Preparation and Test of NH3 Gas Sensor Based on Single-Layer Graphene Film
Abstract
:1. Introduction
2. Materials and Methods
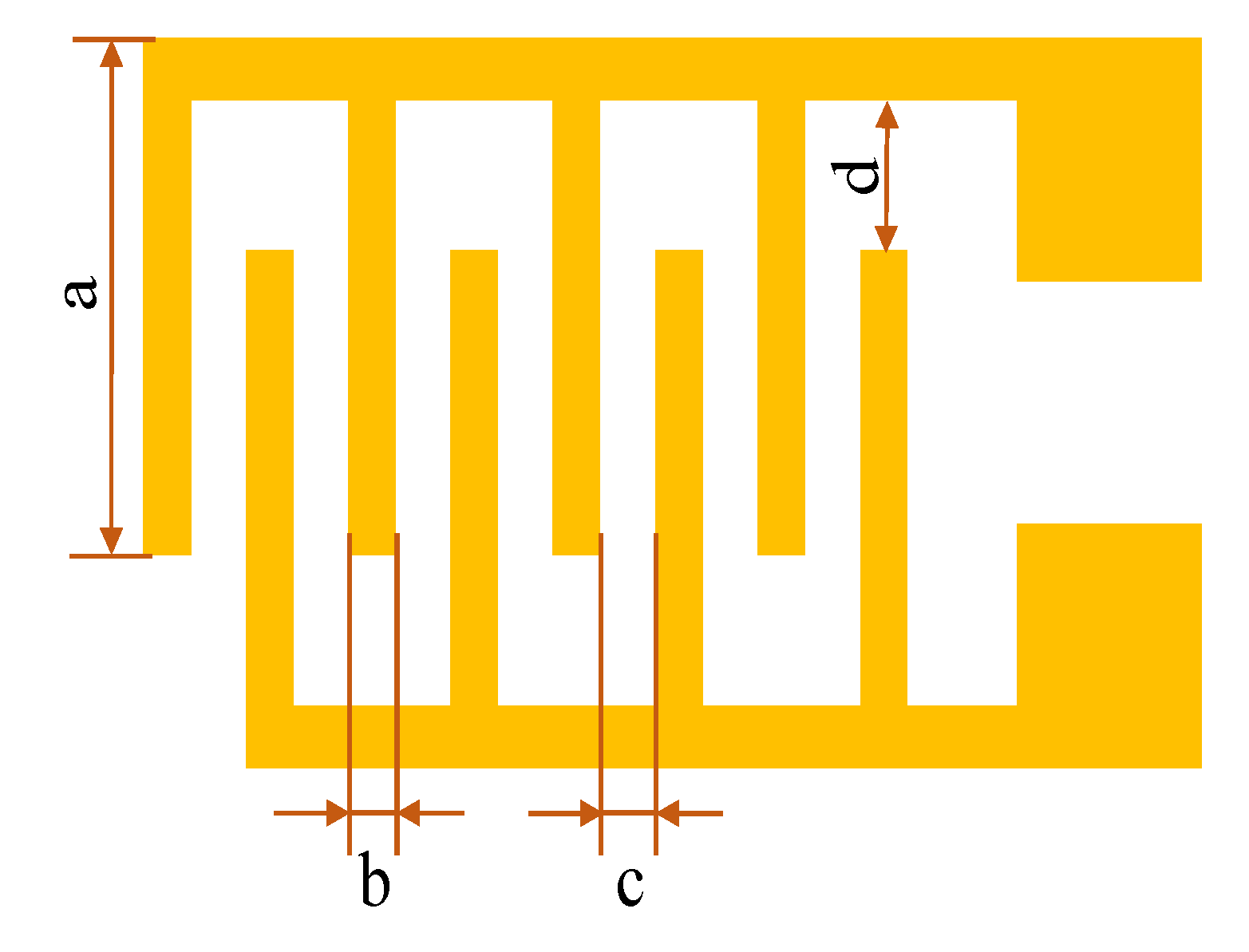
2.1. Fabrication of Devices
2.2. Characterization of the Single Graphene
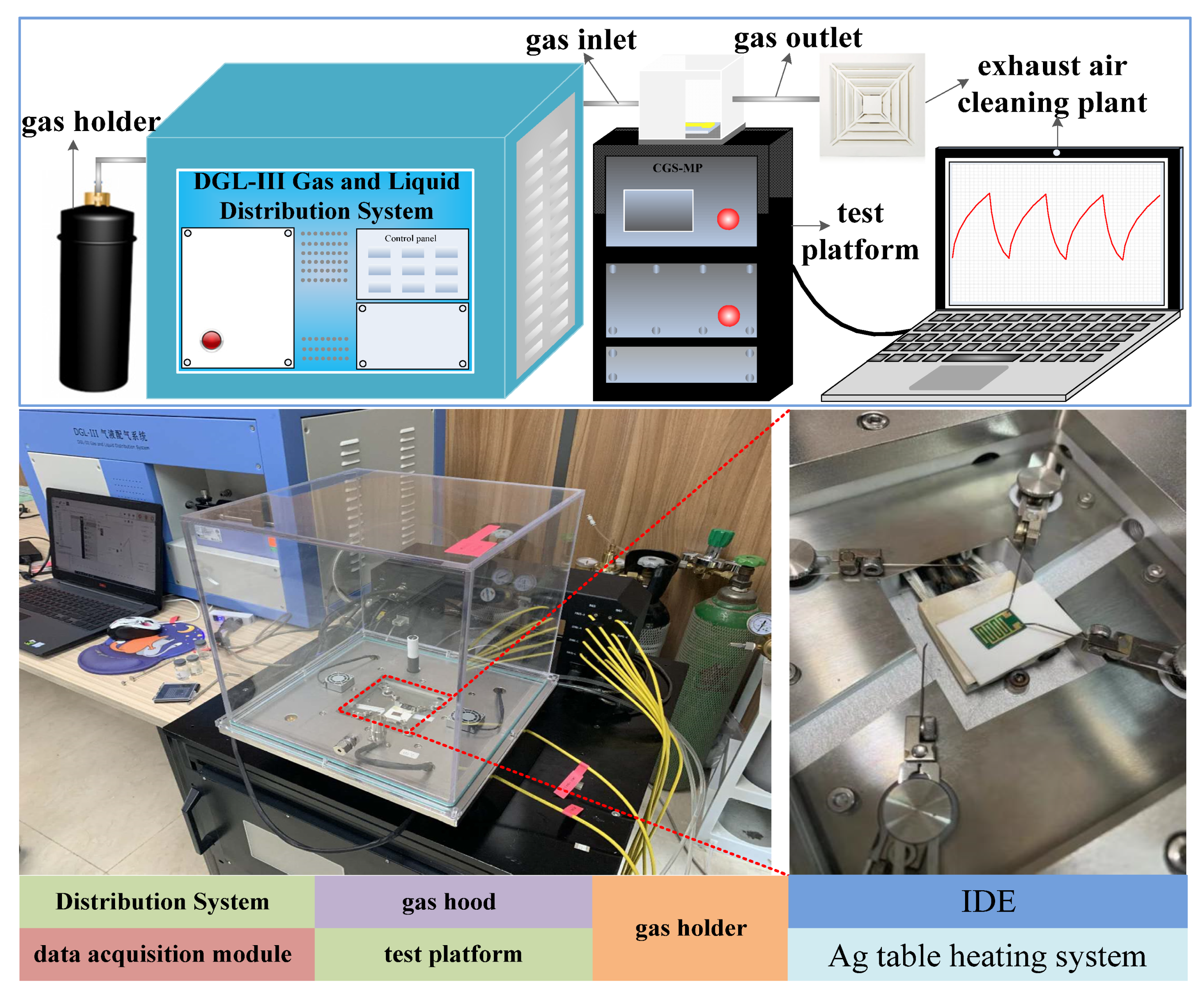
2.3. Experimental Test Platform
3. Results and Discussion
3.1. The Adsorption Mechanism of Graphene
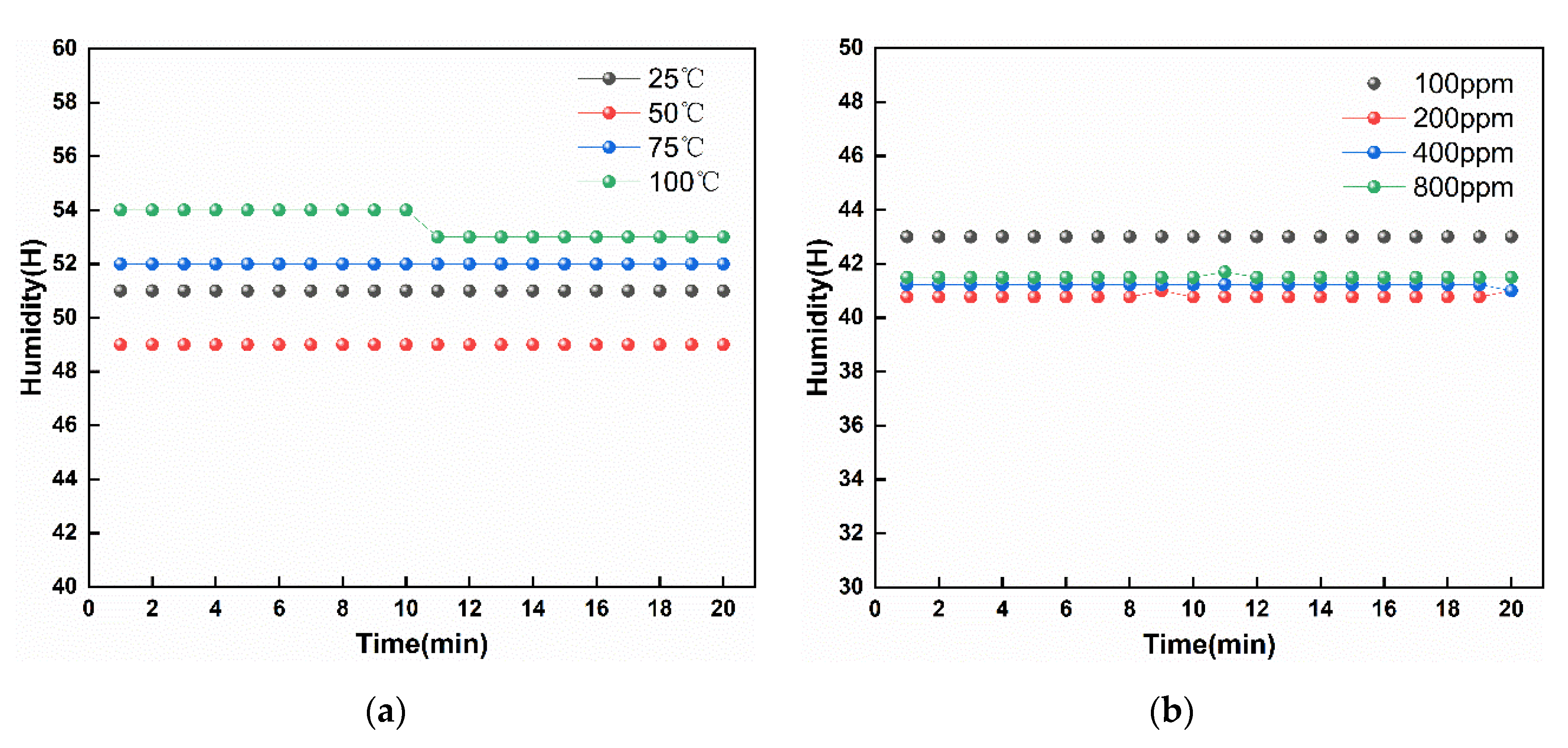
3.2. Humidity Measurement of the Sensor
3.3. The Response of Sensor
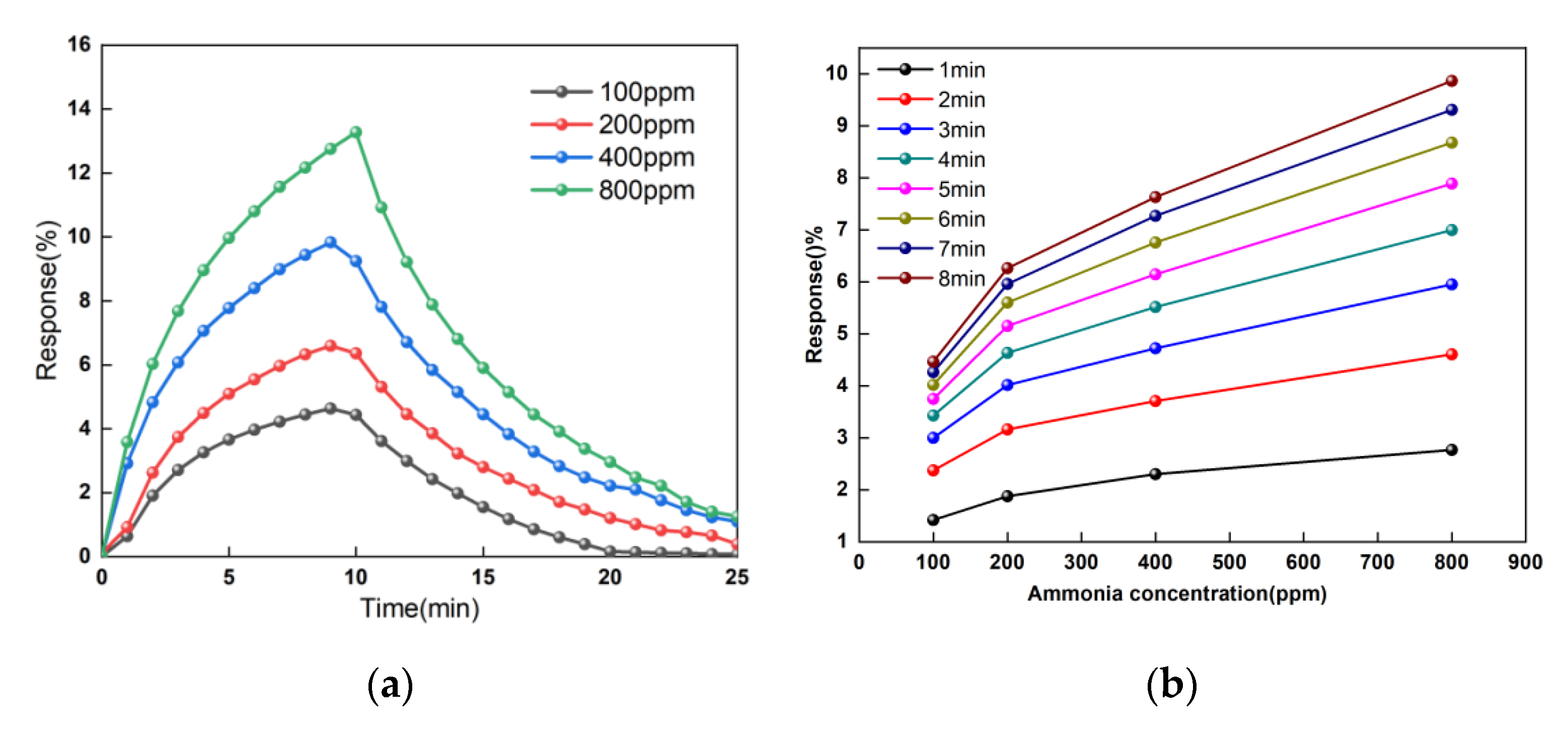
3.4. The Concentrations Response of the Sensor
3.5. The Temperature Response of the Sensor
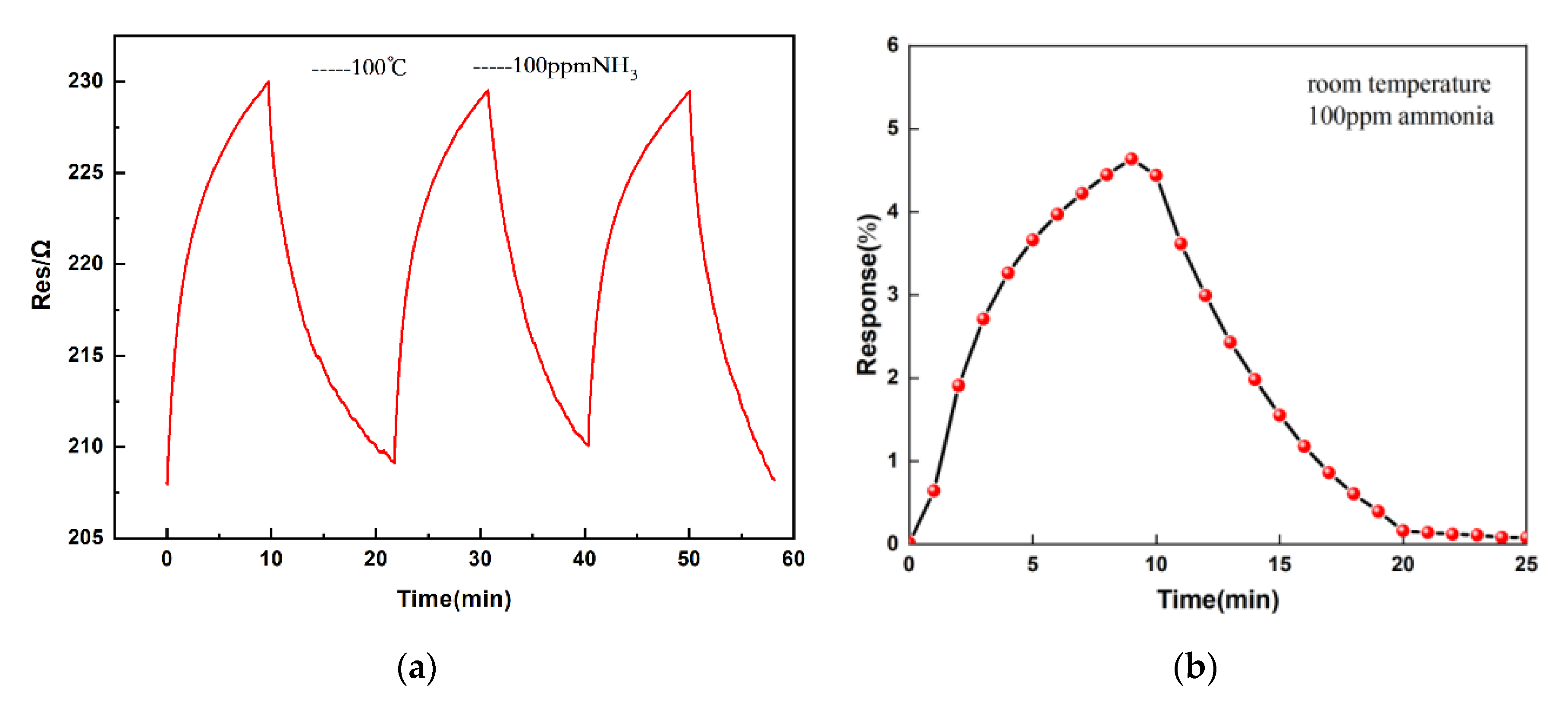
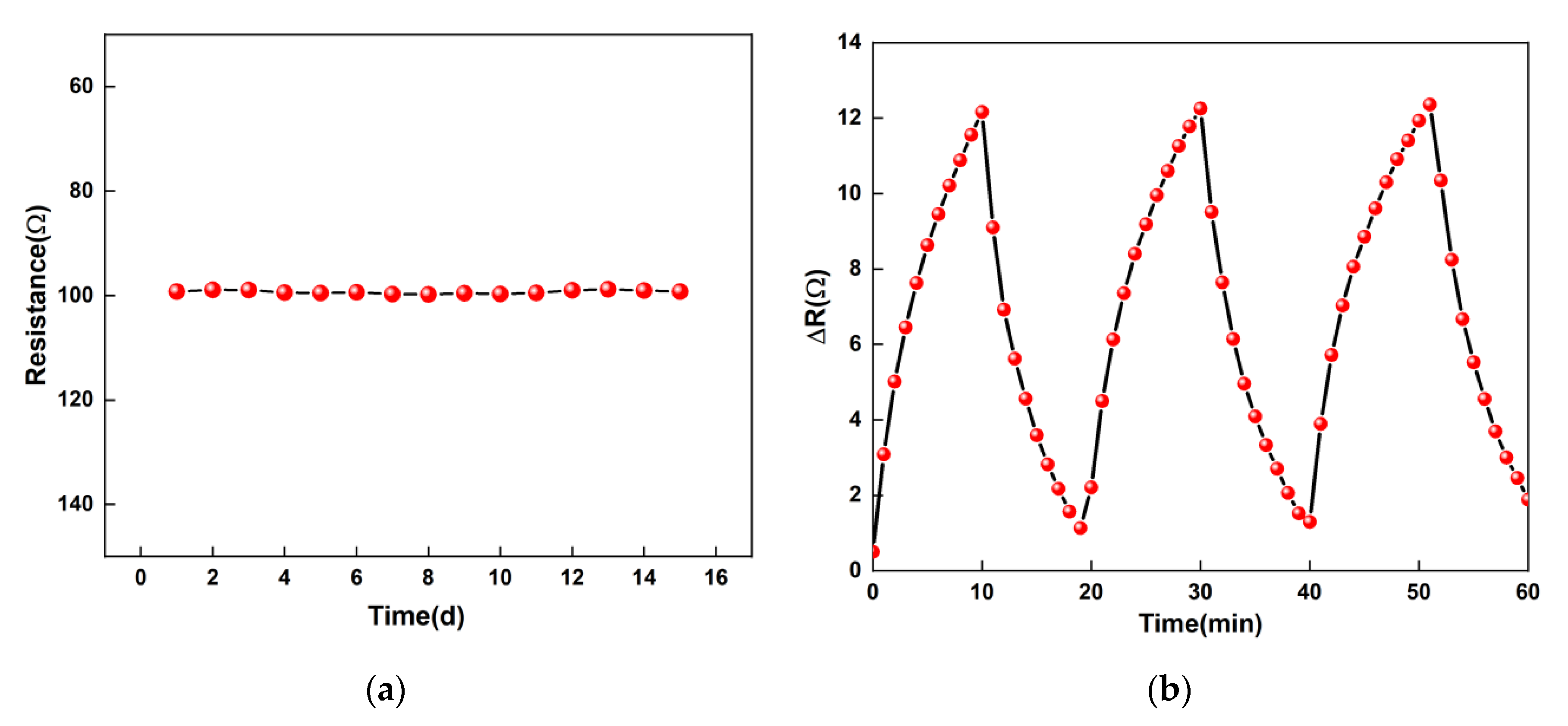
3.6. Repeatability and Stability Tests of the Sensor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yoon, J.W.; Lee, J.H. Toward breath analysis on a chip for disease diagnosis using semiconductor-based chemiresistors: Recent progress and future perspectives. Lab A Chip 2017, 17, 3537–3557. [Google Scholar] [CrossRef]
