Development of an Immunochromatographic Strip Test for the Rapid Detection of Alternariol Monomethyl Ether in Fruit
Abstract
:1. Introduction
2. Results
2.1. Carboxyl Derivative Modification of AME
2.2. Production and Characterization of Anti-AME mAb
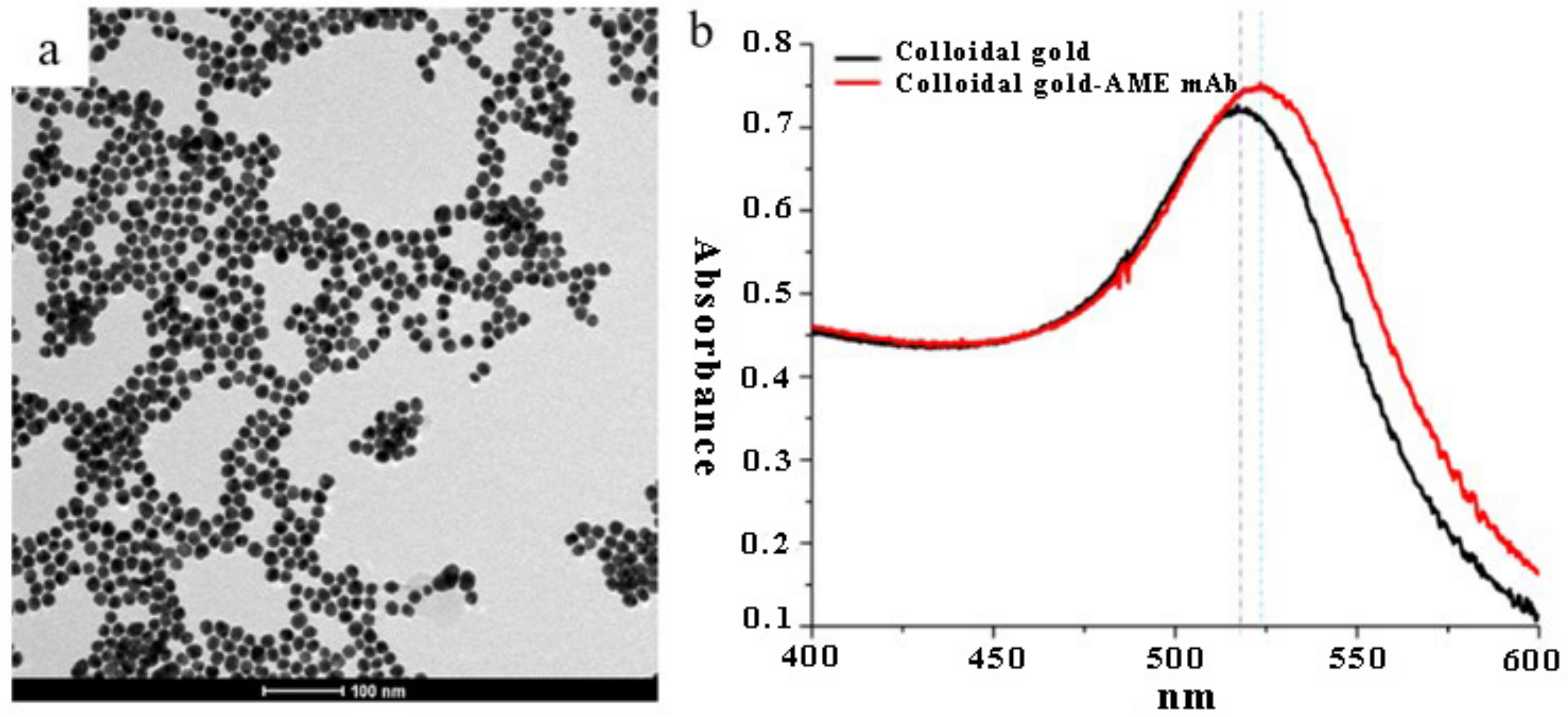
2.3. Characterization of Colloidal Gold Nanoparticles (CGNs) and the CGNs-mAb Conjugates
2.4. Assembly and Competitive Detection Principle of Immunochromatographic Strip
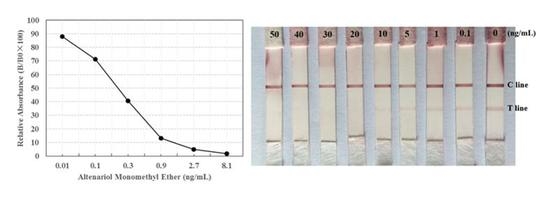
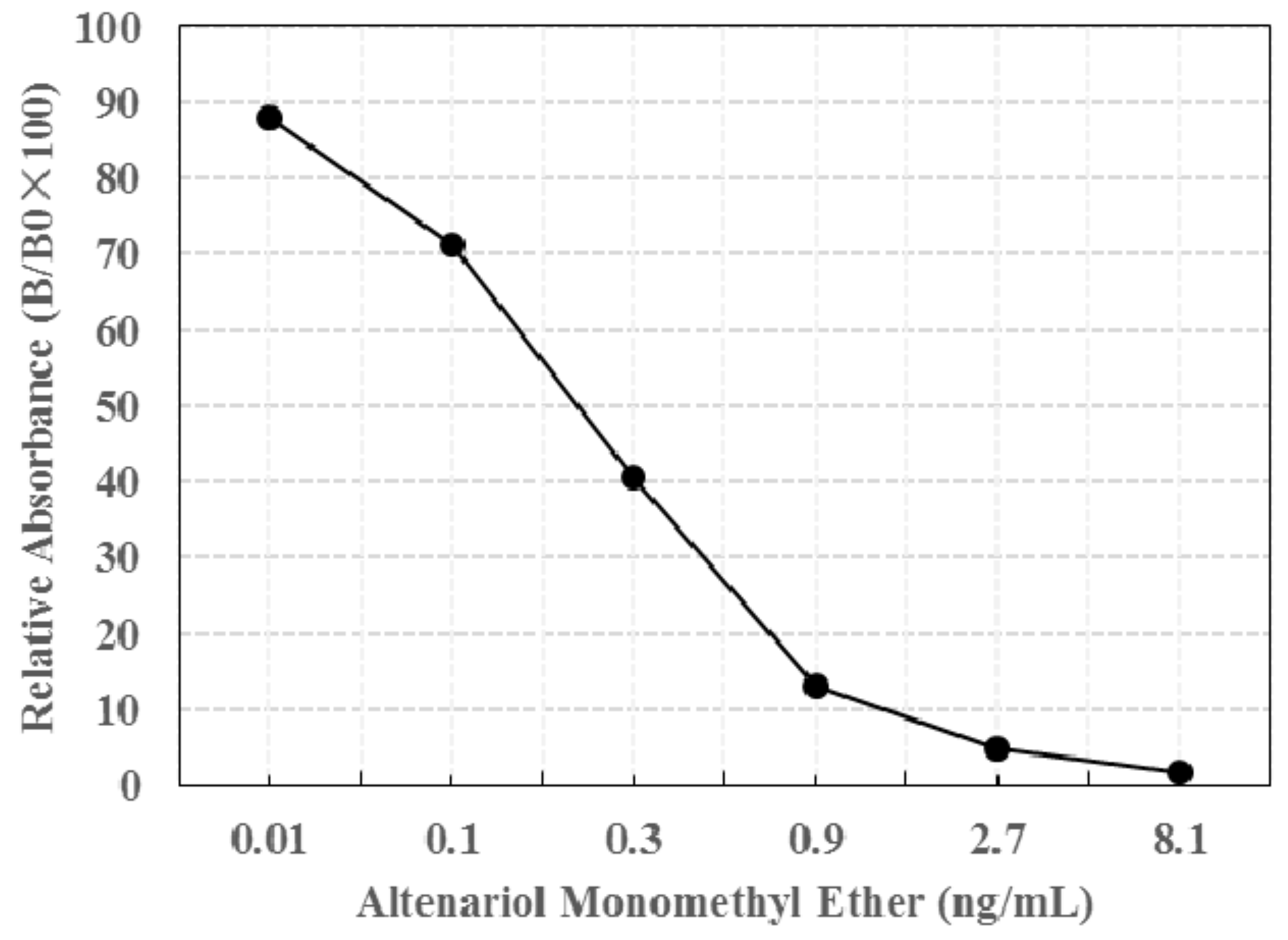
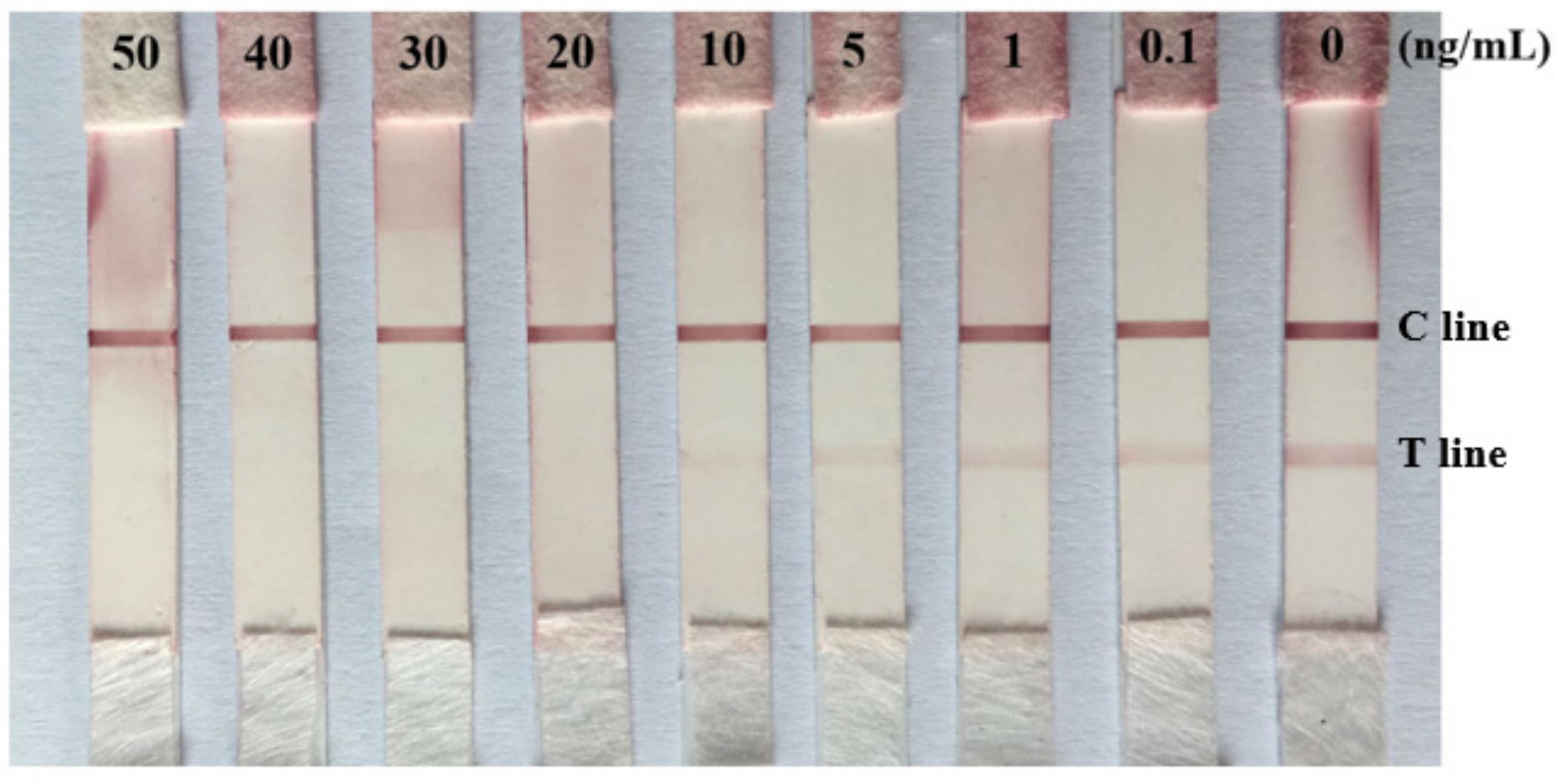
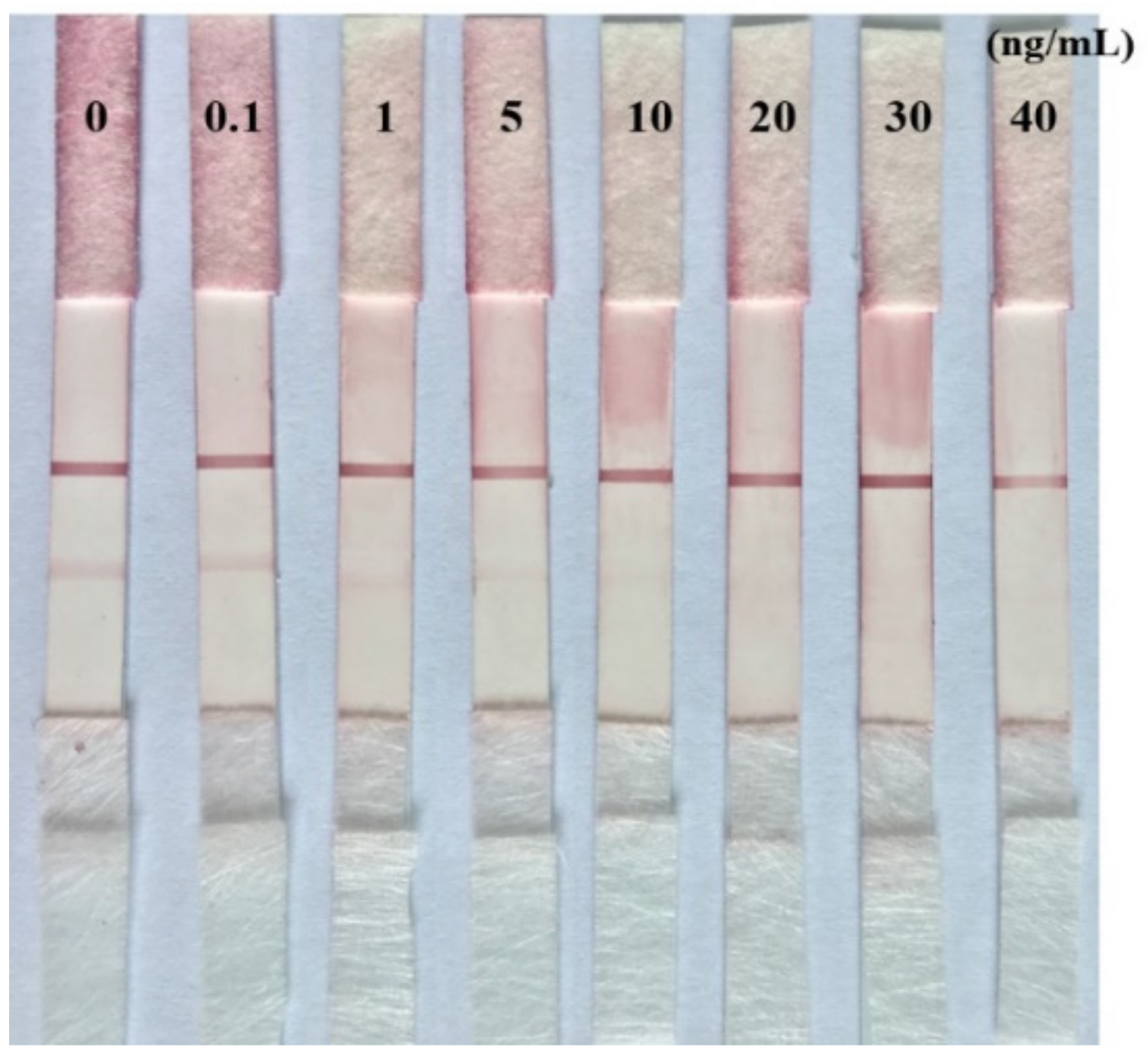
2.5. Limit of Detection (LOD) of the Prepared Immunochromatographic Strip
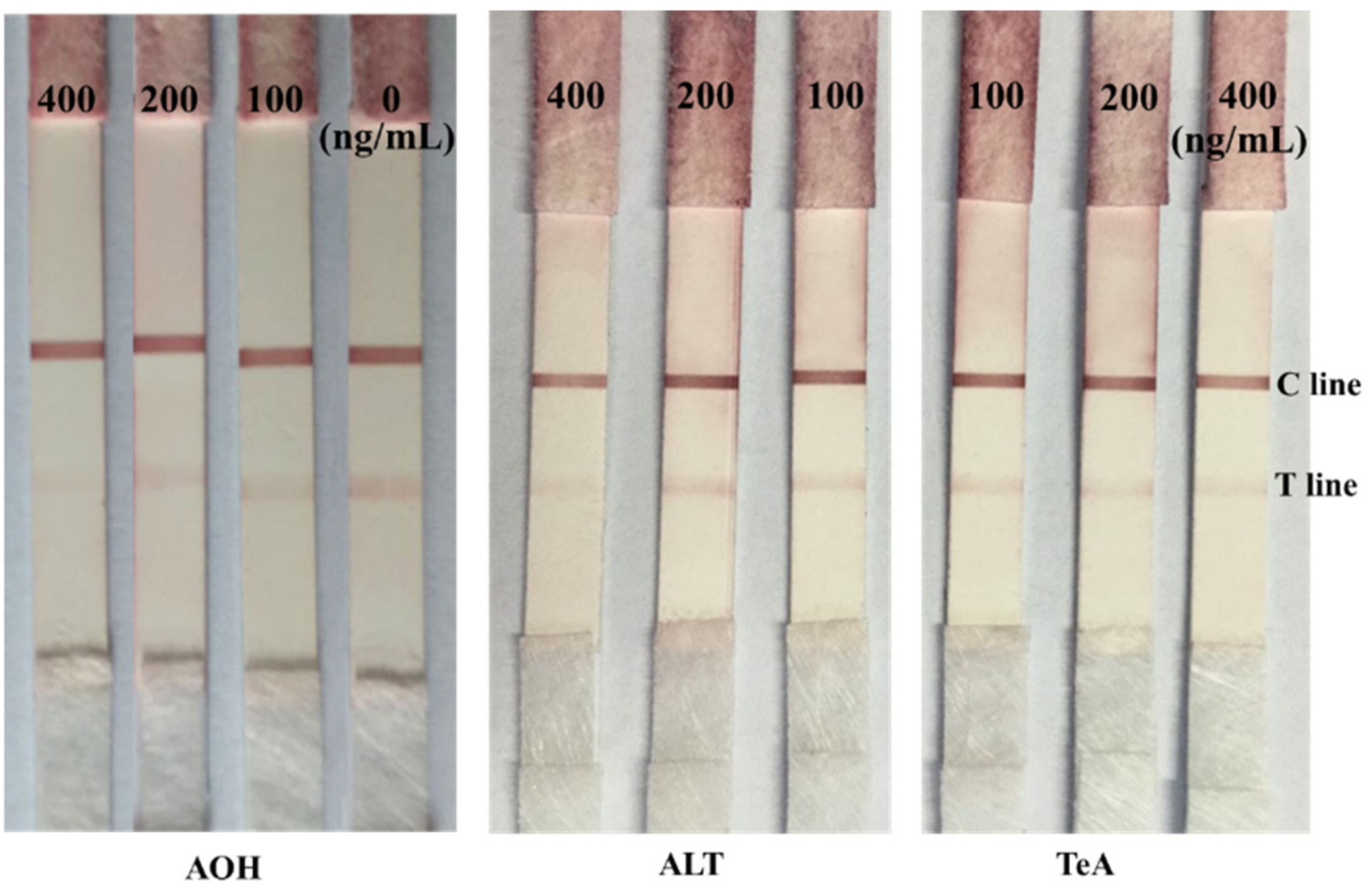
2.6. Cross Reactivity Test of the Prepared Immunochromatographic Strip
2.7. The Stability Performance of the Immunochromatographic Strip for the Detection of AME
2.8. Application of the Immunochromatographic Strip to Spiked Fruit Samples
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Carboxyl Derivative Modification of AME
4.3. Preparation of AME-BSA and AME-OVA
4.4. Preparation of Anti-AME mAb
4.5. Competitive ELISA
4.6. Preparation of Colloidal Gold Nanoparticles (CGNs) and CGNs-mAb Conjugates
4.7. Preparation of Colloidal Gold Immunochromatographic Strip
4.8. Samples and Preparation
