Inhibitory Activities of Blasticidin S Derivatives on Aflatoxin Production by Aspergillus Flavus
Abstract
:1. Introduction
2. Results and Discussion
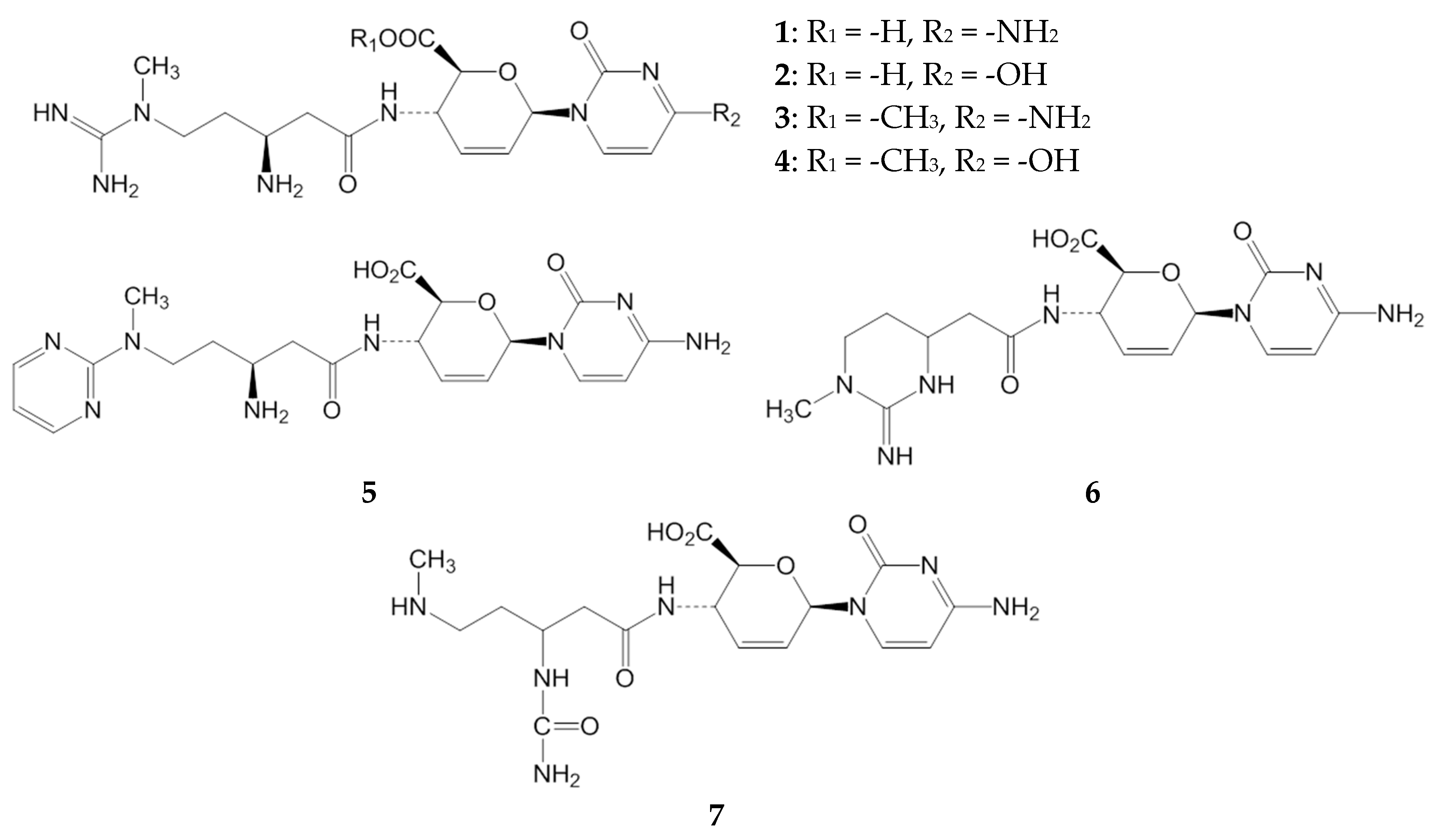
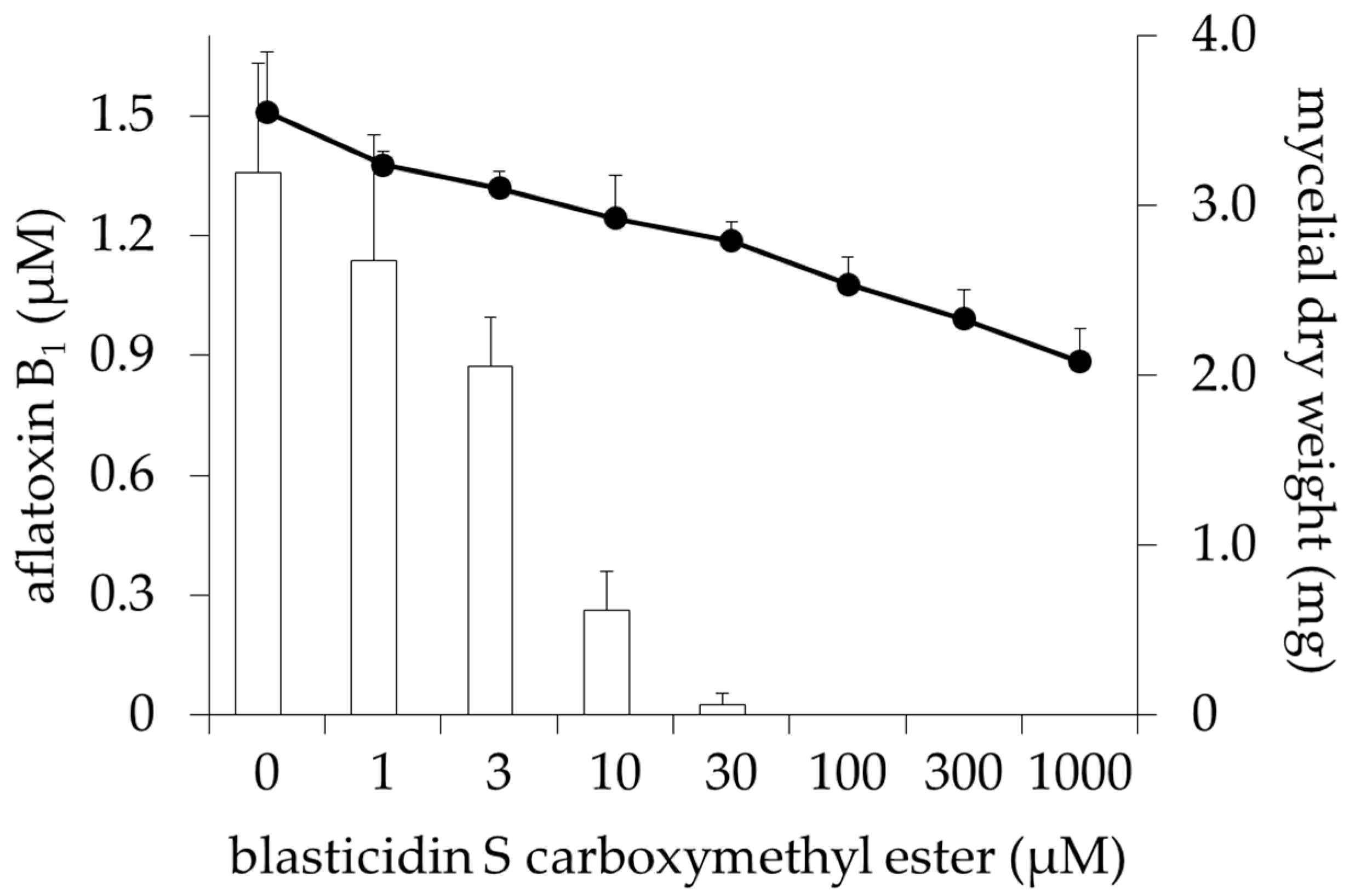
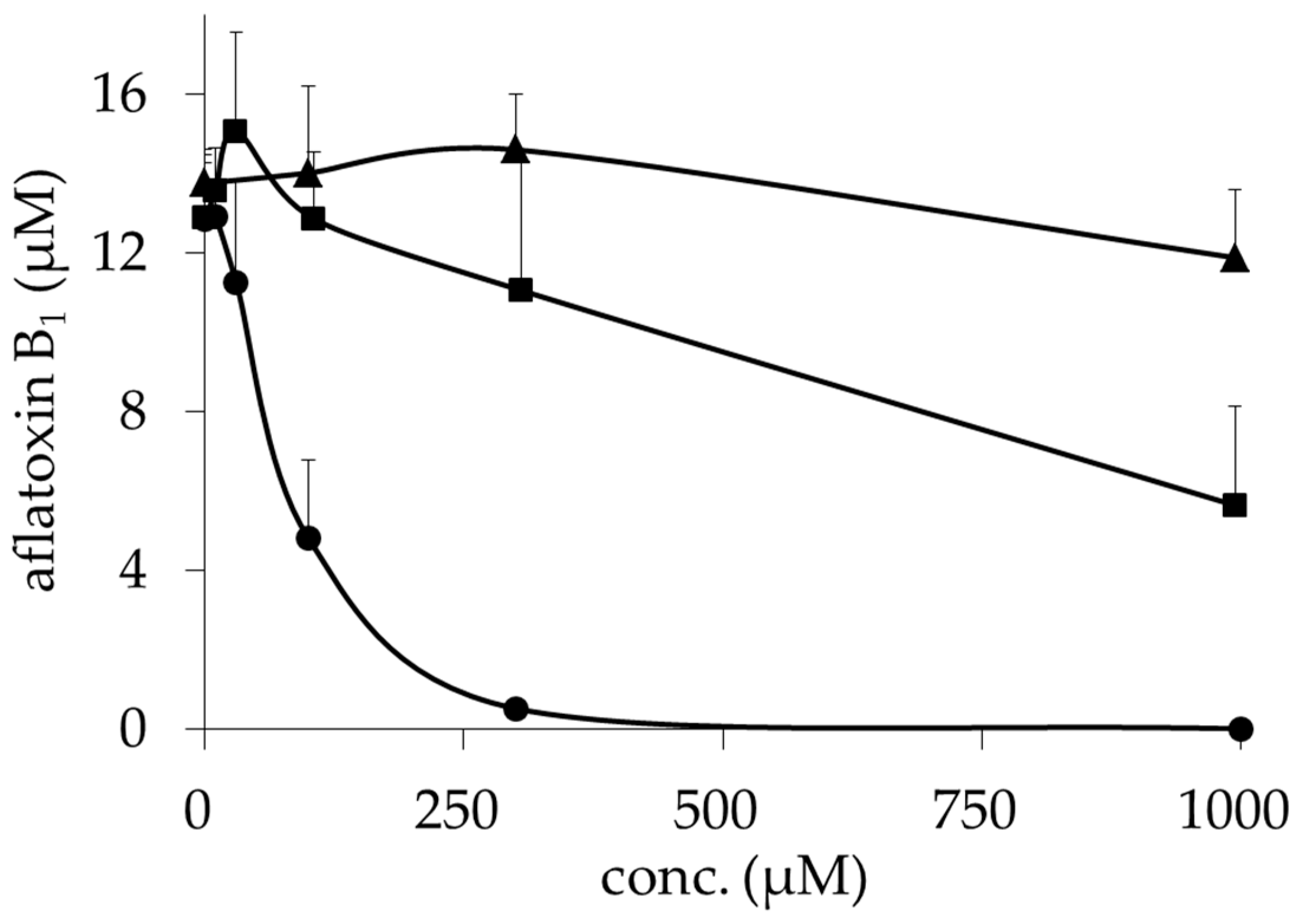
2.1. Inhibitory Activities of BcS Derivatives on Aflatoxin Production
2.2. Antimicrobial Activity of BcS Derivatives
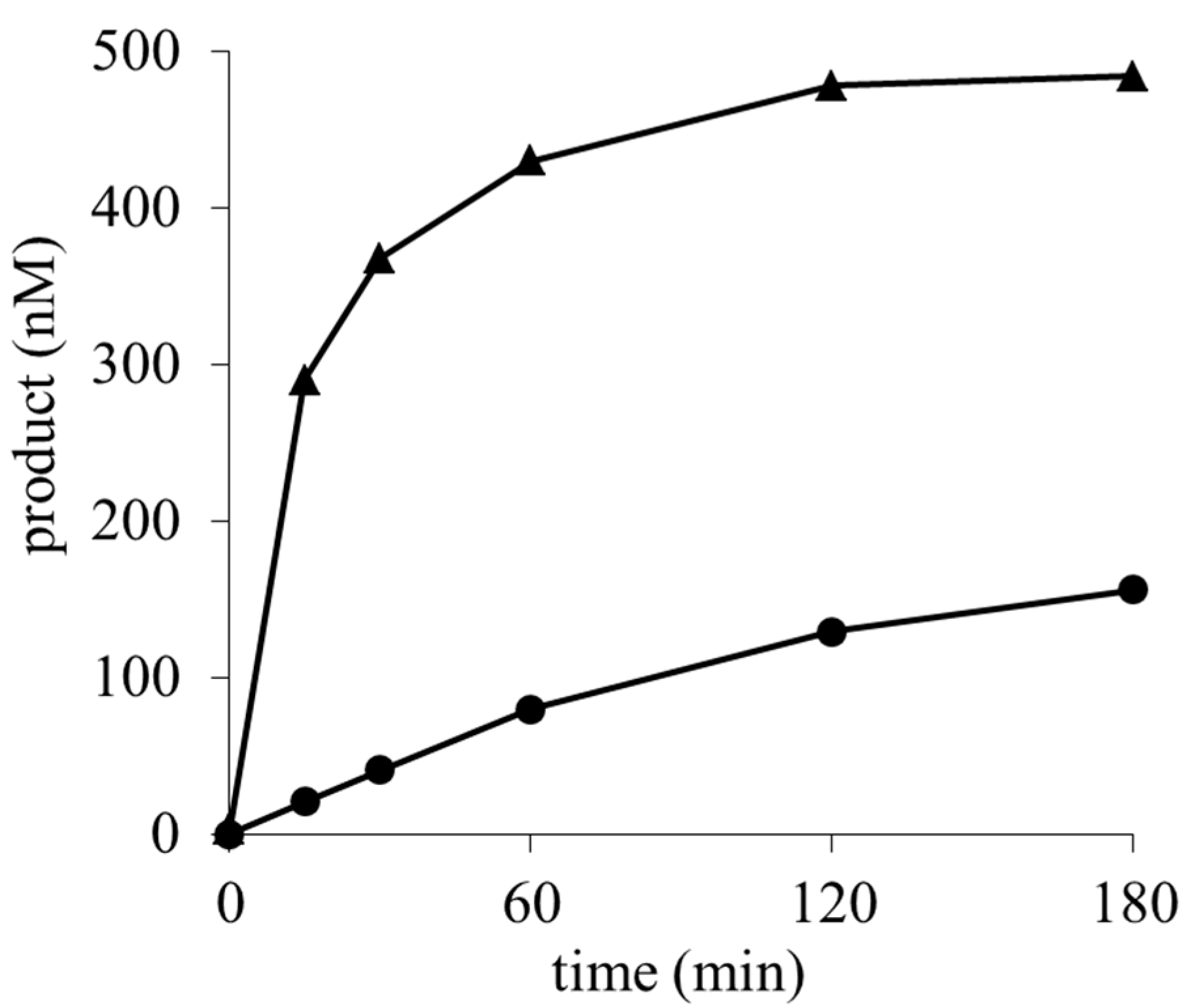
2.3. Determination of the Deaminated Metabolites of Blasticidin S and Its Methyl Ester
3. Conclusions
4. Materials and Methods
4.1. Strains, Growth Media, and Chemicals
4.2. Preparation of BcS Derivatives
4.2.1. Carboxymethyl Esters of Blasticidin S (3) and Deaminohydroxyblasticidin S (4)
4.2.2. Pyrimidinoblasticidin S (5)
4.2.3. Cytomycin (6) and (7)
4.2.4. Chromatographic Condition for Purification of BcS Derivatives
4.3. Analysis of Aflatoxin B1
4.4. Analysis of Antifungal Activity of BcS and Its Derivatives
4.5. Analysis of Antimicrobial Activity of BcS and Its Derivatives
4.6. Assay of BcS Deaminase Activity In Vitro
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef] [PubMed]
