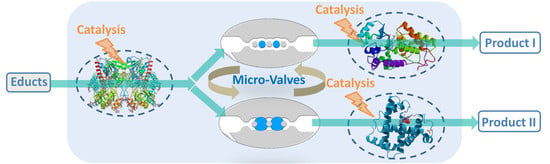
Hydrogel Microvalves as Control Elements for Parallelized Enzymatic Cascade Reactions in Microfluidics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Measurement of Enzyme Activities
2.3. Enzyme Immobilisation in Photopatterned Hydrogel-Enzyme-Dots
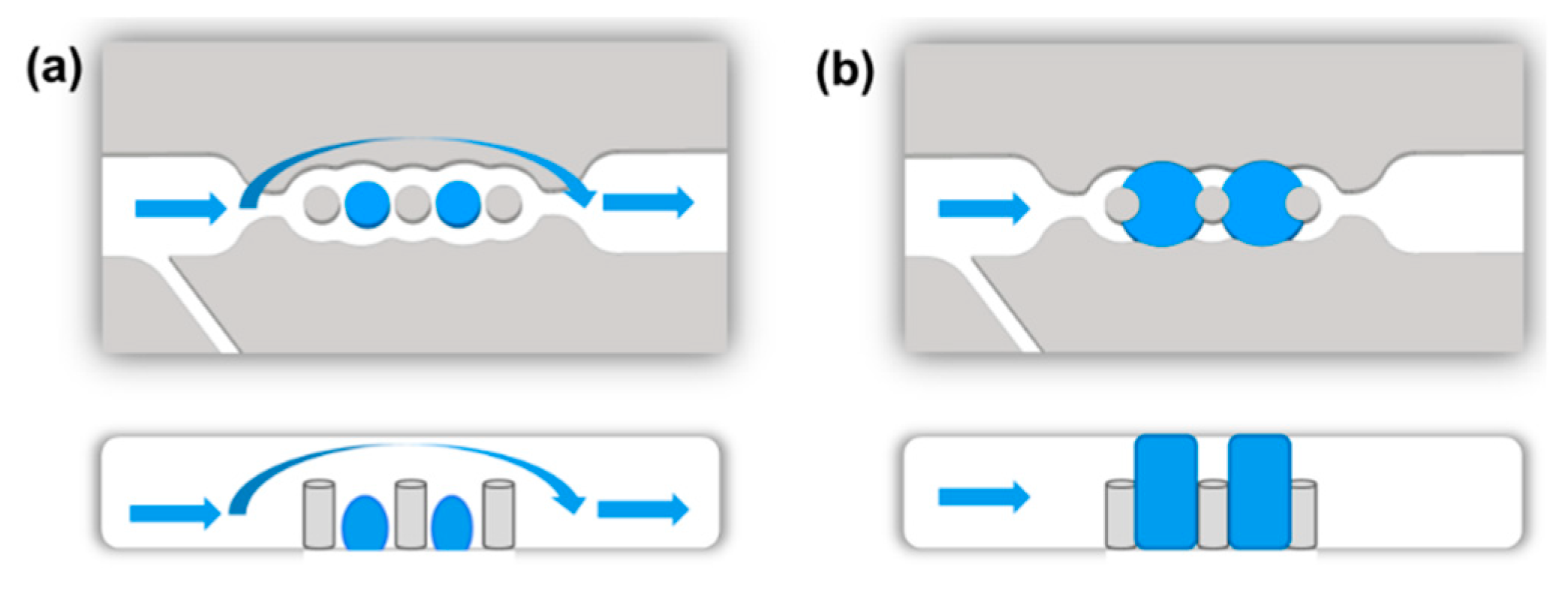
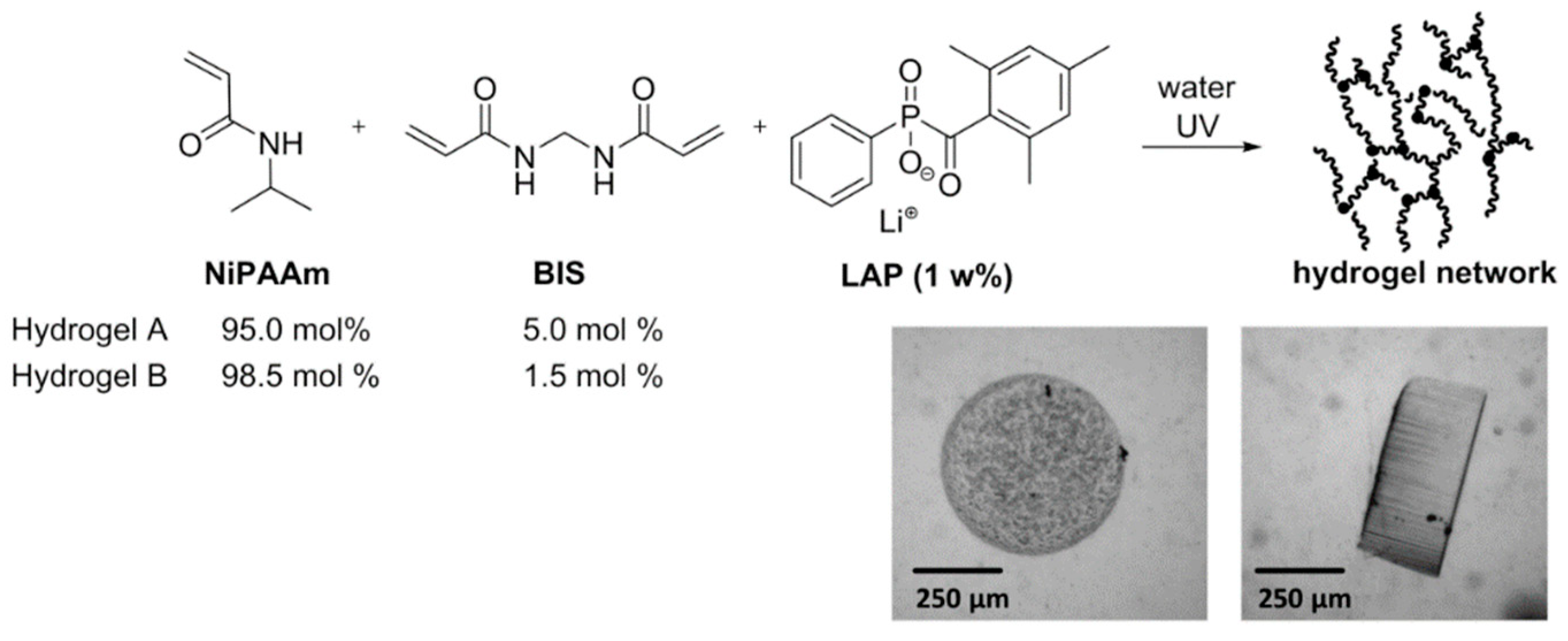
2.4. Synthesis of Hydrogel Microvalves
2.5. Design and Production of the Heat Pad
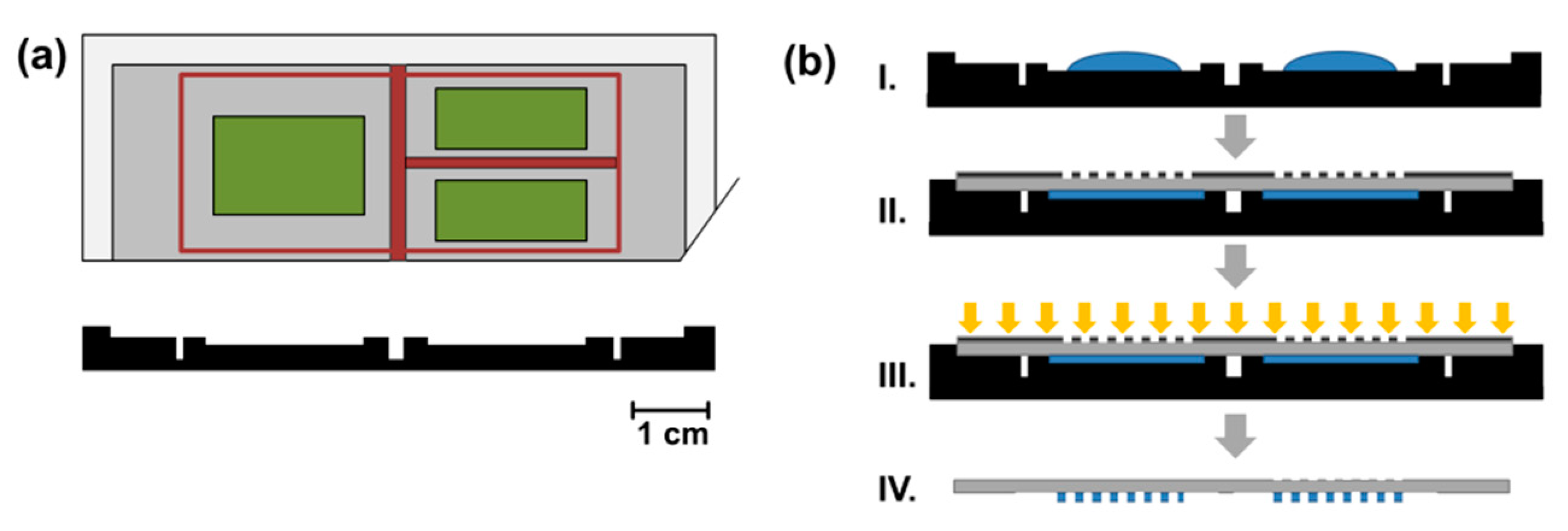
2.6. Production and Assembly of Microfluidic Devices
2.7. Measurement of Enzymatic Activity in Microfluidic Devices
3. Results and Discussion
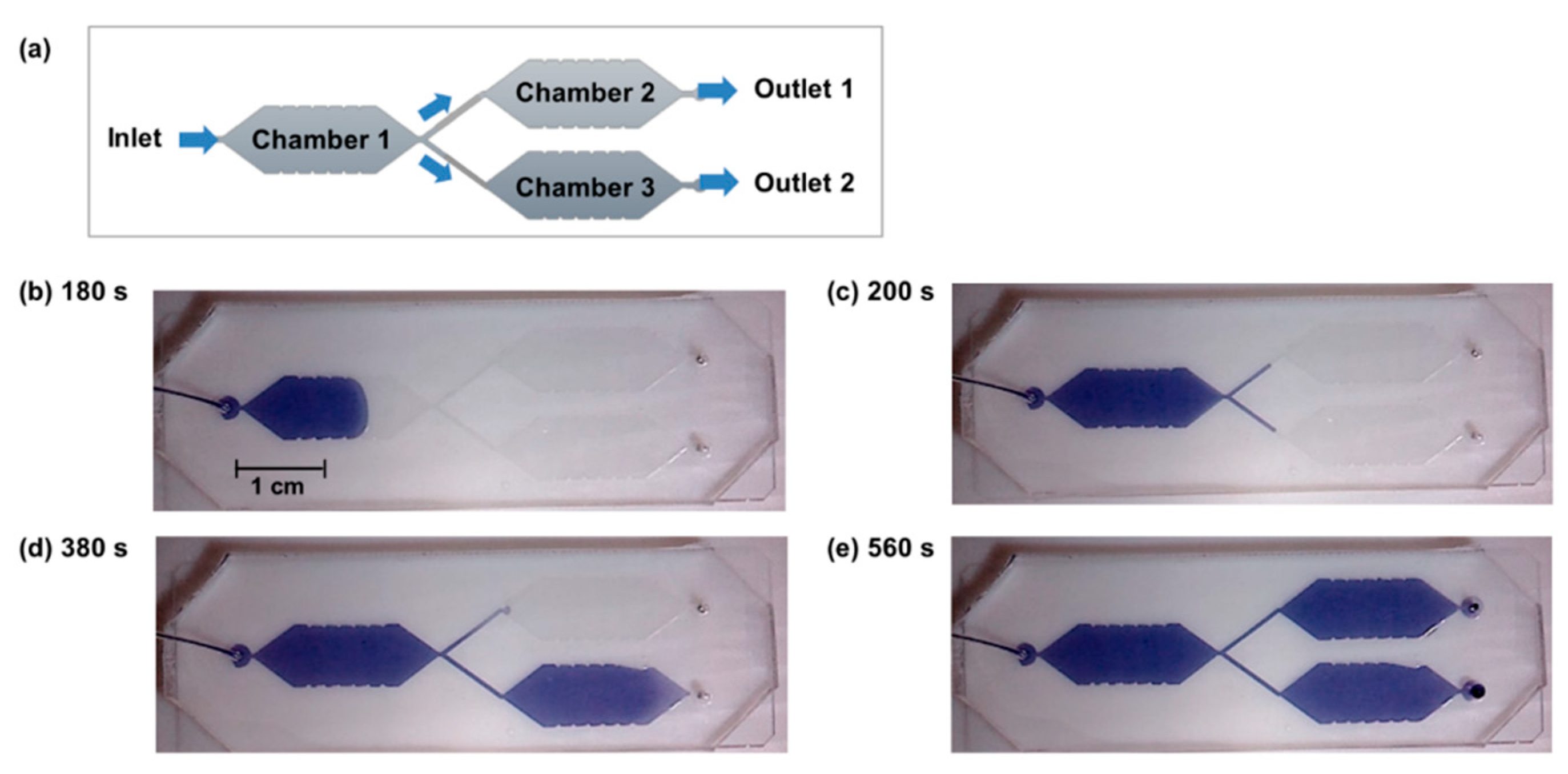
3.1. Microfluidic Device with Parallel Compartments
3.2. Integration of Hydrogel Microvalves in the Microfluidic Device
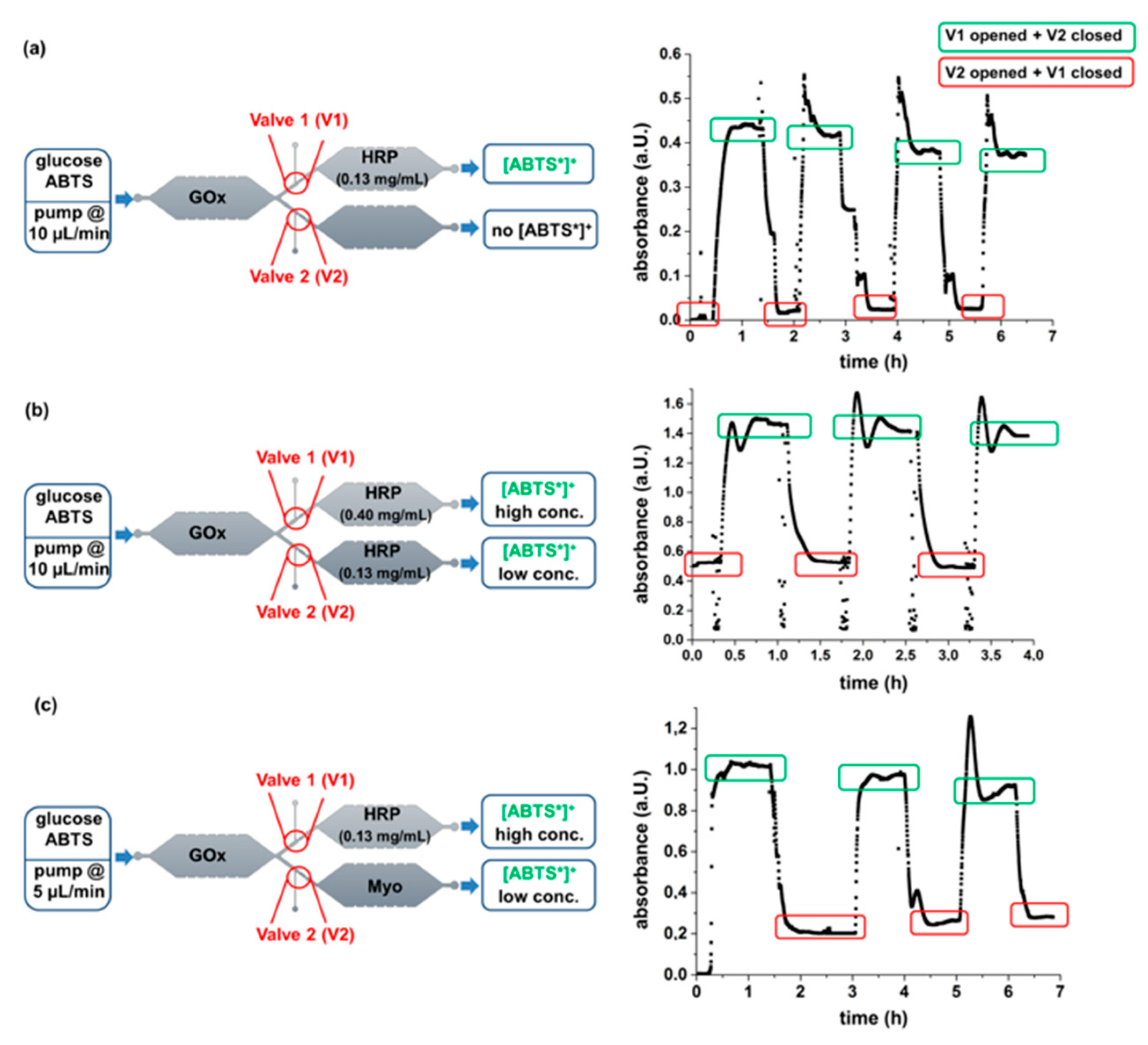
3.3. Parallelized Enzymatic Cascade Reactions in Microfluidic Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van der Helm, M.W.; van der Meer, A.D.; Eijkel, J.C.; van den Berg, A.; Segerink, L.I. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 2016, 4, e1142493. [Google Scholar] [CrossRef] [PubMed]
- Bein, A.; Shin, W.; Jalili-Firoozinezhad, S.; Park, M.H.; Sontheimer-Phelps, A.; Tovaglieri, A.; Chalkiadaki, A.; Kim, H.J.; Ingber, D.E. Microfluidic Organ-on-a-Chip Models of Human Intestine. Cell Mol. Gastroenterol. Hepatol. 2018, 5, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ibanez, A.; Chatrathi, M.P. On-chip integration of enzyme and immunoassays: Simultaneous measurements of insulin and glucose. J. Am. Chem. Soc. 2003, 125, 8444–8445. [Google Scholar] [CrossRef]