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cottrill, B.; Cravedi, J.; Di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L. Scientific opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 9, 2407–2504. [Google Scholar]
- Man, Y.; Liang, G.; Li, A.; Pan, L. Analytical methods for the determination of Alternaria mycotoxins. Chromatographia 2016, 80, 1–14. [Google Scholar] [CrossRef]
- Olsen, M.; Visconti, A. Metabolism of alternariol monomethyl ether by porcine liver and intestinal mucosa in vitro. Toxicol. In Vitro 1988, 2, 27–29. [Google Scholar] [CrossRef]
- Liu, G.T.; Qian, Y.Z.; Zhang, P.; Dong, W.H.; Qi, Y.M.; Guo, H.T. Etiological role of Alternaria alternata in human esophageal cancer. Chin. Med. J. 1992, 105, 394–400. [Google Scholar] [PubMed]
- Pfeiffer, E.; Eschbach, S.; Metzler, M. Alternaria toxins: DNA strand-breaking activity in mammalian cellsin vitro. Mycotoxin Res. 2007, 23, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Marko, D. Mechanisms of the genotoxic effect of Alternaria toxins, Gesellschaft fur Mykotoxin Forschung. In Proceedings of the 29th Mycotoxin Workshop, Stuttgart-Fellbach, Germany, 14–16 May 2007. [Google Scholar]
- Ostry, V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Köppen, R.; Koch, M.; Siegel, D.; Merkel, S.; Maul, R.; Nehls, I. Determination of mycotoxins in foods: Current state of analytical methods and limitations. Appl. Microbiol. Biotechnol. 2010, 86, 1595–1612. [Google Scholar] [PubMed]