- Wu, F. Global impacts of aflatoxin in maize: Trade and human health. World Mycotoxin J. 2015, 8, 137–142. [Google Scholar] [CrossRef]
- Magnussen, A.; Parsi, M.A. Aflatoxins, hepatocellular carcinoma and public health. World J. Gastroenterol. 2013, 19, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Roebuck, B.D.; Wogan, G.N.; Groopman, J.D. Aflatoxin: A 50-year odyssey of mechanistic and translational toxicology. Toxicol. Sci. 2011, 120, S28–S48. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, K.C. Non-aflatoxigenic Aspergillus flavus to prevent aflatoxin contamination in crops: Advantages and limitations. Front. Microbiol. 2014, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Atehnkeng, J.; Ojiambo, P.S.; Ikotun, T.; Sikora, R.A.; Cotty, P.J.; Bandyopadhyay, R. Evaluation of atoxigenic isolates of Aspergillus flavus as potential biocontrol agents for aflatoxin in maize. Food Addit. Contam. Part A 2008, 25, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Sakuda, S.; Yoshinari, T.; Furukawa, T.; Jermnak, J.; Takagi, K.; Iimura, K.; Yamamoto, T.; Suzuki, M.; Nagasawa, H. Search for aflatoxin and trichothecene production inhibitors and analysis of their modes of action. Biosci. Biotechnol. Biochem. 