Individual and Combined Cytotoxic Effects of Co‐Occurring Deoxynivalenol Family Mycotoxins on Human Gastric Epithelial Cells
Abstract
:1. Introduction
2. Results
2.1. Individual Cytotoxicity of Type B Trichothecenes
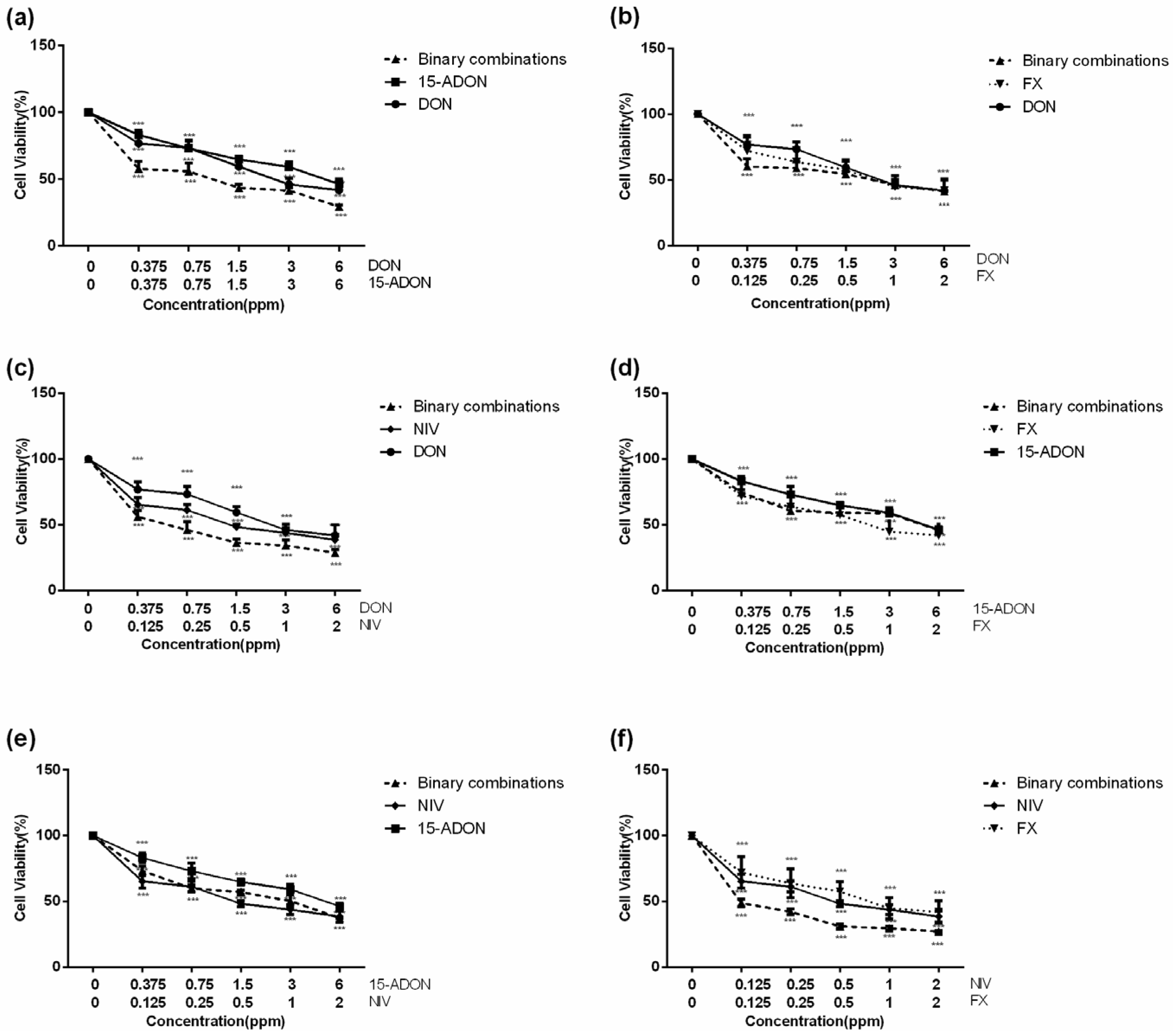
2.2. Combined Toxicity of DON, 15-ADON, FX, and NIV on GES-1 Cells
2.3. The Different Interaction Effects of Type B Trichothecenes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Toxins and Chemicals
5.2. Cell Culture
5.3. Experiment Design
5.4. Assessment of the Effect of Mycotoxin Combinations
5.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.D.G. Emerging food safety issues: An EU perspective. Drug Test. Anal. 2016, 8, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, Q.; Kuča, K.; Dohnal, V.; Tian, Z. Deoxynivalenol: Signaling pathways and human exposure risk assessment—An update. Arch. Toxicol. 2014, 88, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Rotter, B.A.; Prelusky, D.B.; Pestka, J.J. Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Environ. Health 1996, 48, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Nie, D.; Ediage, E.N.; Yang, X.; Wang, J.; Chen, B.; Li, S.; On, S.L.W.; de Saeger, S.; Wu, A. Cumulative health risk assessment of co-occurring mycotoxins of deoxynivalenol and its acetyl derivatives in wheat and maize: Case study, Shanghai, China. Food Chem. Toxicol. 2014, 74, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tan, Y.; Liu, N.; Yan, Z.; Liao, Y.; Chen, J.; de Saeger, S.; Yang, H.; Zhang, Q.; Wu, A. Detoxification of deoxynivalenol via glycosylation represents novel insights on antagonistic activities of trichoderma when confronted with Fusarium graminearum. Toxins 2016, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- De Boevre, M.; Jacxsens, L.; Lachat, C.; Eeckhout, M.; di Mavungu, J.D.; Audenaert, K.; Maene, P.; Haesaert, G.; Kolsteren, P.; de Meulenaer, B.; de Saeger, S. Human exposure to mycotoxins and their masked forms through cereal-based foods in Belgium. Toxicol. Lett. 2013, 218, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Q.; Wang, W.; Ma, J.J.; Yu, C.C.; Lin, X.H.; Yan, W.X. Natural occurrence of masked deoxynivalenol in Chinese wheat and wheat-based products during 2008–2011. World Mycotoxin J. 2012, 5, 221–230. [Google Scholar] [CrossRef]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; Saeger, S.D.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Cirlini, M.; Dall’Asta, C.; Galaverna, G. Hyphenated chromatographic techniques for structural characterization and determination of masked mycotoxins. J. Chromatogr. A 2012, 1255, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Tan, Y.; Wang, S.; Gardiner, D.M.; de Saeger, S.; Liao, Y.; Wang, C.; Fan, Y.; Wang, Z.; Wu, A. Mycotoxigenic potentials of Fusarium species in various culture matrices revealed by mycotoxin profiling. Toxins 2016, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.; Samar, M.; Moltó, G.; Resnik, S.; Pacin, A. Trichothecenes and zearalenone production by Fusarium species isolated from Argentinean black beans. Mycotoxin Res. 2002, 18, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Molto, G.A.; Gonzalez, H.H.; Resnik, S.L.; Pereyra Gonzalez, A. Production of trichothecenes and zearalenone by isolates of Fusarium spp. from Argentinian maize. Food Addit. Contam. 