- Scott, P.; Weber, D.; Kanhere, S. Gas chromatography-mass spectrometry of Alternaria mycotoxins. J. Chromatogr. A 1997, 765, 255–263. [Google Scholar] [CrossRef]
- Hickert, S.; Bergmann, M.; Ersen, S.; Cramer, B.; Humpf, H.-U. Survey of Alternaria toxin contamination in food from the German market, using a rapid HPLC-MS/MS approach. Mycotoxin Res. 2015, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Walravens, J.; Mikula, H.; Rychlik, M.; Asam, S.; Devos, T.; Njumbe Ediage, E.; Diana Di Mavungu, J.; Jacxsens, L.; Van Landschoot, A.; Vanhaecke, L.; et al. Validated UPLC-MS/MS methods to quantitate free and conjugated Alternaria toxins in commercially available tomato products and fruit and vegetable juices in Belgium. J. Agric. Food Chem. 2016, 64, 5101–5109. [Google Scholar] [CrossRef] [PubMed]
- Walravens, J.; Mikula, H.; Rychlik, M.; Asam, S.; Ediage, E.N.; Di Mavungu, J.D.; Van Landschoot, A.; Vanhaecke, L.; De Saeger, S. Development and validation of an ultra-high-performance liquid chromatography tandem mass spectrometric method for the simultaneous determination of free and conjugated Alternaria toxins in cereal-based foodstuffs. J. Chromatogr. A 2014, 1372, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Liu, L.; Song, S.; Suryoprabowo, S.; Li, A.; Kuang, H.; Wang, L.; Xu, C. A gold nanoparticle-based semi-quantitative and quantitative ultrasensitive paper sensor for the detection of twenty mycotoxins. Nanoscale 2016, 8, 5245–5253. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Lee, D.H.; Kim, D.O.; Min, W.K.; Bong, K.T.; Lee, G.G.; Seo, J.H. Production of a monoclonal antibody against ochratoxin A and its application to immunochromatographic assay. J. Agric. Food Chem. 2005, 53, 8447–8451. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Tsao, Z.J.; Wang, J.J.; Yu, F.Y. Development of a monoclonal antibody against ochratoxin A and its application in enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip. Anal. Chem. 2008, 80, 7029–7035. [Google Scholar] [CrossRef] [PubMed]
- Majdinasab, M.; Zeinoddin, M.S.; Zad, S.S.; Li, P.; Zhang, Q.; Li, X.; Tang, X. Ultrasensitive and quantitative gold nanoparticle-based immunochromatographic assay for detection of ochratoxin A in agro-products. J. chromatogr. B 2015, 974, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-B.; Xu, Y.; Li, L.S.; Li, Y.P.; Zhang, H.; He, Q.H. Development of an immunochromatographic strip test for the rapid simultaneous detection of deoxynivalenol and zearalenone in wheat and maize. Food Control 2012, 28, 7–12. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Z.-B.; He, Q.-H.; Deng, S.Z.; Li, L.S.; Li, Y.P. Development of an immunochromatographic strip test for the rapid detection of deoxynivalenol in wheat and maize. Food Chem. 2010, 119, 834–839. [Google Scholar] [CrossRef]