- Kandyla, M.; Pandis, C.; Chatzandroulis, S.; Pissis, P.; Zergioti, I. Direct laser printing of thin-film polyaniline devices. Appl. Phys. A 2012, 110, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Mani, G.K.; Rayappan, J.B.B. A highly selective and wide range ammonia sensor—Nanostructured ZnO:Co thin film. Mater. Sci. Eng. B 2015, 191, 41–50. [Google Scholar] [CrossRef]
- Qi, Q.; Wang, P.-P.; Zhao, J.; Feng, L.-L.; Zhou, L.-J.; Xuan, R.-F.; Liu, Y.-P.; Li, G.-D. SnO2 nanoparticle-coated In2O3 nanofibers with improved NH3 sensing properties. Sens. Actuators B Chem. 2014, 194, 440–446. [Google Scholar] [CrossRef]
- Srivastava, V.; Jain, K. At room temperature graphene/SnO2 is better than MWCNT/SnO2 as NO2 gas sensor. Mater. Lett. 2016, 169, 28–32. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, J.; Fei, T.; Liu, S.; Zhang, T. SnO2 nanoparticles-reduced graphene oxide nanocomposites for NO2 sensing at low operating temperature. Sens. Actuators B Chem. 2014, 190, 472–478. [Google Scholar] [CrossRef]
