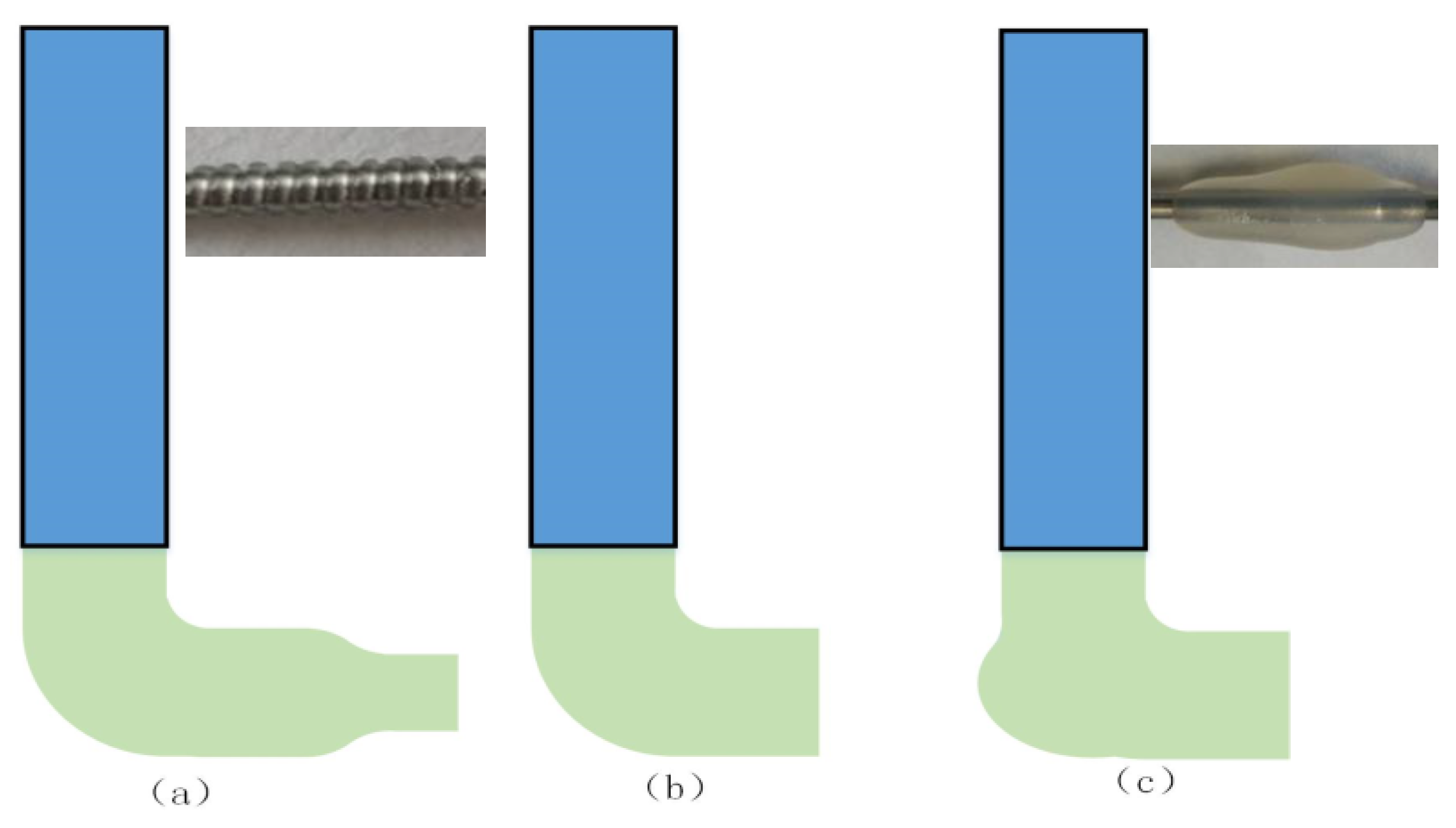
2.2. Fabrication Methods
2.3. Materials
3. Results
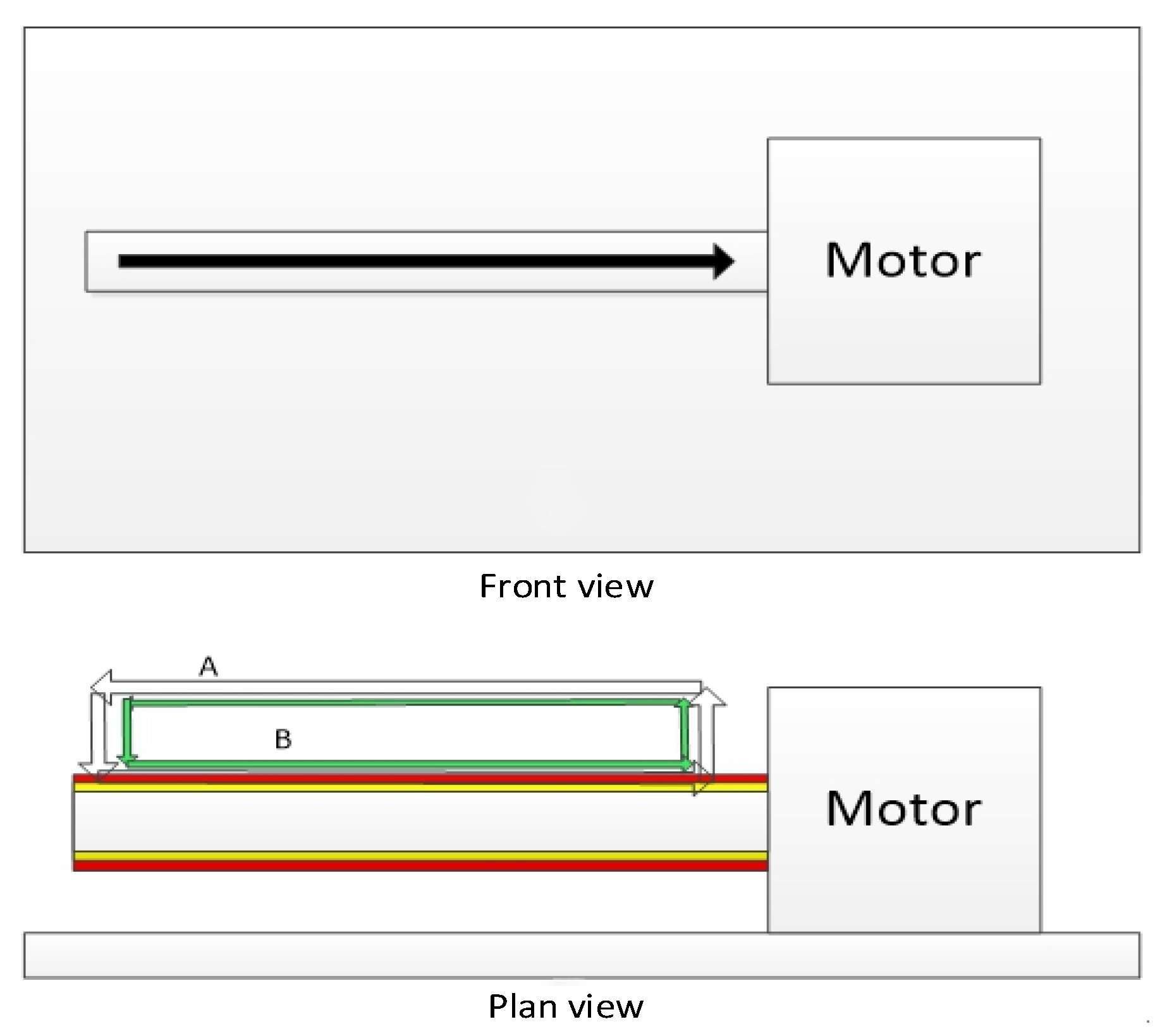
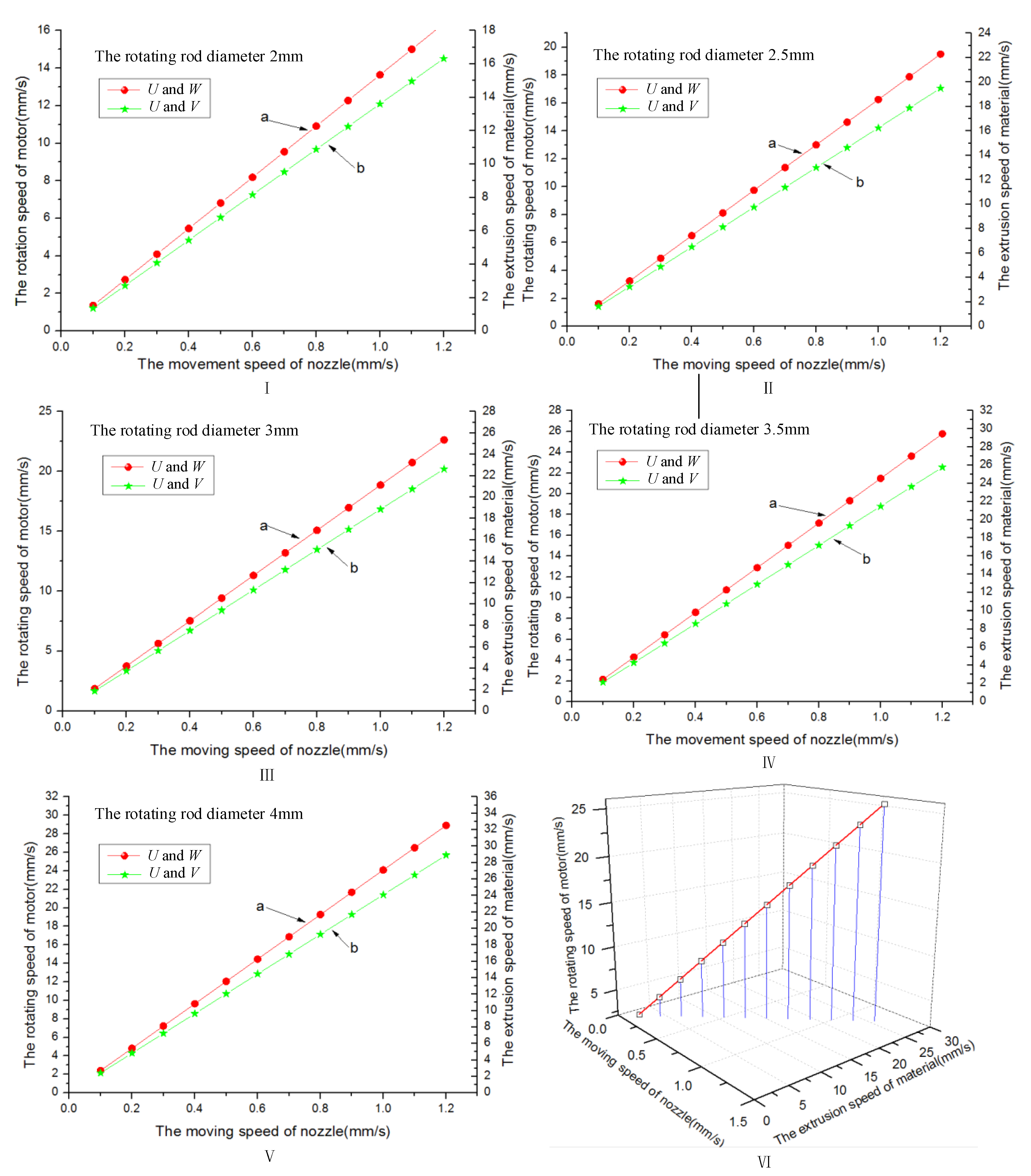
3.1. Multi-Layer Printing Path Planning
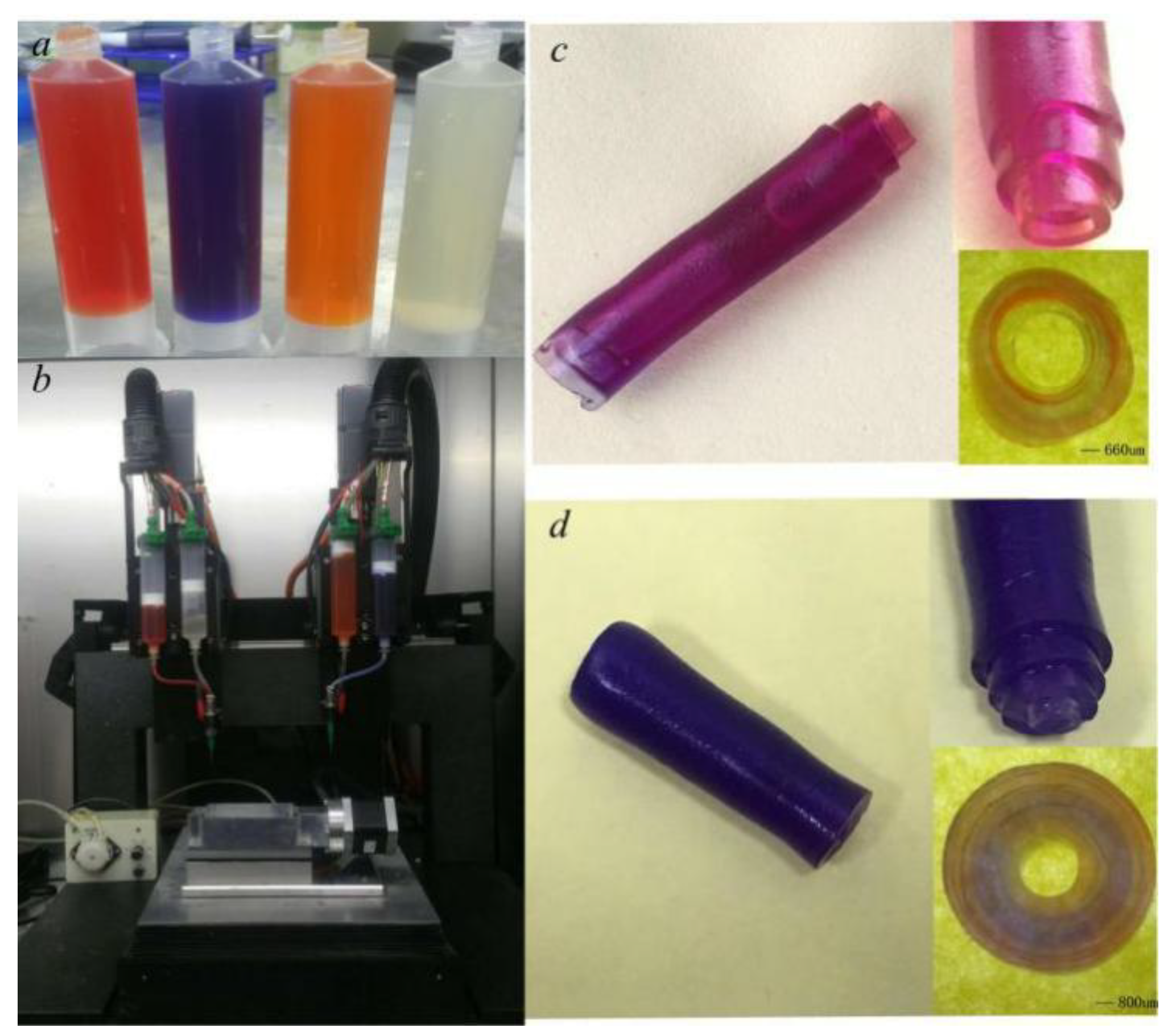
3.2. Multi-Layer Vessel-like Structure Printing Experiment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoch, E.; Tovar, G.E.; Borchers, K. Bioprinting of artificial blood vessels: Current approaches towards a demanding goal. Eur. J. Cardio-Thorac. Surg. 2014, 46, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, H.; Lan, H.; Liu, T. Organ regeneration: Integration application of cell encapsulation and 3D bioprinting. Virtual Phys. Prototyp. 2017, 12, 279–289. [Google Scholar] [CrossRef]
- Akintewe, O.O.; Roberts, E.G.; Rim, N.G.; Ferguson, M.A.; Wong, J.Y. Design Approaches to Myocardial and Vascular Tissue Engineering. Annu. Rev. Biomed. Eng. 2017, 19, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Gao, L.; Ma, L.; Luo, Y.; Yang, H.; Cui, Z. 3D Bioprinting: A Novel Avenue for Manufacturing Tissues and Organs. Engineering 2019, 5, 777–794. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.A.; Liu, W.; Jimenez, A.; Yang, J.; Akpek, A.; Liu, X.; Pi, Q.; Mu, X.; Hu, N.; Schiffelers, R.M.; et al. 3D Bioprinting: From Benches to Translational Applications. Small 2019, 15, e1805510. [Google Scholar] [CrossRef] [PubMed]
- Ulag, S.; Kalkandelen, C.; Oktar, F.N.; Uzun, M.; Sahin, Y.M.; Karademir, B.; Arslan, S.; Ozbolat, I.T.; Mahirogullari, M.; Gunduz, O. 3D Printing Artificial Blood Vessel Constructs Using PCL/Chitosan/Hydrogel Biocomposites. ChemistrySelect 2019, 4, 2387–2391. [Google Scholar] [CrossRef]
- Ruther, F.; Distler, T.; Boccaccini, A.R.; Detsch, R. Biofabrication of vessel-like structures with alginate di-aldehyde—gelatin (ADA-GEL) bioink. J. Mater. Sci. Mater. Med. 2018, 30, 8. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.W.; Tsai, W.B.; Jiang, S. Direct cell encapsulation in biodegradable and functionalizable carboxybetaine hydrogels. Biomaterials 2012, 33, 5706–5712. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Hwang, J.W.; Choi, Y.Y.; Wong, S.F.; Hwang, Y.H.; Lee, D.Y.; Lee, S.-H. In situ formation and collagen-alginate composite encapsulation of pancreatic islet spheroids. Biomaterials 2012, 33, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, Y.; Liu, C.; Yao, H.; Liu, B.; Mi, S. A Novel Strategy for Creating Tissue-Engineered Biomimetic Blood Vessels Using 3D Bioprinting Technology. Materials 2018, 11, 1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delrot, P.; Modestino, M.A.; Gallaire, F.; Psaltis, D.; Moser, C. Inkjet Printing of Viscous Monodisperse Microdroplets by Laser-Induced Flow Focusing. Phys. Rev. Appl. 2016, 6, 024003. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Richards, D.J.; Trusk, T.C.; Visconti, R.P.; Yost, M.J.; Kindy, M.S.; Drake, C.J.; Argraves, W.S.; Markwald, R.R.; Mei, Y. 3D printing facilitated scaffold-free tissue unit fabrication. Biofabrication 2014, 6, 024111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, R.; Zhang, Z.; Chai, W.; Huang, Y.; Chrisey, D.B. Freeform drop-on-demand laser printing of 3D alginate and cellular constructs. Biofabrication 2015, 7, 45011. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zheng, G.; Cheng, X.; Yang, X.; Zhao, G. Simulation Analysis of the Influence of Nozzle Structure Parameters on Material Controllability. Micromachines 2020, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Lian, Q.; Yang, L.; Mao, W.; Liu, S.; Xu, C. Preparation and Endothelialization of Multi-level Vessel-like Network in Enzymated Gelatin Scaffolds. J. Bionic Eng. 2018, 15, 673–681. [Google Scholar] [CrossRef]
- Forget, A.; Derme, T.; Mitterberger, D.; Heiny, M.; Sweeney, C.; Mudili, L.; Dargaville, T.R.; Shastri, V.P. Architecture-inspired paradigm for 3D bioprinting of vessel-like structures using extrudable carboxylated agarose hydrogels. Emergent Mater. 2019, 2, 233–243. [Google Scholar] [CrossRef]
- Daly, A.C.; Davidson, M.D.; Burdick, J.A. 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels. Nat. Commun. 2021, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, H.; Lan, H.; Liu, T.; Liu, X.; Yu, H. 3D Printing of Artificial Blood Vessel: Study on Multi-Parameter Optimization Design for Vascular Molding Effect in Alginate and Gelatin. Micromachines 2017, 8, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Yang, X.; Cheng, X.; Zhao, G.; Zheng, G.; Li, X.; Dong, R. Theoretical and Experimental Research on Multi-Layer Vessel-like Structure Printing Based on 3D Bio-Printing Technology. Micromachines 2021, 12, 1517. https://doi.org/10.3390/mi12121517
Liu H, Yang X, Cheng X, Zhao G, Zheng G, Li X, Dong R. Theoretical and Experimental Research on Multi-Layer Vessel-like Structure Printing Based on 3D Bio-Printing Technology. Micromachines. 2021; 12(12):1517. https://doi.org/10.3390/mi12121517
Chicago/Turabian StyleLiu, Huanbao, Xianhai Yang, Xiang Cheng, Guangxi Zhao, Guangming Zheng, Xuewei Li, and Ruichun Dong. 2021. "Theoretical and Experimental Research on Multi-Layer Vessel-like Structure Printing Based on 3D Bio-Printing Technology" Micromachines 12, no. 12: 1517. https://doi.org/10.3390/mi12121517