2016, 80, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.A.; Boston, R.S.; Payne, G.A. Diverse inhibitors of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2008, 78, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Hirayama, K.; Ueda, K.; Sakai, H.; Yonehara, H. Blasticidin S, a new antibiotic. J. Antibiot. 1958, 11, 1–5. [Google Scholar]
- Yamaguchi, H.; Yamamoto, C.; Tanaka, N. Inhibition of protein synthesis by blasticidin S. I. Studies with cell-free systems from bacterial and mammalian cells. J. Biochem. 1965, 57, 667–677. [Google Scholar] [PubMed]
- Huang, K.T.; Misato, T.; Asuyama, H. Selective toxicity of blasticidin S to Piricularia oryzae and Pellicularia sasakii. J. Antibiot. 1964, 17, 71–74. [Google Scholar] [PubMed]
- Seto, H.; Otake, N.; Yonehara, H. Biological transformation of blasticidin S by Aspergillus fumigatus sp. Agric. Biol. Chem. 1966, 30, 877–886. [Google Scholar] [CrossRef]
- Kimura, M.; Takatsuki, A.; Yamaguchi, I. Blasticidin S deaminase gene from Aspergillus terreus (BSD): A new drug resistance gene for transfection of mammalian cells. Biochim. Biophys. Acta 1994, 1219, 653–659. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Shibata, H.; Seto, H.; Misato, T. Isolation and purification of blasticidin S deaminase from Aspergillus terreus. J. Antibiot. 1975, 28, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, T.; Sakuda, S; Watanabe, M.; Kamata, Y.; Ohnishi, T.; Sugita-Konishi, Y. New metabolic pathway for converting blasticidin S in Aspergillus flavus and inhibitory activity of aflatoxin production by blasticidin S metabolites. J. Agric. Food Chem. 2013, 61, 7925–7931. [Google Scholar] [CrossRef] [PubMed]
- Menzel, H.M.; Lichtenthaler, F.W. Structure–activity relationships in the aminoacyl-4-aminohexosyl-cytosine group of peptidyl transferase inhibitors. Nucleic Acids Res. Spec. Publ. 1975, 1, S155–S158. [Google Scholar]