- Xia, Y.; Si, J.; Li, Z. Fabrication techniques for microfluidic paper-based analytical devices and their applications for biological testing: A review. Biosens. Bioelectron. 2016, 77, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sjoberg, R.; Leyrat, A.A.; Pirone, D.M.; Chen, C.S.; Quake, S.R. Versatile, fully automated, microfluidic cell culture system. Anal. Chem. 2007, 79, 8557–8563. [Google Scholar] [CrossRef] [PubMed]
- Caplin, J.D.; Granados, N.G.; James, M.R.; Montazami, R.; Hashemi, N. Microfluidic Organ-on-a-Chip Technology for Advancement of Drug Development and Toxicology. Adv. Healthcare Mater. 2015, 4, 1426–1450. [Google Scholar] [CrossRef]
- van den Berg, A.; Mummery, C.L.; Passier, R.; van der Meer, A.D. Personalised organs-on-chips: Functional testing for precision medicine. Lab Chip 2019, 19, 198–205. [Google Scholar] [CrossRef]
- Liu, J.; Hansen, C.; Quake, S.R. Solving the “World-to-Chip” Interface Problem with a Microfluidic Matrix. Anal. Chem. 2003, 75, 4718–4723. [Google Scholar] [CrossRef]
- Hadd, A.G.; Raymond, D.E.; Halliwell, J.W.; Jacobson, S.C.; Ramsey, J.M. Microchip device for performing enzyme assays. Anal. Chem. 1997, 69, 3407–3412. [Google Scholar] [CrossRef]
- Valikhani, D.; Bolivar, J.M.; Viefhues, M.; McIlroy, D.N.; Vrouwe, E.X.; Nidetzky, B. A Spring in Performance: Silica Nanosprings Boost Enzyme Immobilization in Microfluidic Channels. ACS Appl. Mater. Interfaces 2017, 9, 34641–34649. [Google Scholar] [CrossRef]
- Fornera, S.; Kuhn, P.; Lombardi, D.; Schlüter, A.D.; Dittrich, P.S.; Walde, P. Sequential Immobilization of Enzymes in Microfluidic Channels for Cascade Reactions. ChemPlusChem 2012, 77, 98–101. [Google Scholar] [CrossRef]
