Typhonium giganteum Lectin Exerts A Pro-Inflammatory Effect on RAW 264.7 via ROS and The NF-κB Signaling Pathway
Abstract
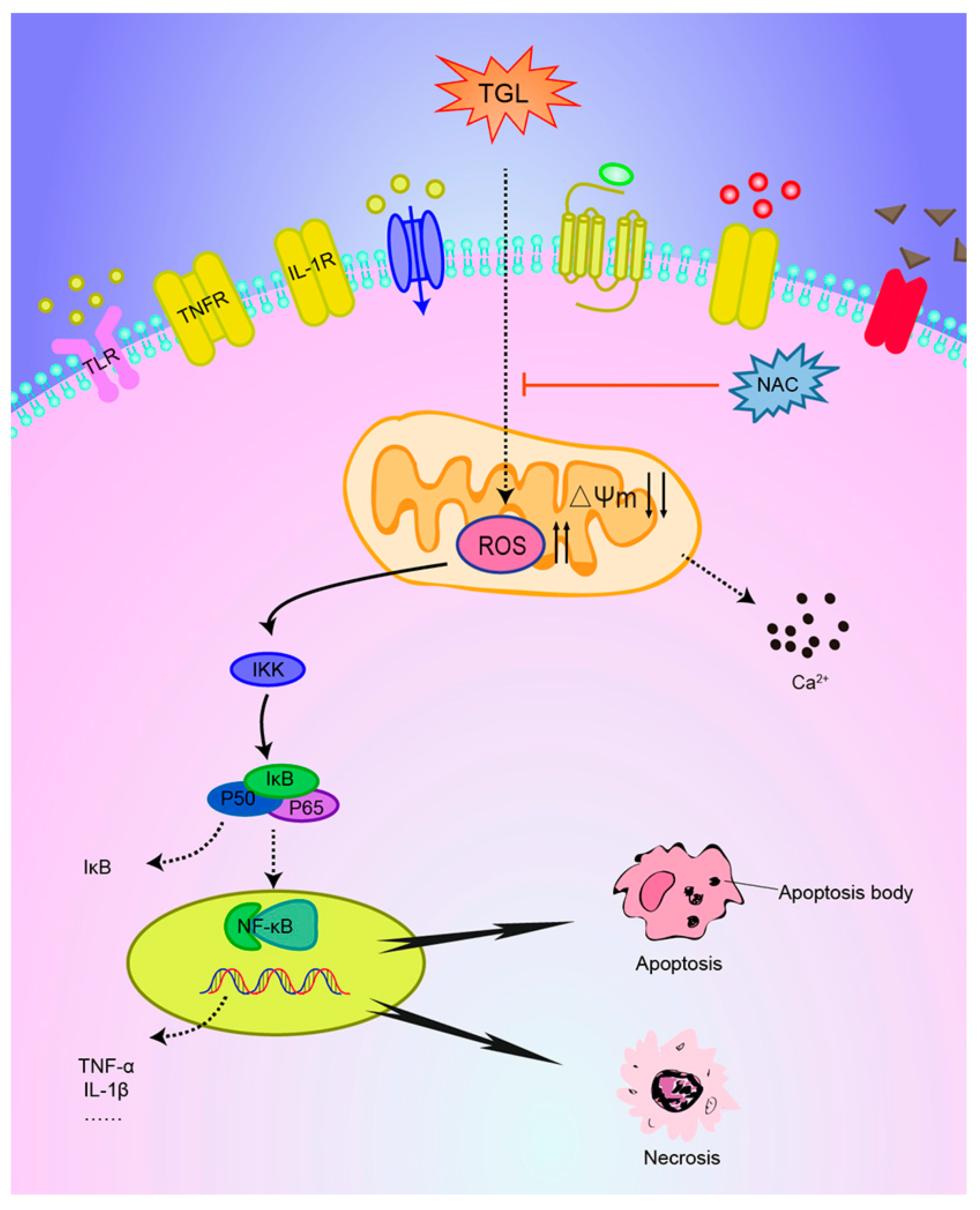
:1. Introduction
2. Results
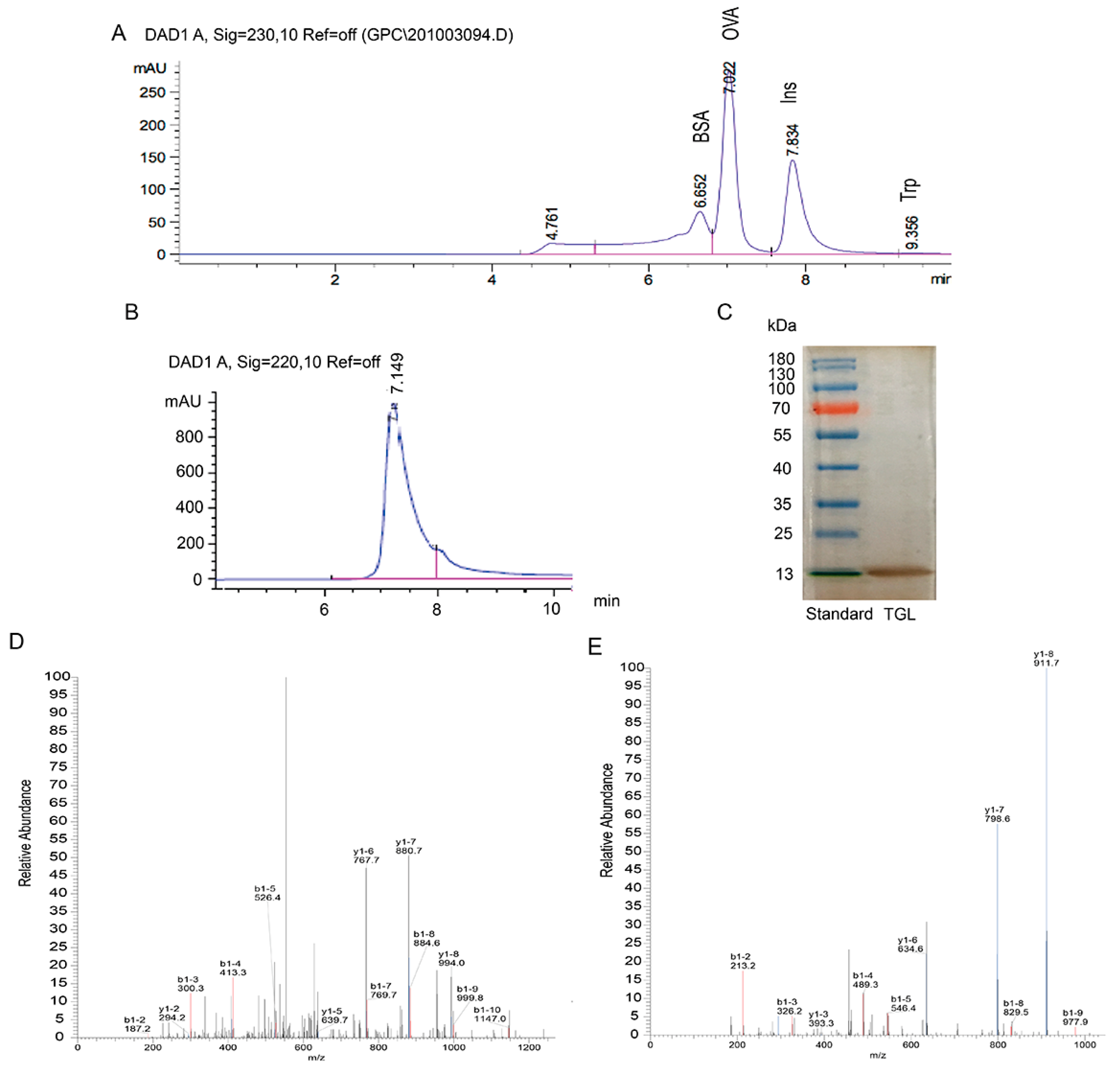
2.1. Extraction, Purification, and Identification of TGL
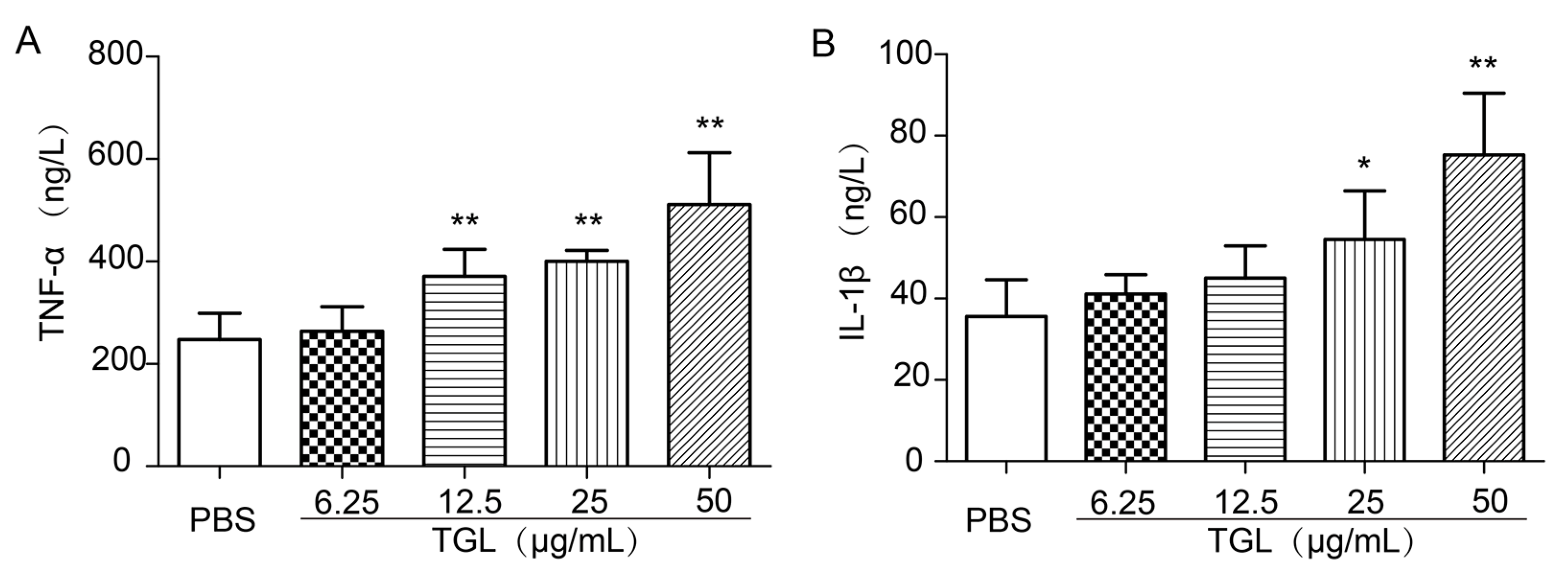
2.2. Cytokines Released from RAW 264.7 Stimulated by Different Doses of TGL
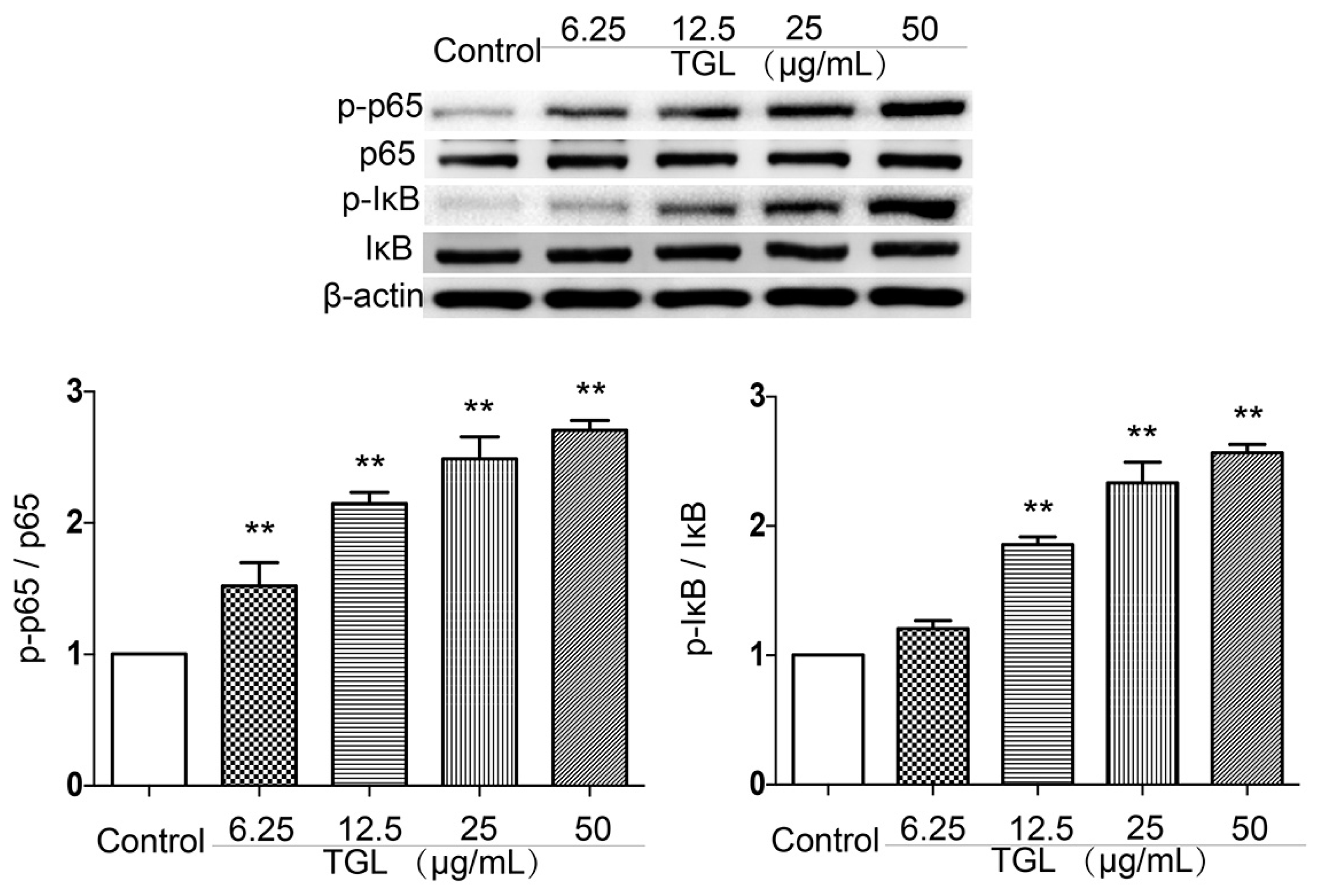
2.3. Western Blot Test of p-p65, p65, p-IκB, IκB
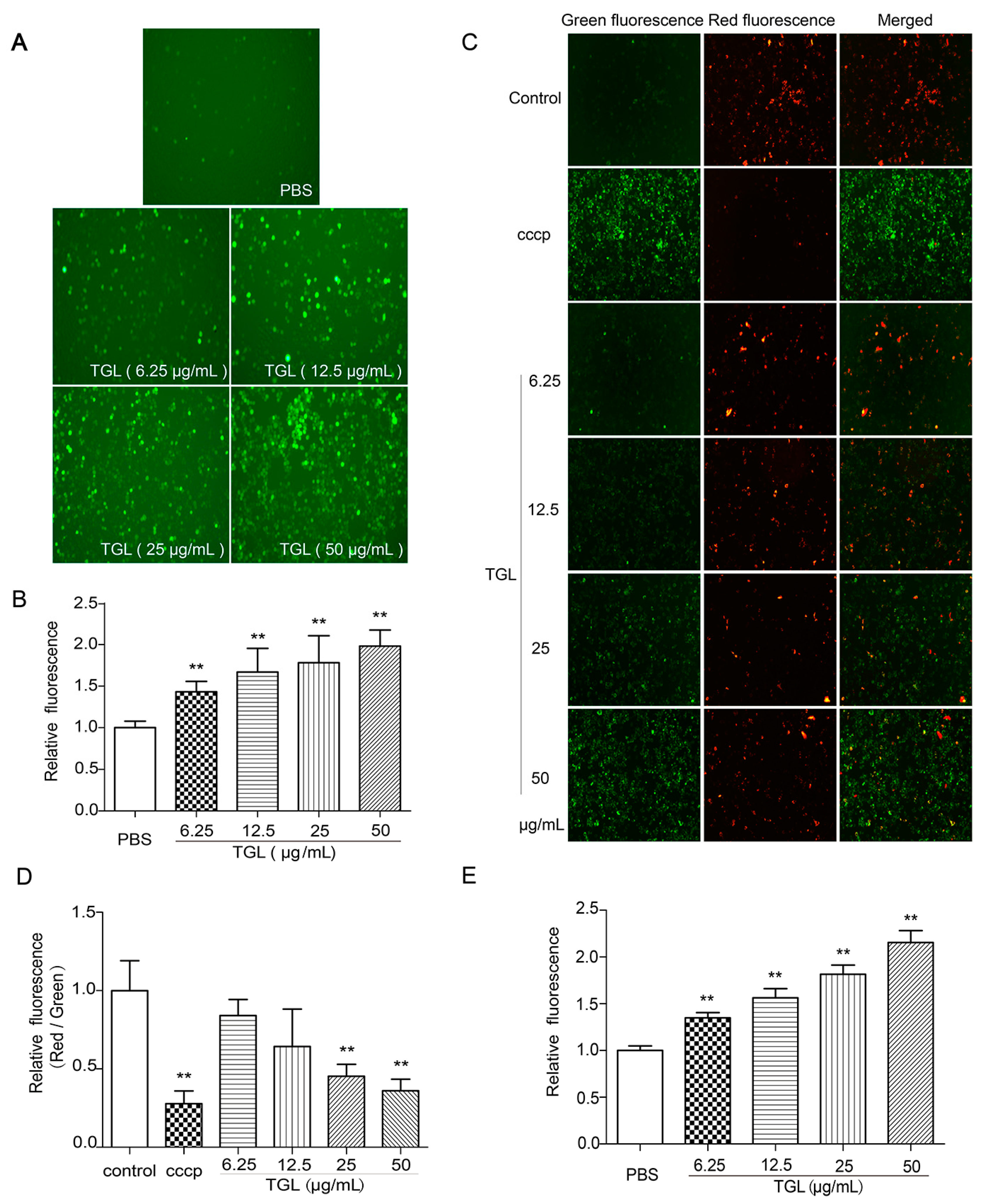
2.4. Measurement of ROS, MMP, and the Cytosolic Free Ca2+
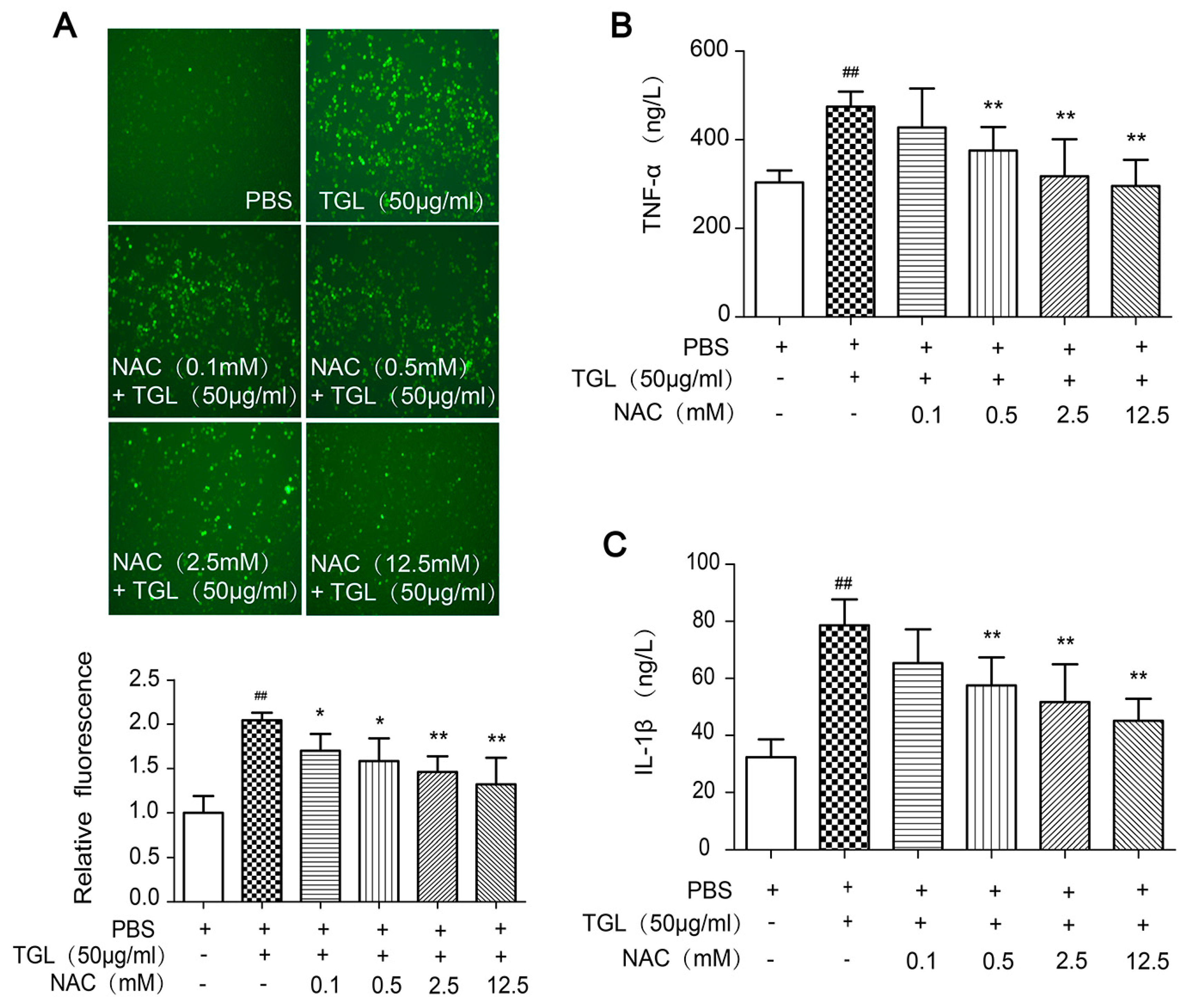
2.5. Cytokines Released after Treatment with NAC (the ROS Scavenger)
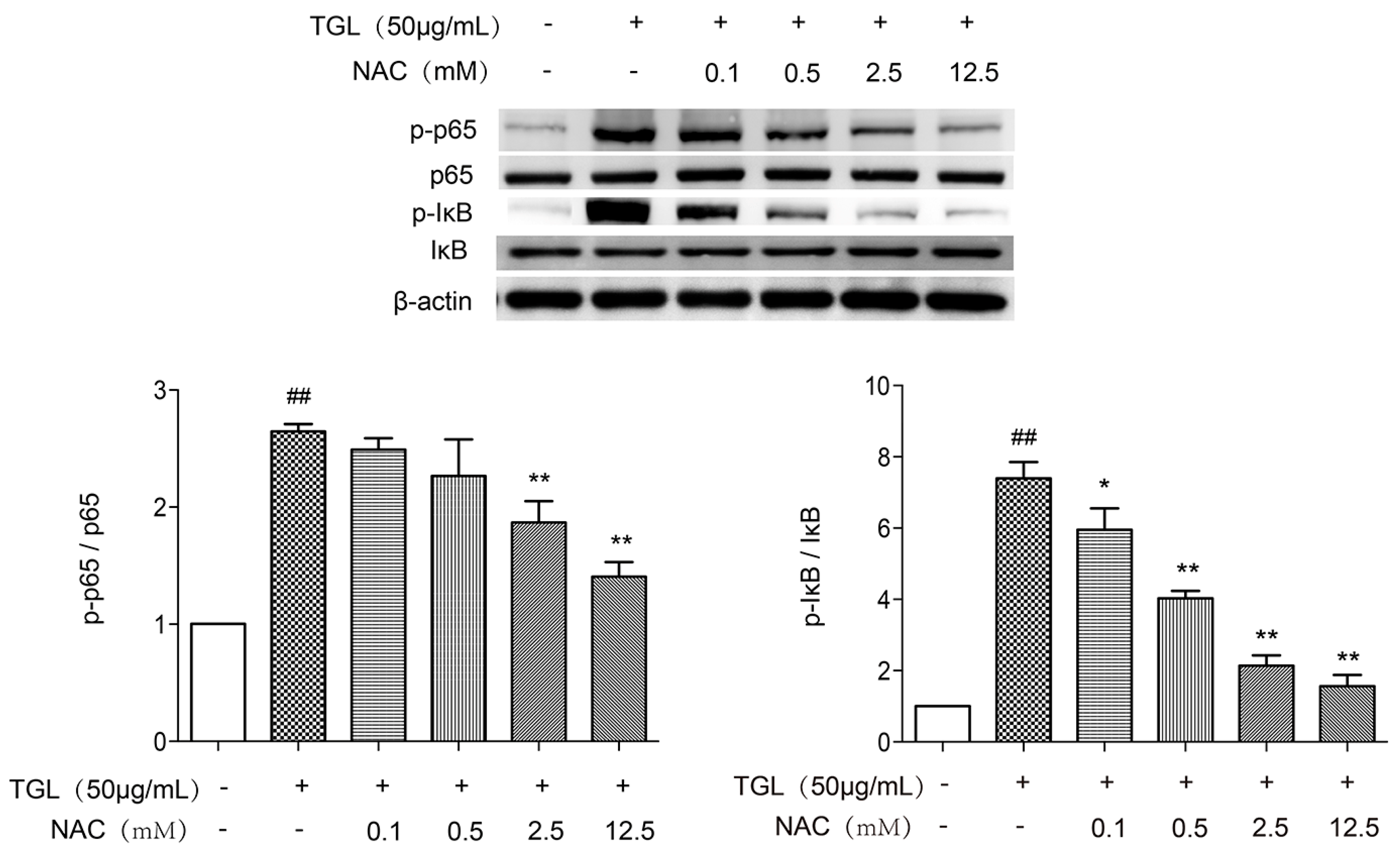
2.6. Western Blot Test of p-p65 and p-IκB after the Treatment with NAC
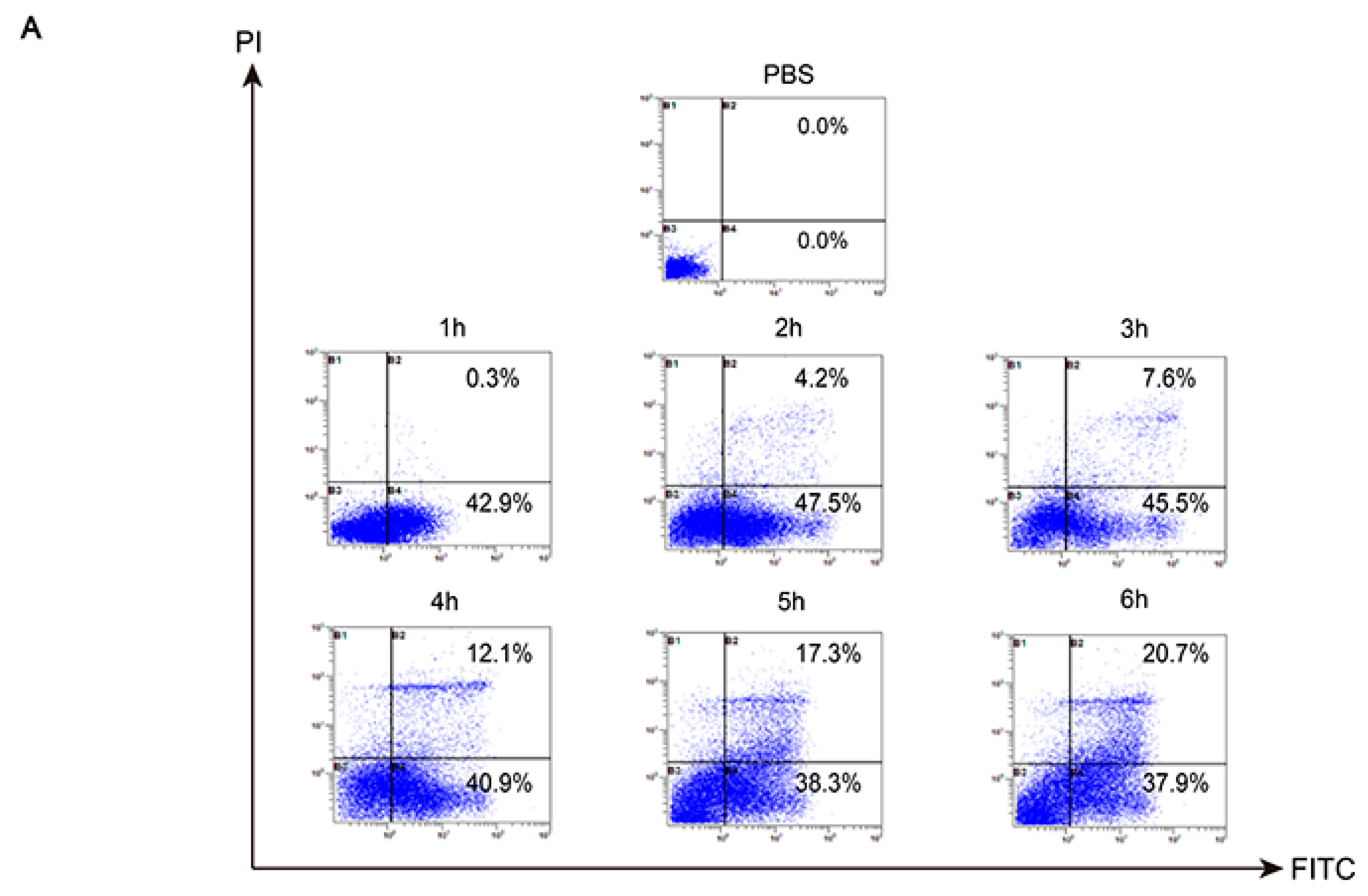
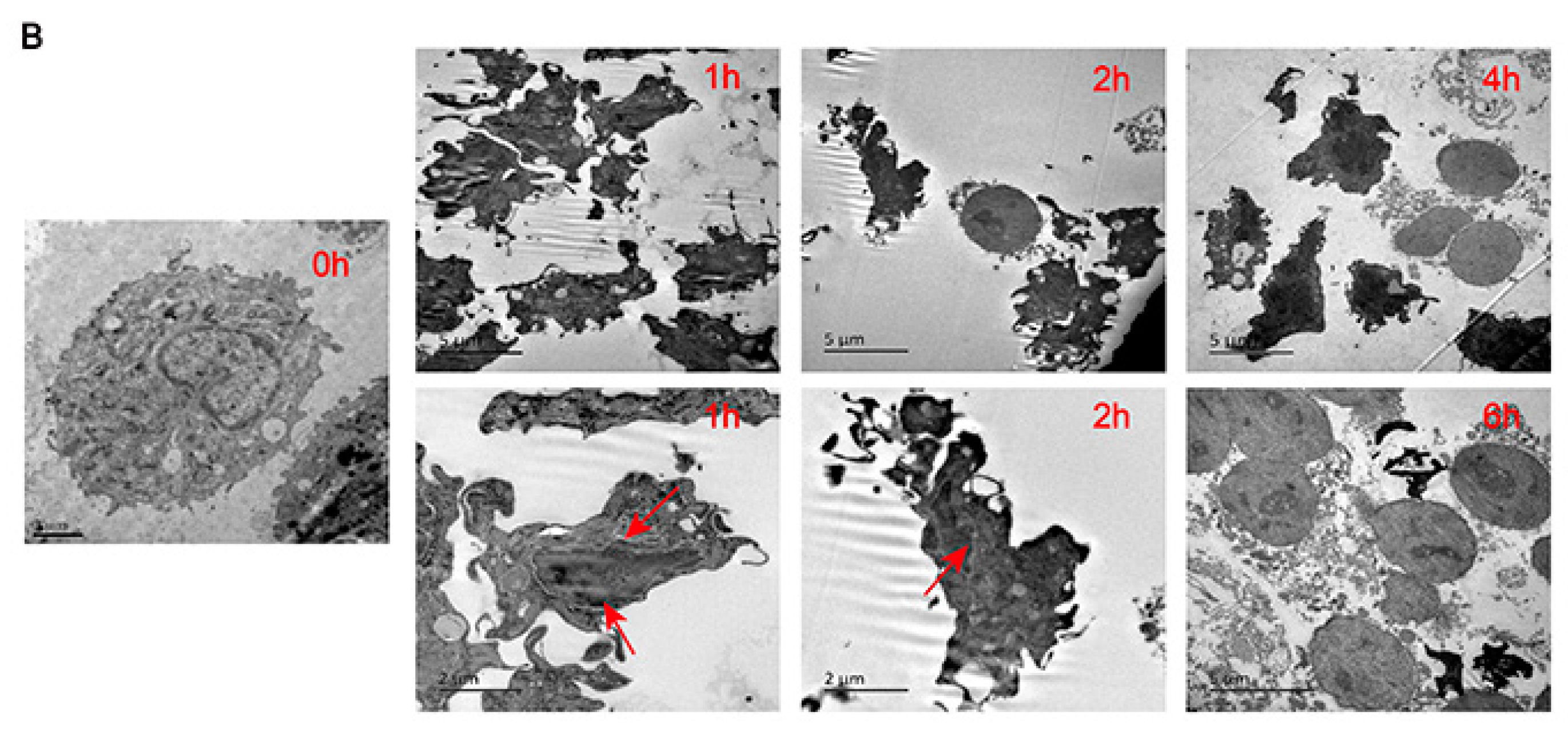
2.7. Analysis of Death Mode of RAW 264.7 Stimulated by TGL
3. Discussion
4. Materials and Methods
4.1. Plant
4.2. Reagents and Antibodies
4.3. Extraction of TGL
4.4. SDS-PAGE and SEC-HPLC
4.5. In-Gel Digestion, LC-Mass Spectromatery (MS)/MS and Database Search [2]
4.6. Cell Culture
4.7. Cytokines Stimulated by Different Doses of TGL
4.8. Western Blot Test of p-p65, p65, p-IκB, IκB
4.9. Measurement of ROS
4.10. Detection of Mitochondrial Membrane Potential (MMP) Variation