- Yamaguchi, H.; Tanaka, N. Inhibition of protein synthesis by blasticidin S. II. Studies on the site of action in E. coli polypeptide synthesizing systems. J. Biochem. 1966, 60, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Otake, N.; Takeuchi, S.; Endo, T.; Yonehara, H. Chemical studies on blasticidin S. Part III. The structure of blasticidin S. Agric. Biol. Chem. 1966, 30, 132–141. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Seto, H.; Misato, T. Substrate binding by blasticidin S deaminase, an aminohydrolase for novel 4-aminopyrimidine nucleosides. Pestic. Biochem. Physiol. 1986, 25, 54–62. [Google Scholar] [CrossRef]
- Yonehara, H.; Takeuchi, S.; Otake, N.; Endo, T.; Sakagami, Y.; Sumiki, Y. Chemical studies on blasticidin S. I. Hydrolysis of blasticidin S. J. Antibiot. 1963, 16, 195–202. [Google Scholar] [PubMed]
| Compound | Aflatoxin Production IC50 (µM) | Growth (Minimum Inhibitory Concentration (µM)) | |||
|---|---|---|---|---|---|
| A. flavus | A. flavus | A. niger | S. cerevisiae | E. coli | |
| BcS (1) | 27 a | >1000 a | 300 a | 3 | 500 |
| DahBcS (2) | 110 a | >1000 a | >1000 a | >1000 | >1000 |
| MeBcS (3) | 5 | >1000 | 300 | 10 | 300 |
| PyBcS (5) | 610 | >1000 | >1000 | >1000 | >1000 |