- Kuchler, A.; Bleich, J.N.; Sebastian, B.; Dittrich, P.S.; Walde, P. Stable and Simple Immobilization of Proteinase K Inside Glass Tubes and Microfluidic Channels. ACS Appl. Mater. Interfaces 2015, 7, 25970–25980. [Google Scholar] [CrossRef] [PubMed]
- Logan, T.C.; Clark, D.S.; Stachowiak, T.B.; Svec, F.; Frechet, J.M.J. Photopatterning enzymes on polymer monoliths in microfluidic devices for steady-state kinetic analysis and spatially separated multi-enzyme reactions. Anal. Chem. 2007, 79, 6592–6598. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Obst, F.; Haefner, S.; Heroldt, T.; Peiter, M.; Simon, F.; Richter, A.; Voit, B.; Appelhans, D. Hydrogel/enzyme dots as adaptable tool for non-compartmentalized multi-enzymatic reactions in microfluidic devices. React. Chem. Eng. 2019, 4, 67–77. [Google Scholar] [CrossRef]
- Obst, F.; Simon, D.; Mehner, P.J.; Neubauer, J.W.; Beck, A.; Stroyuk, O.; Richter, A.; Voit, B.; Appelhans, D. One-step photostructuring of multiple hydrogel arrays for compartmentalized enzyme reactions in microfluidic devices. React. Chem. Eng. 2019, 4, 2141–2155. [Google Scholar] [CrossRef]
- Schwarz, A.; Thomsen, M.S.; Nidetzky, B. Enzymatic Synthesis of beta-Glucosylglycerol Using a Continuous-Flow Microreactor Containing Thermostable beta-Glycoside Hydrolase CelB Immobilized on Coated Microchannel Walls. Biotechnol. Bioeng. 2009, 103, 865–872. [Google Scholar] [CrossRef]
- Hickey, A.M.; Marle, L.; McCreedy, T.; Watts, P.; Greenway, G.M.; Littlechild, J.A. Immobilization of thermophilic enzymes in miniaturized flow reactors. Biochem. Soc. Trans. 2007, 35, 1621–1623. [Google Scholar] [CrossRef]