4.11. Measurement of Cytosolic Free Ca2+ Concentrations
4.12. Cytokines Released after Treatment with NAC (the ROS Scavenger)
4.13. Western Blot Test of p-p65 and p-IκB after Treatment with NAC
4.14. Analysis of the Death Mode of RAW 264.7 Stimulated by TGL via Flow Cytometry and Transmission Electron Microscopy
4.15. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pan, Y.; Yu, H.; Wu, H.; Chen, Y.; Wang, K.; Liu, L.; Jin, Y.; Zhang, C. Correlation between proinflammatory role of a lectin from Typhonium giganteum Engl. and macrophage. Int. J. Clin. Exp. Pathol. 2015, 8, 9854–9862. [Google Scholar]
- Yu, H.L.; Zhao, T.F.; Wu, H.; Pan, Y.Z.; Zhang, Q.; Wang, K.L.; Zhang, C.C.; Jin, Y.P. Pinellia ternata lectin exerts a pro-inflammatory effect on macrophages by inducing the release of pro-inflammatory cytokines, the activation of the nuclear factor-κB signaling pathway and the overproduction of reactive oxygen species. Int. J. Mol. Med. 2015, 36, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.A.; Ainouz, I.L.; De Oliveira, J.T.; Cavada, B.S. Plant lectins, chemical and biological aspects. Mem. Inst. Oswaldo Cruz. 1991, 86, 211–218. [Google Scholar] [CrossRef]
- Dang, L.; Van Damme, E.J. Toxic proteins in plants. Phytochemistry 2015, 117, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Alencar, V.B.; Brito, G.A.; Alencar, N.M.; Assreuy, A.M.; Pinto, V.P.; Teixeira, E.H.; Souza, E.P.; Debray, H.; Ribeiro, R.A.; Cavada, B.S. Helianthus