- Hong, X.Z.; Wang, J.; Hai, Z. Discrimination and prediction of multiple beef freshness indexes based on electronic nose. Sens. Actuators B Chem. 2012, 161, 381–389. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, J.; Ye, S.; Chang, M.X. Application of Electronic Nose and Statistical Analysis to Predict Quality Indices of Peach. Food Bioprocess Technol. 2012, 5, 65–72. [Google Scholar] [CrossRef]
- Wei, Z.B.; Wang, J.; Zhang, W.L. Detecting internal quality of peanuts during storage using electronic nose responses combined with physicochemical methods. Food Chem. 2015, 177, 89–96. [Google Scholar] [PubMed]
- Wu, H.; Bu, X.; Deng, M.; Chen, G.; Zhang, G.; Li, X.; Wang, X.; Liu, W. A Gas Sensing Channel Composited with Pristine and Oxygen Plasma-Treated Graphene. Sensors 2019, 19, 625. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dobonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar]
- Krach, F.; Hertel, S.; Waldmann, D.; Jobst, J.; Krieger, M.; Reshanov, S.; Schöner, A.; Weber, H.B. A switch for epitaxial graphene electronics: Utilizing the silicon carbide substrate as transistor channel. Appl. Phys. Lett. 2012, 100, 122102. [Google Scholar]
- Pop, E.; Varshney, V.; Roy, A.K. Thermal properties of graphene: Fundamentals and applications. MRS Bull. 2012, 37, 1273–1281. [Google Scholar]