- Liu, J.-W.; Lu, C.-C.; Liu, B.-H.; Yu, F.Y. Development of novel monoclonal antibodies-based ultrasensitive enzyme-linked immunosorbent assay and rapid immunochromatographic strip for aflatoxin B1 detection. Food Control 2016, 59, 700–707. [Google Scholar] [CrossRef]
- Wang, J.J.; Liu, B.H.; Hsu, Y.T.; Yu, F.Y. Sensitive competitive direct enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip for detecting aflatoxin M1 in milk. Food Control 2011, 22, 964–969. [Google Scholar] [CrossRef]
- Li, Y.S.; Zhou, Y.; Lu, S.Y.; Guo, D.J.; Ren, H.L.; Meng, X.M.; Zhi, B.H.; Lin, C.; Wang, Z.; Li, X.B.; et al. Development of a one-step test strip for rapid screening of fumonisins B1, B2 and B3 in maize. Food Control 2012, 24, 72–77. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Zhang, Y.; Yang, J.; Wang, F.; Wang, Y.; Deng, R.; Zhang, G. Development of an immunochromatographic strip test for the rapid detection of zearalenone in corn. J. Agric. Food Chem. 2014, 62, 11116–11121. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zhang, X.; Zhao, R.; Wang, L.; Feng, T.; Wei, D. An enzyme-linked immunosorbent assay and a gold-nanoparticle based immunochromatographic test for amatoxins using recombinant antibody. Microchimi. Acta 2016, 183, 2211–2219. [Google Scholar] [CrossRef]