tuberosus agglutinin directly induces neutrophil migration, which can be modulated/inhibited by resident mast cells. Biochem. Cell Biol. 2005, 83, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Alencar, N.M.; Assreuy, A.M.; Alencar, V.B.; Melo, S.C.; Ramos, M.V.; Cavada, B.S.; Cunha, F.Q.; Ribeiro, R.A. The galactose-binding lectin from, Vatairea macrocarpa seeds induces in vivo neutrophil migration by indirect mechanism. Int. J. Biochem. Cell. Biol. 2003, 35, 1674–1681. [Google Scholar] [CrossRef]
- Alencar, N.M.; Assreuy, A.M.; Criddle, D.N.; Souza, E.P.; Soares, P.M.; Havt, A.; Aragão, K.S.; Bezerra, D.P.; Ribeiro, R.A.; Cavada, B.S. Vatairea macrocarpa lectin induces paw edema with leukocyte infiltration. Protein Pept. Lett. 2004, 11, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Alencar, V.B.; Assreuy, A.M.; Alencar, N.M.; Meireles, A.V.; Mota, M.R.; Aragão, K.S.; Cajazeiras, J.B.; Nagano, C.S.; Brito, G.A.; Silva, L.I.; et al. Lectin of Pisum arvense seeds induces in vivo and in vitro neutrophil migration. J. Pharm. Pharmacol. 2005, 57, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.C.; Osterne, V.J.; Santiago, M.Q.; Pinto-Junior, V.R.; Silva-Filho, J.C.; Lossio, C.F.; Nascimento, F.L.; Almeida, R.P.; Teixeira, C.S.; Leal, R.B.; et al. Structural analysis of Centrolobium tomentosum seed lectin with inflammatory activity. Arch. Biochem. Biophys. 