ADRC-Based Control Method for the Vascular Intervention Master–Slave Surgical Robotic System
Abstract
:1. Introduction
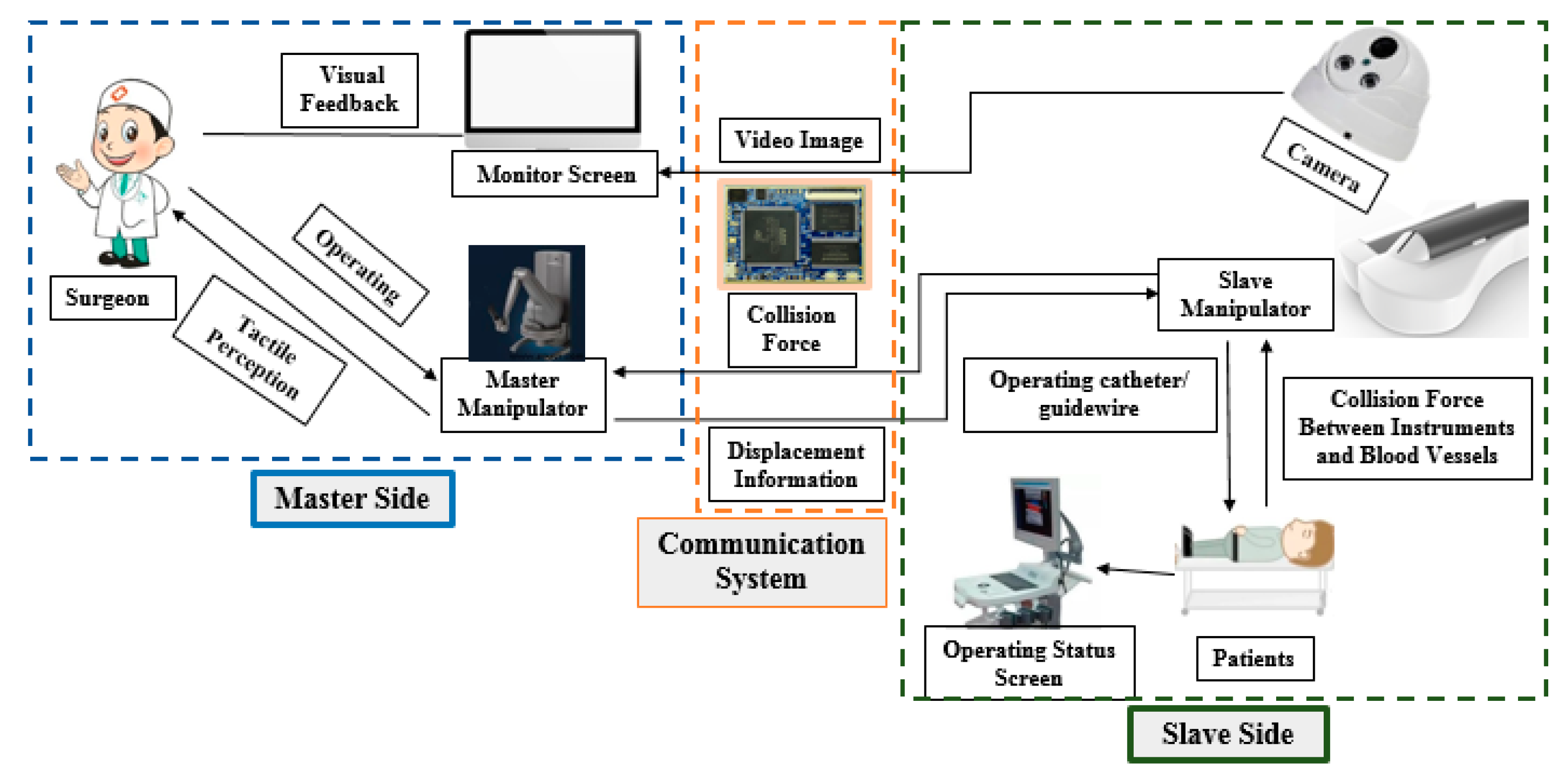
2. Master–Slave VISRS Description
2.1. Principle of the VISRS
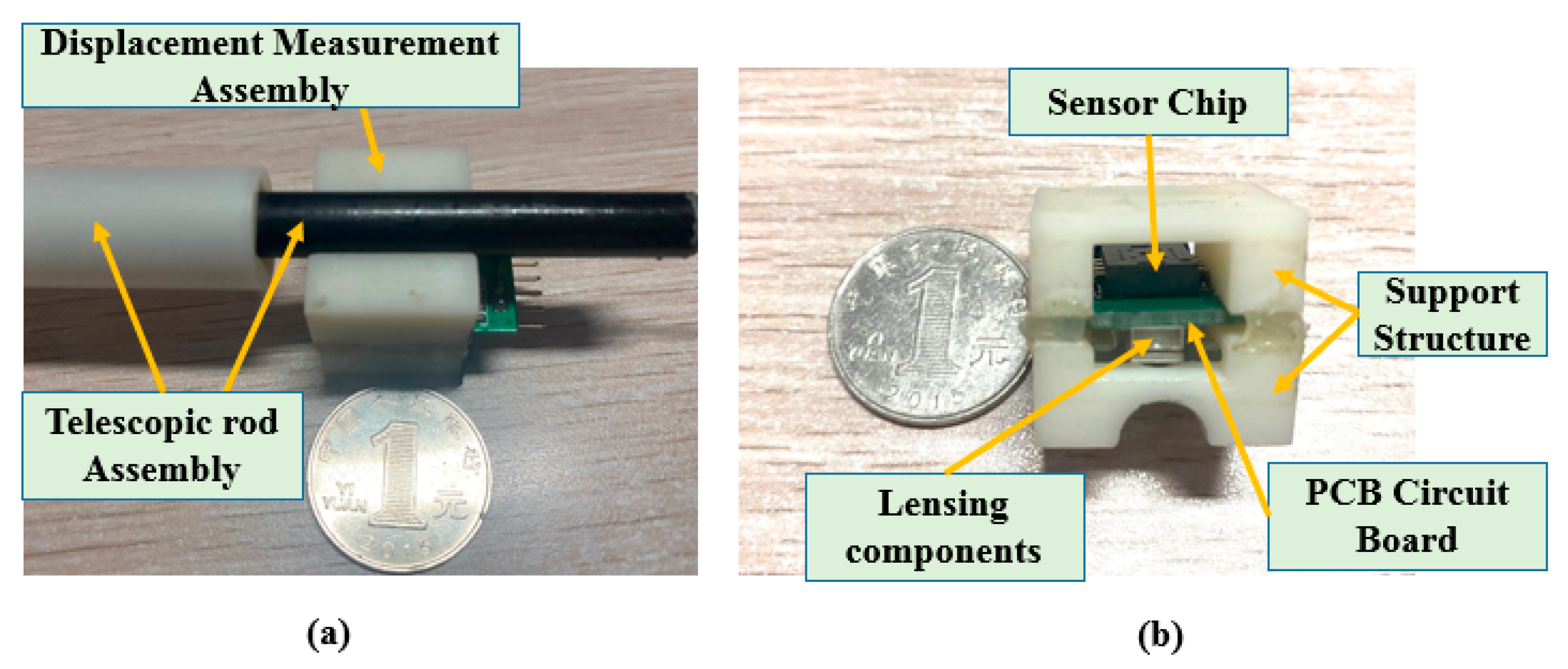
2.2. Description of the Master Manipulator
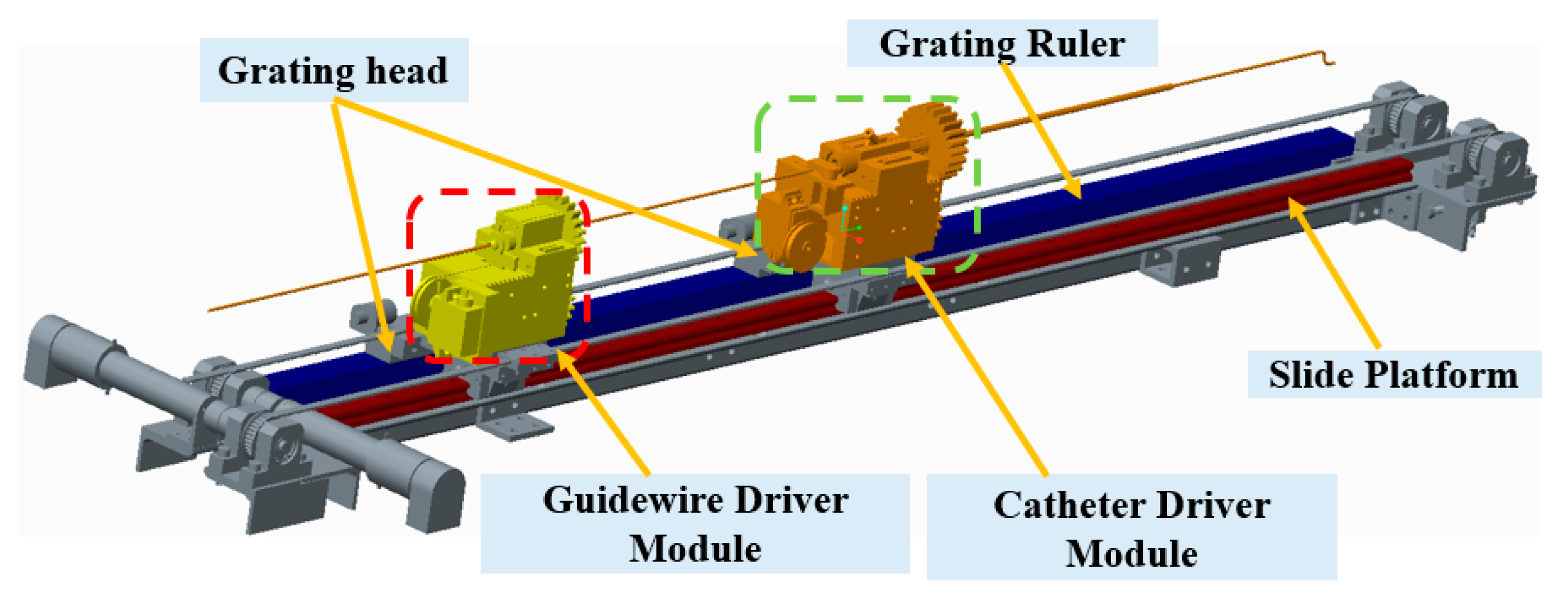
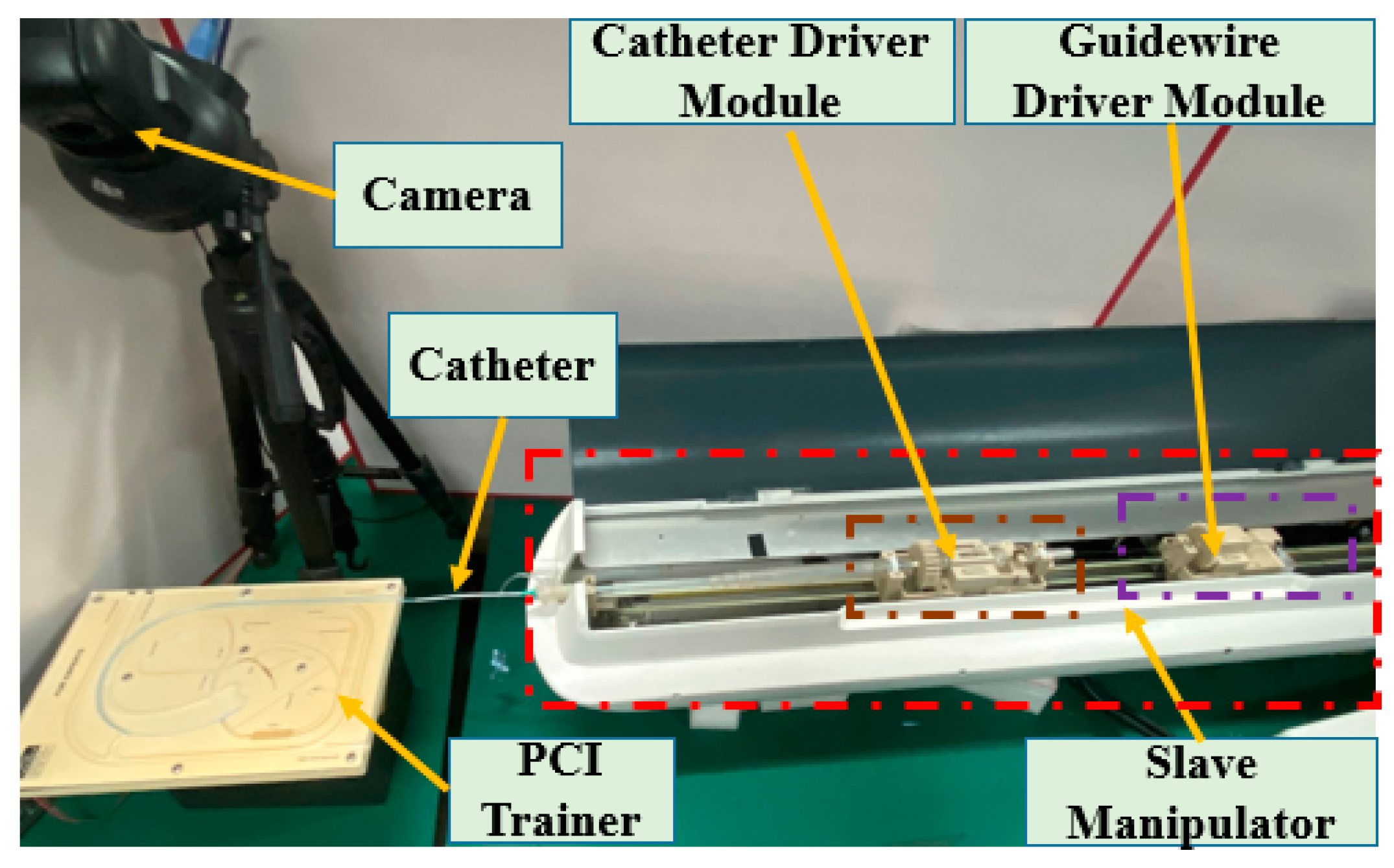
2.3. Description of the Slave Manipulator
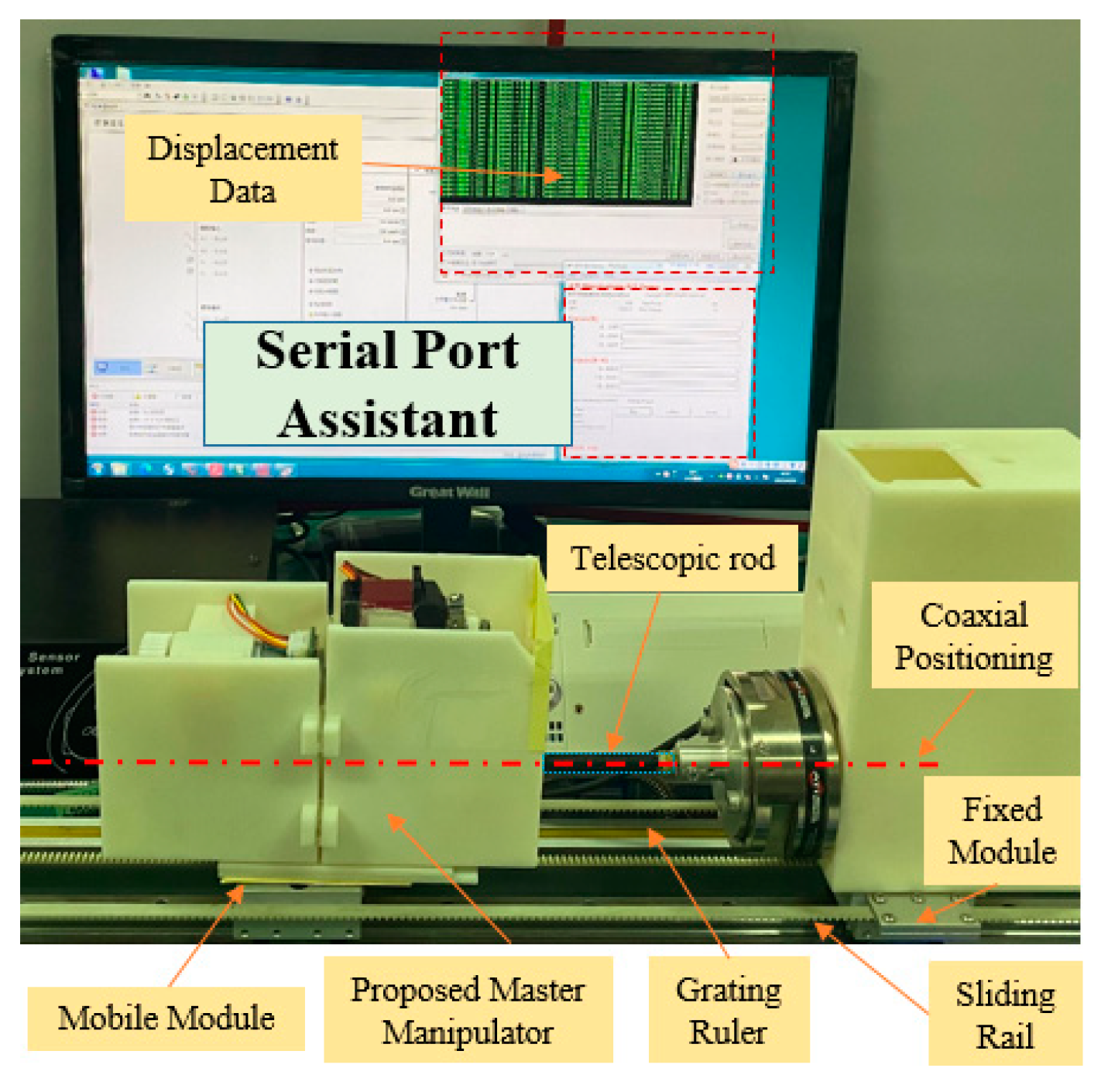
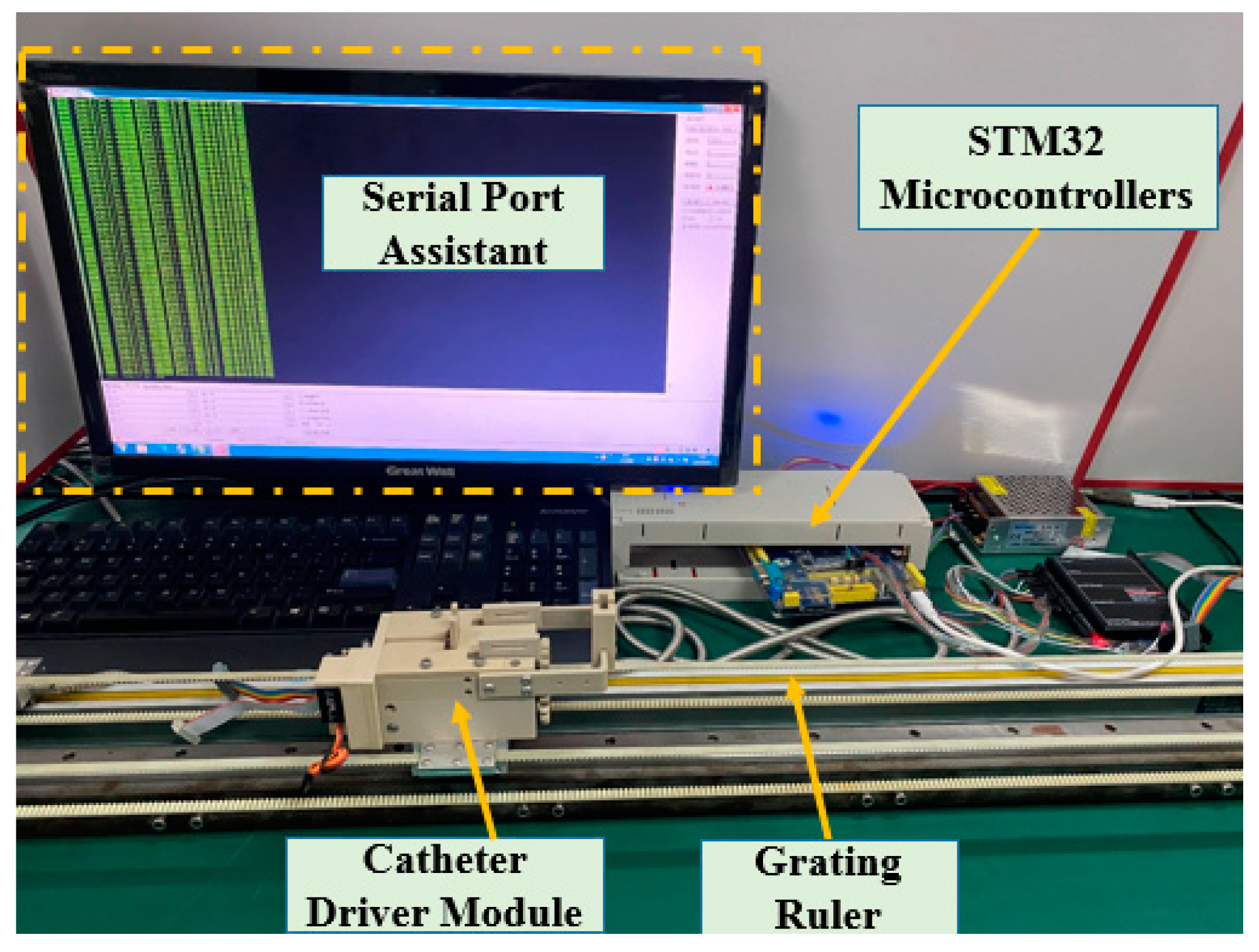
2.4. Calibration Experiments for the DMS of the Master Manipulator
3. Working Principles of the Control Method
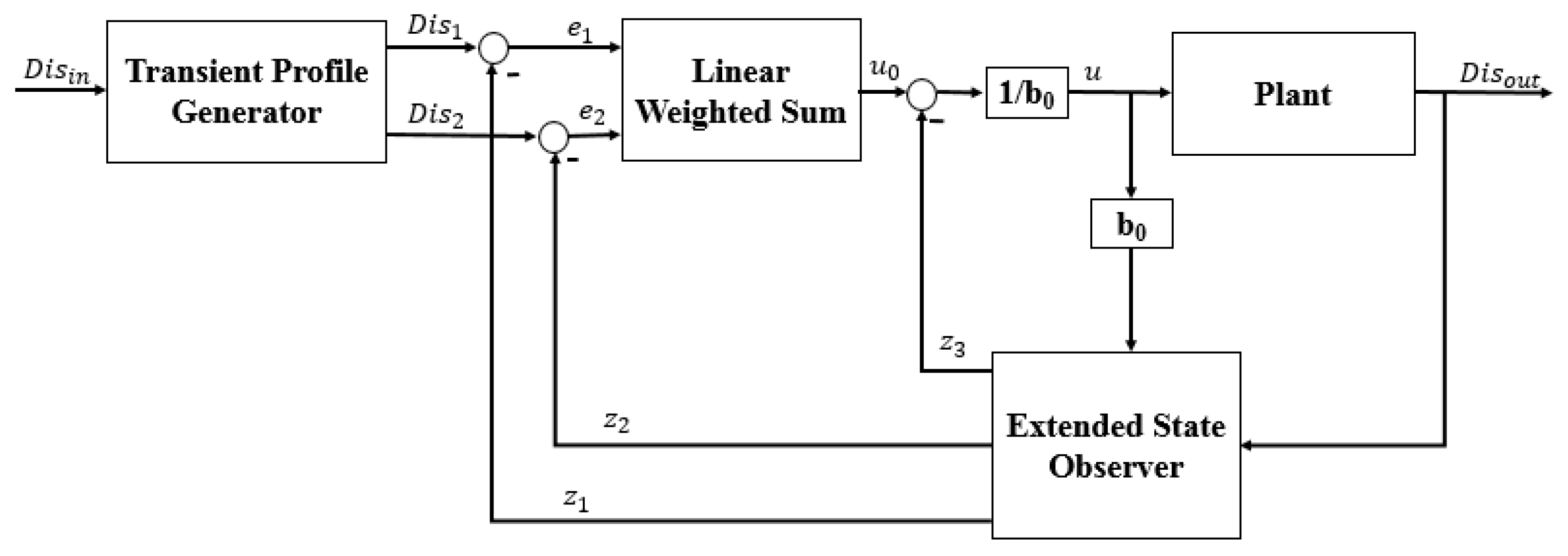
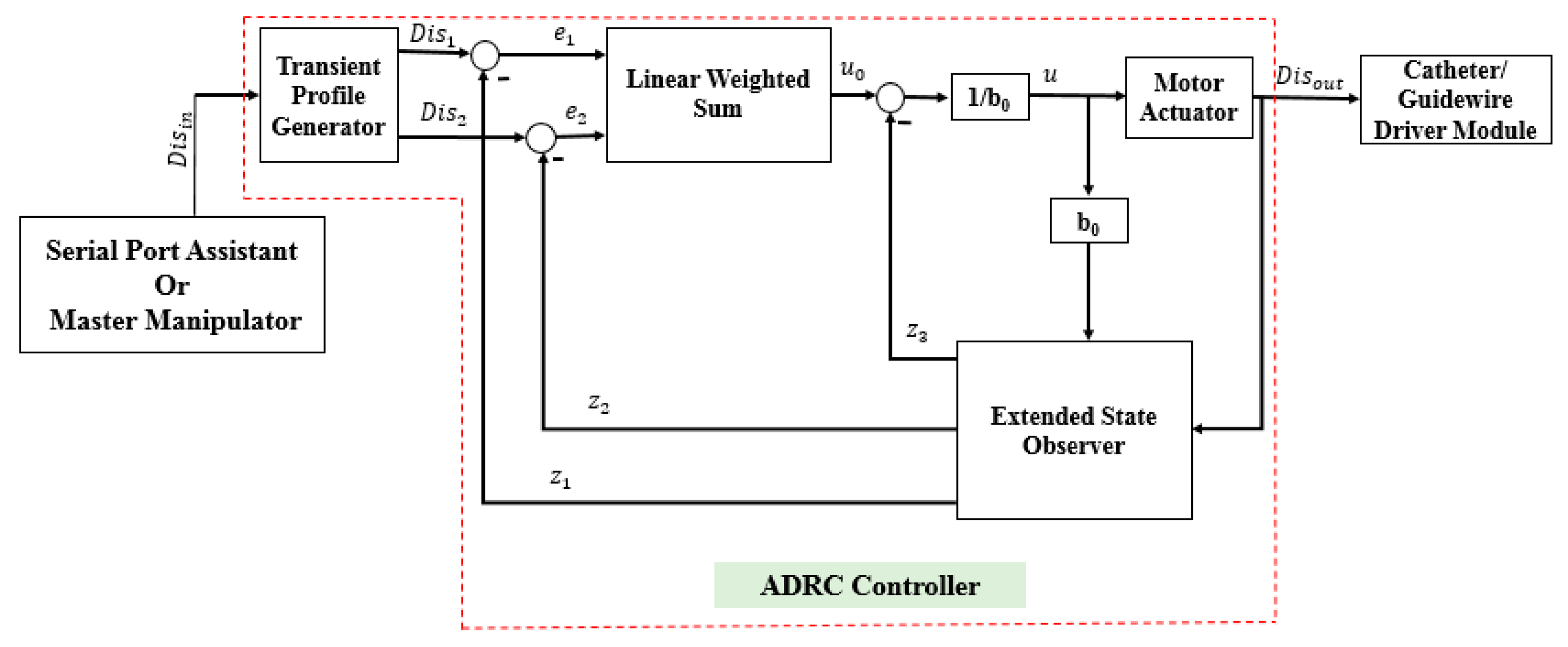
3.1. Description of the ADRC Method
- (1)
- The transient profile generator is depicted by Equation (2):where is the reference value, is the transient value, is the differential value of , is the maximum speed synthesis function, is the speed factor, and is the filter factor. The term is used to control the tracking speed, and can filter the jitter. The output value is .
- (2)
- The extended state observer is demonstrated by Equation (3):where , and are the status observer values of the , and the total disturbance of the system (including the internal and external disturbances), respectively. The , and are the scale parameters, which are calculated using Equation (4). The term is the bandwidth of the state observer. is the compensating parameter. The value of is calculated using Equation (5):
- (3)
- The state error feedback law is given by Equation (6):where is the plant of error feedback controlled, and and are the coefficients of the error and error derivatives, respectively.
- (4)
- The disturbance compensation process is calculated by Equation (7):
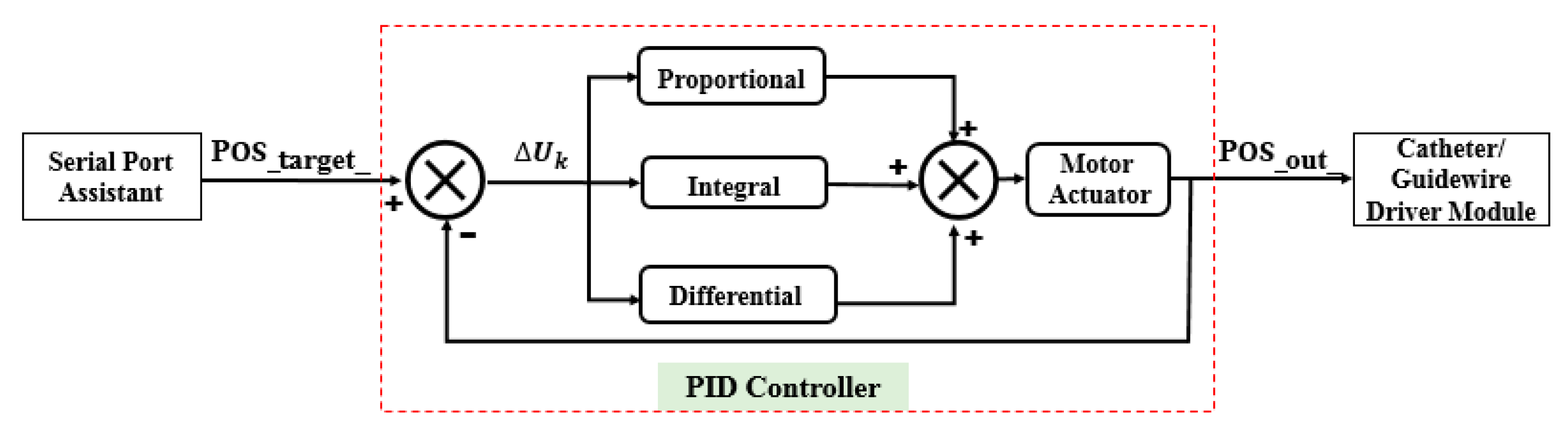