- Gruber, P.; Carvalho, F.; Marques, M.P.C.; O’Sullivan, B.; Subrizi, F.; Dobrijevic, D.; Ward, J.; Hailes, H.C.; Fernandes, P.; Wohlgemuth, R.; et al. Enzymatic synthesis of chiral amino-alcohols by coupling transketolase and transaminase-catalyzed reactions in a cascading continuous-flow microreactor system. Biotechnol. Bioeng. 2018, 115, 586–596. [Google Scholar] [CrossRef]
- Kazenwadel, F.; Franzreb, M.; Rapp, B.E. Synthetic enzyme supercomplexes: Co-immobilization of enzyme cascades. Anal. Methods 2015, 7, 4030–4037. [Google Scholar] [CrossRef]
- Schmidt, S.; Castiglione, K.; Kourist, R. Overcoming the Incompatibility Challenge in Chemoenzymatic and Multi-Catalytic Cascade Reactions. Chemistry 2018, 24, 1755–1768. [Google Scholar] [CrossRef]
- Heo, J.; Crooks, R.M. Microfluidic biosensor based on an array of hydrogel-entrapped enzymes. Anal. Chem. 2005, 77, 6843–6851. [Google Scholar] [CrossRef] [PubMed]
- Eddington, D.T.; Beebe, D.J. Flow control with hydrogels. Adv. Drug Deliv. Rev. 2004, 56, 199–210. [Google Scholar] [CrossRef]
- Oh, K.W.; Ahn, C.H. A review of microvalves. J. Micromech. Microeng. 2006, 16, R13–R39. [Google Scholar] [CrossRef]
- Dong, L.; Jiang, H. Autonomous microfluidics with stimuli-responsive hydrogels. Soft Matter 2007, 3, 1223–1230. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Haefner, S.; Koerbitz, R.; Frank, P.; Elstner, M.; Richter, A. High Integration of Microfluidic Circuits Based on Hydrogel Valves for MEMS Control. Adv. Mater. Technol. 2018, 3, 1700108–1700118. [Google Scholar] [CrossRef]