2016, 596, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Simões, R.C.; Rocha, B.A.; Bezerra, M.J.; Barroso-Neto, I.L.; Pereira-Junior, F.N.; da Mata Moura, R.; do Nascimento, K.S.; Nagano, C.S.; Delatorre, P.; de Freitas Pires, A.; et al. Protein crystal content analysis by mass spectrometry and preliminary X-ray diffraction of a lectin from, Canavalia grandiflora seeds with modulatory role in inflammation. Rapid Commun. Mass Spectrom. 2012, 26, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cheng, Y.; Zhang, B.; Bian, H.J.; Bao, J.K. Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria-mediated, ROS-p38-p53 pathway. Cancer Lett. 2009, 275, 54–60. [Google Scholar] [CrossRef] [PubMed]
- De Melo, C.M.; Paim, B.A.; Zecchin, K.G.; Morari, J.; Chiaratti, M.R.; Correia, M.T.; Barroso Coelho, L.C.; Paiva, P.M. Cramoll 1,4 lectin increases ROS production, calcium levels, and cytokine expression in treated spleen cells of rats. Mol. Cell. Biochem. 2010, 342, 163–169. [Google Scholar]
- Liu, T.; Wu, L.; Wang, D.; Wang, H.; Chen, J.; Yang, C.; Bao, J.; Wu, C. Role of reactive oxygen species mediated MAPK and NF-ĸB activation in Polygonatum cyrtonema lectin induced apoptosis and autophagy in human lung adenocarcinoma A549 cells. J. Biochem. 