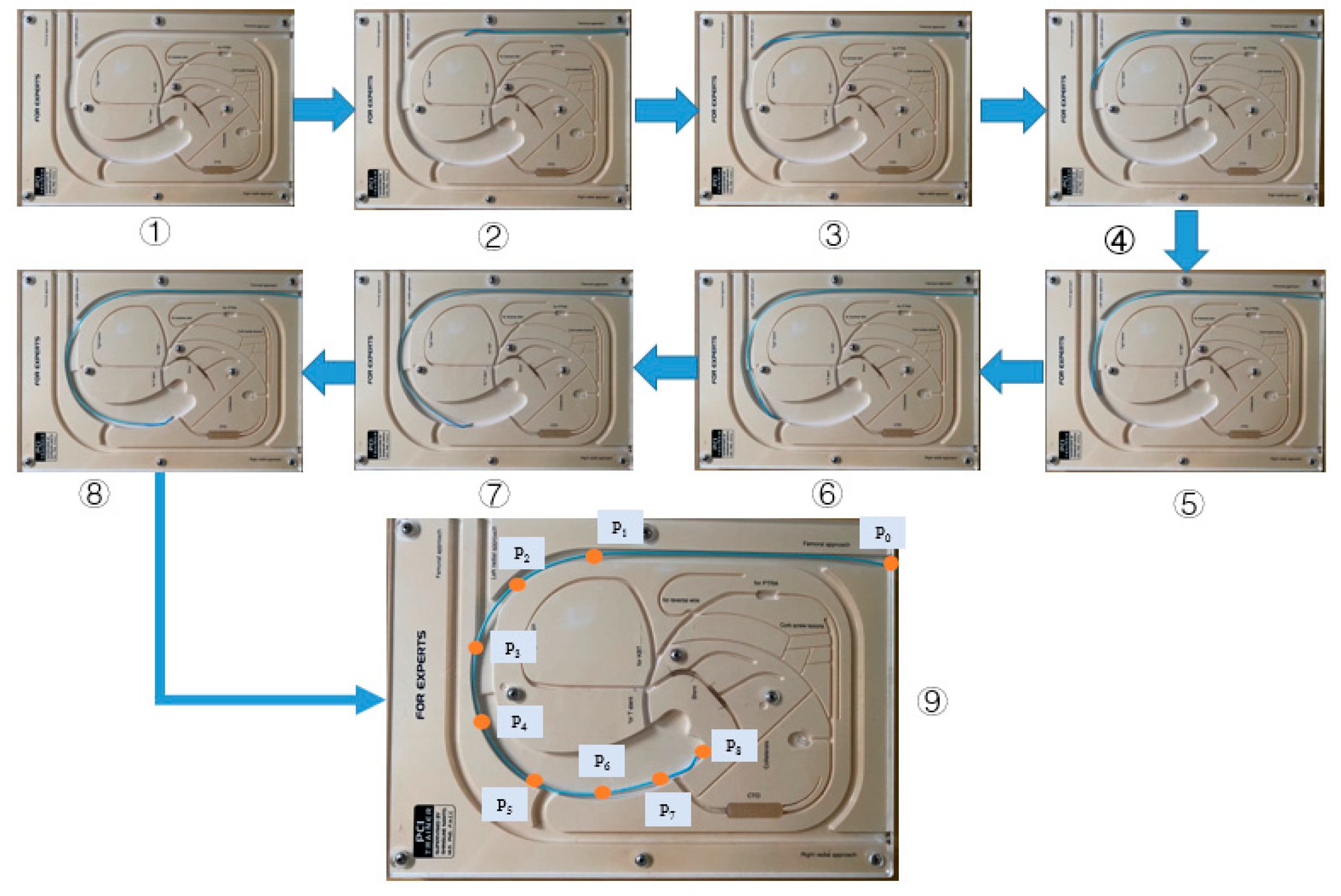
3.2. Comparative Experiments
4. Experiments and Results
5. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roguin, A.; Goldstein, J.; Bar, O.; Goldstein, J.A. Brain and neck tumors among physicians performing interventional procedures. Am. J. Cardiol. 2013, 111, 1368–1372. [Google Scholar] [CrossRef]
- Vano, E.; Kleiman, N.J.; Duran, A.; Romano-Miller, M.; Rehani, M.M. Radiation associated lens opacities in catheterization personnel: Results of a survey and direct assessments. J. Vasc. Interv. Radiol. 2013, 24, 197–204. [Google Scholar] [CrossRef]
- Klein, L.W.; Tra, Y.; Garratt, K.N.; Powell, W.; Lopez-Cruz, G.; Chambers, C.; Goldstein, J.A. Occupational health hazards of interventional cardiologists in the current decade: Results of the 2014 SCAI membership survey. Catheter. Cardiovasc. Interv. 2015, 86, 913–924. [Google Scholar] [CrossRef]
- Wang, H.; Chang, J.; Yu, H.; Liu, H.; Hou, C.; Lu, H. Research on a Novel Vascular Interventional Surgery Robot and Control Method Based on Precise Delivery. IEEE Access 2021, 9, 26568–26582. [Google Scholar] [CrossRef]
- Patel, T.M.; Shah, S.C.; Soni, Y.Y.; Radadiya, R.C.; Patel, G.A.; Tiwari, P.O.; Pancholy, S.B. Comparison of robotic percutaneous coronary intervention with traditional percutaneous coronary intervention: A propensity score–matched analysis of a large cohort. Circ. Cardiovasc. Interv. 2020, 13, 1941–7632. [Google Scholar] [CrossRef] [PubMed]
- Riga, C.V.; Bicknell, C.D.; Rolls, A.; Cheshire, N.J.; Hamady, M.S. Robot-assisted fenestrated endovascular aneurysm repair (FEVAR) using the Magellan system. J. Vasc. Interv. Radiol. 