- Bäcker, M.; Raue, M.; Schusser, S.; Jeitner, C.; Breuer, L.; Wagner, P.; Poghossian, A.; Förster, A.; Mang, T.; Schöning, M.J. Microfluidic chip with integrated microvalves based on temperature- and pH-responsive hydrogel thin films. Phys. Status Solidi A 2012, 209, 839–845. [Google Scholar] [CrossRef]
- Chen, G.; Svec, F.; Knapp, D.R. Light-actuated high pressure-resisting microvalve for on-chip flow control based on thermo-responsive nanostructured polymer. Lab Chip 2008, 8, 1198–1204. [Google Scholar] [CrossRef]
- Sershen, S.R.; Mensing, G.A.; Ng, M.; Halas, N.J.; Beebe, D.J.; West, J.L. Independent Optical Control of Microfluidic Valves Formed from Optomechanically Responsive Nanocomposite Hydrogels. Adv. Mater. 2005, 17, 1366–1368. [Google Scholar] [CrossRef]
- Schiphorst, J.; Coleman, S.; Stumpel, J.E.; Ben Azouz, A.; Diamond, D.; Schenning, A.P.H.J. Molecular Design of Light-Responsive Hydrogels, For in Situ Generation of Fast and Reversible Valves for Microfluidic Applications. Chem. Mat. 2015, 27, 5925–5931. [Google Scholar] [CrossRef]
- Franke, M.; Leubner, S.; Dubavik, A.; George, A.; Savchenko, T.; Pini, C.; Frank, P.; Melnikau, D.; Rakovich, Y.; Gaponik, N.; et al. Immobilization of pH-sensitive CdTe Quantum Dots in a Poly(acrylate) Hydrogel for Microfluidic Applications. Nanoscale Res Lett. 2017, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.H.; Choi, Y.Y.; Park, J.Y.; Woo, D.H.; Lee, K.B.; Kim, J.H.; Lee, S.H. Electrically-driven hydrogel actuators in microfluidic channels: Fabrication, characterization, and biological application. Lab Chip 2010, 10, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Satarkar, N.S.; Zhang, W.; Eitel, R.E.; Hilt, J.Z. Magnetic hydrogel nanocomposites as remote controlled microfluidic valves. Lab Chip 2009, 9, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A.; Yuandong, G.; Loftness, P.E.; Siegel, R.A.; Ziaie, B. A hydrogel-actuated environmentally sensitive microvalve for active flow control. J. Microelectromech. Syst. 2003, 12, 613–621. [Google Scholar] [CrossRef]
- Paschew, G.; Schreiter, J.; Voigt, A.; Pini, C.; Chávez, J.P.; Allerdißen, M.; Marschner, U.; Siegmund, S.; Schüffny, R.; Jülicher, F.; et al. Autonomous Chemical Oscillator Circuit Based on Bidirectional Chemical-Microfluidic Coupling. Adv. Mater. Technol. 2016, 1, 1600005. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Xu, X.-D.; Cheng, S.-X.; Zhuo, R.-X. Strategies to improve the response rate of thermosensitive PNIPAAm hydrogels. Soft Matter 2008, 4, 385–391. [Google Scholar] [CrossRef]
- Yu, Q.; Bauer, J.M.; Moore, J.S.; Beebe, D.J. Responsive biomimetic hydrogel valve for microfluidics. Appl. Phys. Lett. 2001, 78, 2589–2591. [Google Scholar] [CrossRef]
- Yu, C.; Mutlu, S.; Selvaganapathy, P.; Mastrangelo, C.H.; Svec, F.; Fréchet, J.M.J. Flow Control Valves for Analytical Microfluidic Chips without Mechanical Parts Based on Thermally Responsive Monolithic Polymers. Anal. Chem. 2003, 75, 1958–1961. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Mauk, M.; Hong, K.-S.; Li, M.; Yang, S.; Bau, H.H. Self-Actuated, Thermo-Responsive Hydrogel Valves for Lab on a Chip. Biomed. Microdevices 2005, 7, 313–322. [Google Scholar] [CrossRef]
- Klatt, S.; Allerdissen, M.; Körbitz, R.; Voit, B.; Arndt, K.F.; Richter, A. Hydrogel-Based Microfluidic Systems. Adv. Sci. Tech. 2012, 81, 90–95. [Google Scholar] [CrossRef]