2016, 160, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Mukhopadhyay, S.; Behera, B.; Bhol, C.S.; Dey, S.; Das, D.N.; Sinha, N.; Bissoyi, A.; Pramanik, K.; Maiti, T.K.; et al. Antitumor effect of soybean lectin mediated through reactive oxygen species-dependent pathway. Life Sci. 2014, 111, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Son, Y.O.; Divya, S.P.; Roy, R.V.; Hitron, J.A.; Wang, L.; Kim, D.; Dai, J.; Asha, P.; Zhang, Z.; et al. Luteolin inhibits Cr(VI)-induced malignant cell transformation of human lung epithelial cells by targeting ROS mediated multiple cell signaling pathways. Toxicol. Appl. Pharmacol. 2014, 281, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Tajeddine, N. How do reactive oxygen species and calcium trigger mitochondrial membrane permeabilisation? Biochim. Biophys. Acta. 2016, 1860, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Cho, H.J.; Yu, S.N.; Kim, S.H.; Yu, H.S.; Park, Y.M.; Mirkheshti, N.; Kim, S.Y.; Song, C.S.; Chatterjee, B.; et al. Interplay of reactive oxygen species, intracellular Ca2+ and mitochondrial homeostasis in the apoptosis of prostate cancer cells by deoxypodophyllotoxin. J. Cell. Biochem. 2013, 114, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liu, H.; Jiao, H.; Wang, L.; Chen, L.; Liang, J.; Zhao, M.; Zhang, X. Neuroprotective effect of ginkgolide K on glutamate-induced cytotoxicity in PC 12 cells via inhibition of ROS generation