2013, 24, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Khan, E.M.; Frumkin, W.; Ng, G.A.; Neelagaru, S.; Abi-Samra, F.M.; Lee, J.; Giudici, M.; Gohn, D.; Winkle, R.A.; Sussman, J.; et al. First experience with a novel robotic remote catheter system: Amigo mapping trial. J. Interv. Card. Electrophysiol. 2013, 37, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Faddis, M.N.; Blume, W.; Finney, J.; Hall, A.; Rauch, J.; Sell, J.; Bae, K.T.; Talcott, M.; Lindsay, B. Novel, magnetically guided catheter for endocardial mapping and radiofrequency catheter ablation. Circulation 2002, 106, 2980–2985. [Google Scholar] [CrossRef] [Green Version]
- Millan, B.; Nagpal, S.; Ding, M.; Lee, J.Y.; Kapoor, A. A Scoping Review of Emerging and Established Surgical Robotic Platforms With Applications in Urologic Surgery. Soc. Int. Urol. J. 2021, 2, 300–310. [Google Scholar] [CrossRef]
- Shi, P.; Guo, S.; Zhang, L.; Jin, X.; Hirata, H.; Tamiya, T.; Kawanishi, M. Design and Evaluation of a Haptic Robot-assisted Catheter Operating System with Collision Protection Function. IEEE Sens. J. 2021, 21, 20807–20816. [Google Scholar] [CrossRef]
- Yin, X.; Guo, S.; Xiao, N.; Tamiya, T.; Hirata, H.; Ishihara, H. Safety Operation Consciousness Realization of a MR Fluids-Based Novel Haptic Interface for Teleoperated Catheter Minimally Invasive Neurosurgery. IEEE/ASME Trans. Mechatron. 2016, 21, 1043–1054. [Google Scholar] [CrossRef]
- Woo, J.; Song, H.; Cha, H.; Yi, B. Advantage of Steerable Catheter and Haptic Feedback for a 5-DOF Vascular Intervention Robot System. Appl. Sci. 2019, 9, 4305. [Google Scholar] [CrossRef] [Green Version]
- Bao, X.; Guo, S.; Xiao, N.; Li, Y.; Yang, C.; Jiang, Y. A cooperation of catheters and guidewires-based novel remote-controlled vascular interventional robot. Biomed. Microdevices 2018, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Guo, S.; Xiao, N.; Li, Y.; Yang, C.; Shen, R.; Cui, J.; Jiang, Y.; Liu, X.; Liu, K. Operation