- Geiger, E.J.; Pisano, A.P.; Svec, F. A Polymer-Based Microfluidic Platform Featuring On-Chip Actuated Hydrogel Valves for Disposable Applications. J. Microelectromech. Syst. 2010, 19, 944–950. [Google Scholar] [CrossRef]
- Wu, J.; Kodzius, R.; Cao, W.; Wen, W. Extraction, amplification and detection of DNA in microfluidic chip-based assays. Microchim. Acta 2013, 181, 1611–1631. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Corstjens, P.L.A.M.; Mauk, M.G.; Bau, H.H. A disposable microfluidic cassette for DNA amplification and detection. Lab Chip 2006, 6, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.C.; Ye, H.; Kuan, Y.K.; Li, M.-H.; Ying, J.Y. Integrated two-step gene synthesis in a microfluidic device. Lab Chip 2009, 9, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.W.; Ong, C.N. Lag-time measurement of antioxidant capacity using myoglobin and 2, 2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid): Rationale, application, and limitation. Anal Biochem. 1999, 275, 217–223. [Google Scholar] [CrossRef]
- Gräfe, D.; Gaitzsch, J.; Appelhans, D.; Voit, B. Cross-linked polymersomes as nanoreactors for controlled and stabilized single and cascade enzymatic reactions. Nanoscale 2014, 6, 10752–10761. [Google Scholar] [CrossRef]
- Raevskaya, A.; Lesnyak, V.; Haubold, D.; Dzhagan, V.; Stroyuk, O.; Gaponik, N.; Zahn, D.R.T.; Eychmüller, A. A Fine Size Selection of Brightly Luminescent Water-Soluble Ag–In–S and Ag–In–S/ZnS Quantum Dots. J. Phys. Chem. C 2017, 121, 9032–9042. [Google Scholar] [CrossRef]
- Ng, J.M.; Gitlin, I.; Stroock, A.D.; Whitesides, G.M. Components for integrated poly(dimethylsiloxane) microfluidic systems. Electrophoresis 2002, 23, 3461–3473. [Google Scholar] [CrossRef]