and Ca2+ influx. Neurotoxicology 2012, 33, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Vajravijayan, S.; Pletnev, S.; Pletnev, V.Z.; Nandhagopal, N.; Gunasekaran, K. Structural analysis of β-prism lectin from Colocasia esculenta (L.) S chott. Int. J. Biol. Macromol. 2016, 91, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Ghosh, S.; Gupta, P.; Mukherjee, S.; Chattopadhyay, S. Inflammation-induced ROS generation causes pancreatic cell death through modulation of Nrf2/NF-κB and SAPK/JNK pathway. Free Radic. Res. 2015, 49, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Barth, C.R.; Funchal, G.A.; Luft, C.; de Oliveira, J.R.; Porto, B.N.; Donadio, M.V. Carrageenan-induced inflammation promotes ROS generation and neutrophil extracellular trap formation in a mouse model of peritonitis. Eur. J. Immunol. 2016, 46, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Hertzberg, H.; Butschi, A.; Bleuler-Martinez, S.; Aebi, M.; Deplazes, P.; Künzler, M.; Štefanić, S. Inhibition of Haemonchus contortus larval development by fungal lectins. Parasites Vectors. 2015, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- Bleuler-Martínez, S.; Butschi, A.; Garbani, M.; Wälti, M.A.; Wohlschlager, T.; Potthoff, E.; Sabotiĉ, J.; Pohleven, J.; Lüthy, P.; Hengartner, M.O.; et al. A lectin-mediated resistance of higher fungi against predators and parasites. Mol. Ecol. 2011, 20, 3056–3070. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.; Cabrero, P.; Basterrechea, J.E.; Tejero, J.; Cordoba-Diaz, D.; Girbes, T. Isolation and Molecular C.haracterization of Two Lectins from Dwarf Elder (Sambucus ebulus L.) Blossoms Related to the Sam n1 Allergen. Toxins 2013, 5, 1767–1779. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Pan, W.L.; Wong, J.H.; Chan, Y.S.; Ye, X.J.; Ng, T.B. A new Phaseolus vulgaris lectin induces selective toxicity on human liver carcinoma Hep G2 cells. Arch. Toxicol. 2011, 85, 1551–1563. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.R.; Nabi, M.M.; Nurujjaman, M.; Abu Reza, M.; Alam, A.H.; Uz Zaman, R.; Khalid-Bin-Ferdaus, K.M.; Amin, R.; Khan, M.M.; Hossain, M.A.; et al. Momordica charantia Seed Lectin: Toxicity Bacterial Agglutination and Antitumor Properties. Appl. Biochem. Biotechnol. 2015, 175, 2616–2628. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Zhivotovsky, B. Caspases and cancer. Cell Death Differ. 2011, 18, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Upton, J.W.; Kaiser, W.J.; Mocarski, E.S. Virus Inhibition of RIP3-Dependent Necrosis. Cell Host Microbe 2010, 7, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Lucas, K.; Maes, M. Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: Possible treatments targeting the TLR4 pathway. Mol. Neurobiol. 