evaluation in-human of a novel remote-controlled vascular interventional robot. Biomed. Microdevices 2018, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Wang, C.; Xie, L.; Zhou, S.; Gu, L.; Xie, H. A novel remote-controlled robotic system for cerebrovascular intervention. Int. J. Med. Robot. Comput. Assist. Surg. 2018, 14, e1943. [Google Scholar] [CrossRef]
- Omisore, O.; Han, S.; Ren, L.; Wang, G.; Ou, F.; Li, H.; Wang, L. Towards Characterization and Adaptive Compensation of Backlash in a Novel Robotic Catheter System for Cardiovascular Interventions. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 824–838. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, S.; Guo, S.; Tamiya, T. A Magnetorheological Fluids-Based Robot-Assisted Catheter/Guidewire Surgery System for Endovascular Catheterization. Micromachines 2021, 12, 640. [Google Scholar] [CrossRef]
- Guo, J.; Jin, X.; Guo, S.; Fu, Q. A Vascular Interventional Surgical Robotic System Based on Force-Visual Feedback. IEEE Sens. J. 2019, 19, 11081–11089. [Google Scholar] [CrossRef]
- Guo, J.; Jin, X.; Guo, S. Study of the Operational Safety of a Vascular Interventional Surgical Robotic System. Micromachines 2018, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Lee, D.Y. Hydraulically Steerable Micro Guidewire Capable of Distal Sharp Steering. IEEE Trans. Biomed. Eng. 2021, 68, 728–735. [Google Scholar] [CrossRef]
- Pancadi, L.; Dirix, P.; Fanelli, A.; Lima, A.M.; Stergiopulos, N.; Mosimann, P.J.; Ghezzi, D.; Sakar, M.S. Flow Driven Robotic Navigation of Micro-engineered Endovascular Probes. Nat. Commun. 2020, 11, 1–14. [Google Scholar]