- Greiner, R.; Allerdissen, M.; Voigt, A.; Richter, A. Fluidic microchemomechanical integrated circuits processing chemical information. Lab Chip 2012, 12, 5034–5044. [Google Scholar] [CrossRef]
- Richter, A.; Howitz, S.; Kuckling, D.; Arndt, K.-F. Influence of volume phase transition phenomena on the behavior of hydrogel-based valves. Sens. Actuat. B-Chem. 2004, 99, 451–458. [Google Scholar] [CrossRef]
- Young, E.W.; Beebe, D.J. Fundamentals of microfluidic cell culture in controlled microenvironments. Chem. Soc. Rev. 2010, 39, 1036–1048. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obst, F.; Beck, A.; Bishayee, C.; Mehner, P.J.; Richter, A.; Voit, B.; Appelhans, D. Hydrogel Microvalves as Control Elements for Parallelized Enzymatic Cascade Reactions in Microfluidics. Micromachines 2020, 11, 167. https://doi.org/10.3390/mi11020167
Obst F, Beck A, Bishayee C, Mehner PJ, Richter A, Voit B, Appelhans D. Hydrogel Microvalves as Control Elements for Parallelized Enzymatic Cascade Reactions in Microfluidics. Micromachines. 2020; 11(2):167. https://doi.org/10.3390/mi11020167
Chicago/Turabian StyleObst, Franziska, Anthony Beck, Chayan Bishayee, Philipp J. Mehner, Andreas Richter, Brigitte Voit, and Dietmar Appelhans. 2020. "Hydrogel Microvalves as Control Elements for Parallelized Enzymatic Cascade Reactions in Microfluidics" Micromachines 11, no. 2: 167. https://doi.org/10.3390/mi11020167
APA StyleObst, F., Beck, A., Bishayee, C., Mehner, P. J., Richter, A., Voit, B., & Appelhans, D. (2020). Hydrogel Microvalves as Control Elements for Parallelized Enzymatic Cascade Reactions in Microfluidics. Micromachines, 11(2), 167. https://doi.org/10.3390/mi11020167