- Pumera, M. Electrochemistry of graphene: New horizons for sensing and energy storage. Chem. Rec. 2009, 9, 211–223. [Google Scholar]
- Nahlik, J.; Voves, J.; Laposa, A.; Kroutil, J. The Study of Graphene Gas Sensor. Key Eng. Mater. 2014, 605, 495–498. [Google Scholar]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar]
- Tai, H.; Jiang, Y.; Xie, G.; Yu, J.; Chen, X. Fabrication and gas sensitivity of polyaniline–titanium dioxide nanocomposite thin film. Sens. Actuators B Chem. 2007, 125, 644–650. [Google Scholar]
- Varghese, S.S.; Lonkar, S.; Singh, K.K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuators B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Gautam, M.; Jayatissa, A.H. Gas sensing properties of graphene synthesized by chemical vapor deposition. Mater. Sci. Eng. C 2011, 31, 1405–1411. [Google Scholar] [CrossRef]
- Gautam, M.; Jayatissa, A.H. Ammonia gas sensing behavior of graphene surface decorated with gold nanoparticles. Solid-State Electron. 2012, 78, 159–165. [Google Scholar] [CrossRef]
- Zhao, M.; Yan, L.; Zhang, X.; Xu, L.; Song, Z.; Chen, P.; Dong, F.; Chu, W. Room temperature NH3 detection of Ti/graphene devices promoted by visible light illumination. J. Mater. Chem. C 2017, 5, 1113–1120. [Google Scholar] [CrossRef]
- Joshi, A.; Gangal, S.A.; Gupta, S.K. Ammonia sensing properties of polypyrrole thin films at room temperature. Sens. Actuators B Chem. 2011, 156, 938–942. [Google Scholar] [CrossRef]
- Davaji, B.; Cho, H.D.; Malakoutian, M.; Lee, J.K.; Panin, G.; Kang, T.W.; Lee, C.H. A patterned single layer graphene resistance temperature sensor. Sci. Rep. 2017, 7, 8811. [Google Scholar] [CrossRef]
- Brandenburg, A.; Kita, J.; Groß, A.; Moos, R. Novel tube-type LTCC transducers with buried heaters and inner interdigitated electrodes as a platform for gas sensing at various high temperatures. Sens. Actuators B Chem. 2013, 189, 80–88. [Google Scholar] [CrossRef]
- Blume, S.O.P.; Ben-Mrad, R.; Sullivan, P.E. Modelling the capacitance of multi-layer conductor-facing interdigitated electrode structures. Sens. Actuators B Chem. 2015, 213, 423–433. [Google Scholar]