- Zhou, X.; Bian, G.; Xie, X.; Hou, Z.; Qu, X.; Guan, S. Analysis of Interventionalists’ Natural Behaviors for Recognizing Motion Patte ems of Endovascular Tools during Percutaneous Coronary Interventions. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Guo, S.; Li, M.; Tamiya, T. A Marker-based Contactless Catheter-sensing Method to Detect Surgeons’ Operations for Catheterization Training Systems. Biomed. Microdevices 2018, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, S.; Wang, Y.; Cui, J.; Ma, Y.; Zeng, Y.; Liu, X.; Jiang, Y.; Li, Y.; Shi, L.; et al. A CNNs-based Prototype Method of Unstructured Surgical State Perception and Navigation for an Endovascular Surgery Robot. Med. Biol. Eng. Comput. 2019, 57, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xing, H.; Guo, S.; Wang, Y.; Cui, J.; Ma, Y.; Liu, Y.; Liu, Y.; Feng, J.; Li, Y. A novel noncontact detection method of surgeon’s operation for a master-slave endovascular surgery robot. Med. Biol. Eng. Comput. 2020, 58, 871–885. [Google Scholar] [CrossRef]
- Guo, S.; Cui, J.; Zhao, Y.; Wang, Y.; Ma, Y.; Gao, W.; Mao, G.; Hong, S. Machine learning-based Operation Skills Assessment with Vascular Difficulty Index for Vascular Intervention Surgery. Med. Biol. Eng. Comput. 2020, 58, 1707–1721. [Google Scholar] [CrossRef]
- Guo, S.; Wang, Y.; Zhao, Y.; Cui, J.; Ma, Y.; Mao, G.; Hong, S. A Surgeon’s Operating Skills-based Non-interference Operation Detection Method for Novel Vascular Interventional Surgery Robot Systems. IEEE Sens. J. 2019, 20, 3879–3891. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, J.; Xie, L.; Cui, G. A generalized predictive control for remote cardiovascular surgical systems. ISA Trans. 2020, 104, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Guo, S.; Guo, J.; Shi, P.; Tamiya, T.; Hirata, H. Development of a Tactile Sensing Robot-Assisted System for Vascular Interventional Surgery. IEEE Sens. J. 2021, 21, 12284–12294. [Google Scholar] [CrossRef]