2013, 48, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.M.; Pervaiz, S. TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J. 2006, 20, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Banning, A.; Kny, M.; Böl, G.F. Redox events in interleukin-1 signaling. Arch. Biochem. Biophys. 2004, 423, 66–73. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wu, H.; Yu, H.; Zhang, X.; Cui, G.; Wang, K.; Mao, S.; Pan, Y. Typhonium giganteum Lectin Exerts A Pro-Inflammatory Effect on RAW 264.7 via ROS and The NF-κB Signaling Pathway. Toxins 2017, 9, 275. https://doi.org/10.3390/toxins9090275
Wang W, Wu H, Yu H, Zhang X, Cui G, Wang K, Mao S, Pan Y. Typhonium giganteum Lectin Exerts A Pro-Inflammatory Effect on RAW 264.7 via ROS and The NF-κB Signaling Pathway. Toxins. 2017; 9(9):275. https://doi.org/10.3390/toxins9090275
Chicago/Turabian StyleWang, Wei, Hao Wu, Hongli Yu, Xingde Zhang, Guojing Cui, Kuilong Wang, Shanhu Mao, and Yaozong Pan. 2017. "Typhonium giganteum Lectin Exerts A Pro-Inflammatory Effect on RAW 264.7 via ROS and The NF-κB Signaling Pathway" Toxins 9, no. 9: 275. https://doi.org/10.3390/toxins9090275
APA StyleWang, W., Wu, H., Yu, H., Zhang, X., Cui, G., Wang, K., Mao, S., & Pan, Y. (2017). Typhonium giganteum Lectin Exerts A Pro-Inflammatory Effect on RAW 264.7 via ROS and The NF-κB Signaling Pathway. Toxins, 9(9), 275. https://doi.org/10.3390/toxins9090275