- Wang, J.; Zhang, P.; Qi, J.-Q.; Yao, P.-J. Silicon-based micro-gas sensors for detecting formaldehyde. Sens. Actuators B Chem. 2009, 136, 399–404. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy in monolayer graphene and graphite: Electron-phonon coupling and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Das, A.; Pisana, S.; Chakraborty, B.; Piscanec, S.; Saha, S.K.; Waghmare, U.V.; Novoselov, K.S.; Krishnamurthy, H.R.; Geim, A.K.; Ferrari, A.C.; et al. Monitoring dopants by Raman scattering in an electrochemically top-gated graphene transistor. Nat. Nanotechnol. 2008, 3, 210–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Zhang, J.; Su, H.; Li, J.; Sun, L.; Wang, Z.; Xu, F.; Liu, C.; Lopatin, S.; Zhu, Y.; et al. Towards super-clean graphene. Nat. Commun. 2019, 10, 1912. [Google Scholar] [CrossRef] [PubMed]
- Purdie, D.G.; Pugno, N.M.; Taniguchi, T.; Watanabe, K.; Ferrari, A.C.; Lombardo, A. Cleaning interfaces in layered materials heterostructures. Nat. Commun. 2018, 9, 5387. [Google Scholar] [CrossRef] [PubMed]