| cytomycin (6) | >1000 | >1000 | >1000 | >1000 | >1000 |
| 7 | >1000 | >1000 | >1000 | >1000 | >1000 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshinari, T.; Sugita-Konishi, Y.; Ohnishi, T.; Terajima, J. Inhibitory Activities of Blasticidin S Derivatives on Aflatoxin Production by Aspergillus Flavus. Toxins 2017, 9, 176. https://doi.org/10.3390/toxins9060176
Yoshinari T, Sugita-Konishi Y, Ohnishi T, Terajima J. Inhibitory Activities of Blasticidin S Derivatives on Aflatoxin Production by Aspergillus Flavus. Toxins. 2017; 9(6):176. https://doi.org/10.3390/toxins9060176
Chicago/Turabian StyleYoshinari, Tomoya, Yoshiko Sugita-Konishi, Takahiro Ohnishi, and Jun Terajima. 2017. "Inhibitory Activities of Blasticidin S Derivatives on Aflatoxin Production by Aspergillus Flavus" Toxins 9, no. 6: 176. https://doi.org/10.3390/toxins9060176
APA StyleYoshinari, T., Sugita-Konishi, Y., Ohnishi, T., & Terajima, J. (2017). Inhibitory Activities of Blasticidin S Derivatives on Aflatoxin Production by Aspergillus Flavus. Toxins, 9(6), 176. https://doi.org/10.3390/toxins9060176