- Jin, X.; Guo, S.; Guo, J.; Shi, P.; Tamiya, T.; Kawanishi, M.; Hirata, H. Total Force Analysis and Safety Enhancing for Operating both Guidewire and Catheter in Endovascular Surgery. IEEE Sens. J. 2021, 21, 22499–22509. [Google Scholar] [CrossRef]
- Yang, C.; Guo, S.; Bao, X.; Xiao, N.; Shi, L.; Li, Y.; Jiang, Y. A Vascular Interventional Surgical Robot Based on Surgeon’s Operating Skills. Med. Biol. Eng. Comput. 2019, 57, 1999–2010. [Google Scholar] [CrossRef]
- Wang, K.; Lu, Q.; Chen, B.; Shen, Y.; Li, H.; Liu, M.; Xu, Z. Endovascular Intervention Robot with Multi-manipulators for Surgical Procedures: Dexterity, adaptability and practicability. Robot. Comput.-Integr. Manuf. 2019, 56, 75–84. [Google Scholar] [CrossRef]
- Haidegger, T.; Benyó, B.; Kovács, L.; Benyó, Z. Force Sensing and Force Control for Surgical Robots. IFAC Proc. 2009, 42, 401–406. [Google Scholar] [CrossRef]
- Haidegger, T. Probabilistic Method to Improve the Accuracy of Computer-Integrated Surgical Systems. Acta Polytech. Hung. 2019, 16, 119–141. [Google Scholar] [CrossRef]
- Jayender, J.; Patel, R.; Nikumb, S. Robot-assisted Active Catheter Insertion: Algorithms and Experiments. Int. J. Robot. Res. 2009, 28, 1101–1117. [Google Scholar] [CrossRef]
- Guo, J.; Yang, S.; Guo, S.; Meng, C.; Qi, L. Study on Robust Control for the Vascular Interventional Surgical Robot. In Proceedings of the 2019 IEEE International Conference on Mechatronics and Automation (ICMA), Tianjin, China, 4–7 August 2019; pp. 1361–1366. [Google Scholar] [CrossRef]
- Han, J. From PID to Active Disturbance Rejection Control. IEEE Trans. Ind. Electron. 2009, 56, 900–906. [Google Scholar] [CrossRef]