- Punetha, D.; Kar, M.; Pandey, S.K. A new type low-cost, flexible and wearable tertiary nanocomposite sensor for room temperature hydrogen gas sensing. Sci. Rep. 2020, 10, 2151. [Google Scholar] [CrossRef]
- Romero, H.E.; Joshi, P.; Gupta, A.K.; Gutierrez, H.R.; Cole, M.W.; Tadigadapa, S.A.; Eklund, P.C. Adsorption of ammonia on graphene. Nanotechnology 2009, 20, 245501. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.; Gu, C.; Yu, K.; Meng, F.; Liu, J. A novel highly sensitive gas ionization sensor for ammonia detection. Sens. Actuators A Phys. 2009, 150, 218–223. [Google Scholar] [CrossRef]
- Cadore, A.R.; Mania, E.; Alencar, A.B.; Rezende, N.P.; de Oliveira, S.; Watanabe, K.; Taniguchi, T.; Chacham, H.; Campos, L.C.; Lacerda, R.G. Enhancing the response of NH3 graphene-sensors by using devices with different graphene-substrate distances. Sens. Actuators B Chem. 2018, 266, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Reina, A.; Jia, X.T.; Ho, J.; Nezich, D.; Kong, J. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Fan, X.; Elgammal, K.; Anderson, D.S.; Mikael, Ö.; Anna, D.; Max, C.L.; Frank, N. Humidity and CO2 gas sensing properties of double-layer graphene. Carbon 2018, 127, 576–587. [Google Scholar] [CrossRef]