- Molinelli, A.; Grossalber, K.; Führer, M.; Baumgartner, S.; Sulyok, M.; Krska, R. Development of qualitative and semiquantitative immunoassay-based rapid strip tests for the detection of T-2 toxin in wheat and oat. J. Agric. Food Chem. 2008, 56, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, C.; Wen, K.; Jiang, H.; Shen, J.; Zhang, S.; Wang, Z. Comparison of Fluorescent Microspheres and Colloidal Gold as Labels in Lateral Flow Immunochromatographic Assays for the Detection of T-2 Toxin. Molecules 2016, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xing, G.; Yang, J.; Wang, F.; Deng, R.; Zhang, G.; Hu, X.; Zhang, Y. Development of an immunochromatographic test strip for simultaneous qualitative and quantitative detection of ochratoxin A and zearalenone in cereal. J. Agric. Food Chem. 2016, 96, 3673–3678. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, V.; Nara, S.; Singh, K.; Singh, H.; Shrivastav, T.G. A competitive immunochromatographic strip assay for 17-α-hydroxy progesterone using colloidal gold nanoparticles. Clin. Chim. Acta 2012, 413, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.; Lv, X.; Iqbal, J.; Peng, G.; Song, D.; Zhang, C.; Deng, Y. Microchip based and immunochromatographic strip assays for the visual detection of interleukin-6 and of tumor necrosis factor α using gold nanoparticles as labels. Microchim. Acta 2015, 182, 597–604. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, N.; Xian, H.; Wei, D.; Shi, L.; Feng, X. A single-step solid phase extraction for the simultaneous determination of 8 mycotoxins in fruits by ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1429, 22–29. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, Y.; Liang, G.; Jia, F.; Li, A.; Fu, H.; Wang, M.; Pan, L. Development of an Immunochromatographic Strip Test for the Rapid Detection of Alternariol Monomethyl Ether in Fruit. Toxins 2017, 9, 152. https://doi.org/10.3390/toxins9050152
Man Y, Liang G, Jia F, Li A, Fu H, Wang M, Pan L. Development of an Immunochromatographic Strip Test for the Rapid Detection of Alternariol Monomethyl Ether in Fruit. Toxins. 2017; 9(5):152. https://doi.org/10.3390/toxins9050152
Chicago/Turabian StyleMan, Yan, Gang Liang, Fuchao Jia, An Li, Hailong Fu, Meng Wang, and Ligang Pan. 2017. "Development of an Immunochromatographic Strip Test for the Rapid Detection of Alternariol Monomethyl Ether in Fruit" Toxins 9, no. 5: 152. https://doi.org/10.3390/toxins9050152
APA StyleMan, Y., Liang, G., Jia, F., Li, A., Fu, H., Wang, M., & Pan, L. (2017). Development of an Immunochromatographic Strip Test for the Rapid Detection of Alternariol Monomethyl Ether in Fruit. Toxins, 9(5), 152. https://doi.org/10.3390/toxins9050152