- Peng, H.; Wu, M.; Lu, H.; Wang, J.; Xiao, J.; Huang, Y.; Shi, D. A Distributed Strategy to Attitude Following of the Multi-DOF Parallel Electrical Manipulator Systems. IEEE Trans. Ind. Electron. 2021, 69, 1630–1640. [Google Scholar] [CrossRef]
- Lakomy, K.; Madonski, R.; Dai, B.; Yang, J.; Kicki, P.; Ansari, M.; Li, S. Active Disturbance Rejection Control Design with Suppression of Sensor Noise Effects in Application to DC-DC Buck Power Converter. IEEE Trans. Ind. Electron. 2021, 69, 816–824. [Google Scholar] [CrossRef]
- Chi, R.; Hui, Y.; Huang, B.; Hou, Z. Active Disturbance Rejection Control for Nonaffined Globally Lipschitz Nonlinear Discrete-time Systems. IEEE Trans. Autom. Control. 2021, 1, 1. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, H.; Wu, M.; Zhang, W. Active Disturbance Rejection Decoupling Control for Three-Degree-of- Freedom Six-Pole Active Magnetic Bearing Based on BP Neural Network. IEEE Trans. Appl. Supercond. 2020, 30, 1–5. [Google Scholar] [CrossRef]
- Lu, W.; Li, Q.; Lu, K.; Lu, Y.; Guo, L.; Yan, W.; Xu, F. Load Adaptive PMSM Drive System Based on an Improved ADRC for Manipulator Joint. IEEE Access 2021, 9, 33369–33384. [Google Scholar] [CrossRef]
- Sun, L.; Xue, W.; Li, D.; Zhu, H.; Su, Z. Quantitative Tuning of Active Disturbance Rejection Controller for FOPDT Model with Application to Power Plant Control. IEEE Trans. Ind. Electron. 2021, 69, 805–815. [Google Scholar] [CrossRef]
- Guo, S.; Song, Y.; Yin, X.; Zhang, L.; Tamiya, T.; Hirata, H.; Ishihara, H. A Novel Robot-Assisted Endovascular Catheterization System with Haptic Force Feedback. IEEE Trans. Robot. 2019, 35, 685–696. [Google Scholar] [CrossRef]
- Yin, X.; Guo, S.; Song, Y. Magnetorheological Fluids Actuated Haptic-based Tele-operated Catheter Operating System. Micromachines 2018, 9, 465. [Google Scholar] [CrossRef] [Green Version]