- Anupama, G.; Dattatray, J.L.; Panchakarla, L.S.; Govindaraj, A. NO2 and humidity sensing characteristics of few-layer graphenes. J. Exp. Nanosci. 2009, 4, 313–322. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, T.; Liu, R.; Lei, C.; Wang, K.; Li, Z.; Li, Y. Preparation and Test of NH3 Gas Sensor Based on Single-Layer Graphene Film. Micromachines 2020, 11, 965. https://doi.org/10.3390/mi11110965
Liang T, Liu R, Lei C, Wang K, Li Z, Li Y. Preparation and Test of NH3 Gas Sensor Based on Single-Layer Graphene Film. Micromachines. 2020; 11(11):965. https://doi.org/10.3390/mi11110965
Chicago/Turabian StyleLiang, Ting, Ruifang Liu, Cheng Lei, Kai Wang, Zhiqiang Li, and Yongwei Li. 2020. "Preparation and Test of NH3 Gas Sensor Based on Single-Layer Graphene Film" Micromachines 11, no. 11: 965. https://doi.org/10.3390/mi11110965
APA StyleLiang, T., Liu, R., Lei, C., Wang, K., Li, Z., & Li, Y. (2020). Preparation and Test of NH3 Gas Sensor Based on Single-Layer Graphene Film. Micromachines, 11(11), 965. https://doi.org/10.3390/mi11110965