- Bao, X.; Guo, S.; Shi, L.; Xiao, N. Design and Evaluation of Sensorized Robot for Minimally Vascular Interventional Surgery. Microsyst. Technol. 2019, 25, 2759–2766. [Google Scholar] [CrossRef]
- Jie, Y.; Liu, Z.; Zeng, W. Isothermal Extrusion Speed Curve Design for Porthole Die of Hollow Aluminium Profile Based on PID Algorithm and Finite Element Simulations. Trans. Nonferrous Met. Soc. China 2021, 31, 1939–1950. [Google Scholar]
- Gao, Z. Scaling and Bandwidth-Parameterization Based Controller Tuning. In Proceedings of the 2003 American Control Conference, Denver, CO, USA, 4–6 June 2003; pp. 4989–4996. [Google Scholar] [CrossRef]
- McDonald, P.; Ashton, K.; Barratt, R.; Doyle, S.; Imeson, D.; Meir, A.; Risser, G. Clinical realism: A new literary genre and a potential tool for encouraging empathy in medical students. BMC Med. Educ. 2015, 15, 112. [Google Scholar] [CrossRef] [Green Version]
- Nagy, T.; Haidegger, T. Towards Standard Approaches for the Evaluation of Autonomous Surgical Subtask Execution. In Proceedings of the 25th International Conference on Intelligent Engineering Systems, Budapest, Hungary, 7–9 July 2021. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Guo, S.; Guo, J.; Meng, F.; Chen, Z. ADRC-Based Control Method for the Vascular Intervention Master–Slave Surgical Robotic System. Micromachines 2021, 12, 1439. https://doi.org/10.3390/mi12121439
Zhou W, Guo S, Guo J, Meng F, Chen Z. ADRC-Based Control Method for the Vascular Intervention Master–Slave Surgical Robotic System. Micromachines. 2021; 12(12):1439. https://doi.org/10.3390/mi12121439
Chicago/Turabian StyleZhou, Wei, Shuxiang Guo, Jin Guo, Fanxu Meng, and Zhengyang Chen. 2021. "ADRC-Based Control Method for the Vascular Intervention Master–Slave Surgical Robotic System" Micromachines 12, no. 12: 1439. https://doi.org/10.3390/mi12121439