1997, 14, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; Oswald, I.P. Current situation of mycotoxin contamination and co-occurrence in animal feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.; Ghareeb, K.; Böhm, J.; Zentek, J. The toxicological impacts of the Fusarium mycotoxin, deoxynivalenol, in poultry flocks with special reference to immunotoxicity. Toxins 2013, 5, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Stoev, S.D. Food safety and increasing hazard of mycotoxin occurrence in foods and feeds. Crit. Rev. Food Sci. Nutr. 2013, 53, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Braber, S.; Varasteh, S.; Alizadeh, A.; Garssen, J.; Fink-Gremmels, J. The intestinal barrier as an emerging target in the toxicological assessment of mycotoxins. Arch. Toxicol. 2017, 91, 1007–1029. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Oswald, I.P. Effect of deoxynivalenol and other type B trichothecenes on the intestine: A review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef] [PubMed]
- Akbari, P.; Braber, S.; Gremmels, H.; Koelink, P.J.; Verheijden, K.A.T.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol: A trigger for intestinal integrity breakdown. FASEB J. 2014, 28, 2414–2429. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Kolf-Clauw, M.; Gauthier, T.; Abrami, R.; Abiola, F.A.; Oswald, I.P.; Puel, O. New insights into mycotoxin mixtures: The toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol. Appl. Pharmacol. 2013, 272, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Puel, O.; Oswald, I.P. Toxicological interactions between the mycotoxins deoxynivalenol, nivalenol and their acetylated derivatives in intestinal epithelial cells. Arch. Toxicol. 2015, 89, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Mimoun, S.; Murate, L.S.; Loiseau, N.; Lippi, Y.; Bracarense, A.-P.F.L.; Liaubet, L.; Schatzmayr, G.; Berthiller, F.; Moll, W.-D.; Oswald, I.P. Intestinal toxicity of the masked mycotoxin deoxynivalenol-3-β-d-glucoside. Arch. Toxicol. 2016, 90, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, T.; Wang, X.; Santos, J.S.D.; Fysikopoulos, A.; Tadrist, S.; Canlet, C.; Artigot, M.P.; Loiseau, N.; Oswald, I.P.; Puel, O. Trypacidin, a spore-borne toxin from Aspergillus fumigatus, is cytotoxic to lung cells. PLoS ONE 2012, 7, e29906. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.C. Drug combination studies and their synergy quantification using the Chou–Talalay method—Letter. Cancer Res. 2015, 75, 2400. [Google Scholar] [CrossRef] [PubMed]

| Mycotoxin | Dose-Effect Parameters | EC30 | EC70 | ||
|---|---|---|---|---|---|
| Dm | m | r | |||
| DON | 2.99 | 0.61 | 0.9814 | 0.75 | 11.99 |
| 15-ADON | 4.77 | 0.59 | 0.9904 | 1.13 | 20.05 |
| NIV | 0.58 | 0.42 | 0.9820 | 0.08 | 4.36 |
| FX | 0.85 | 0.48 | 0.9873 | 0.15 | 4.97 |
| DON + 15ADON | 1.94 | 0.46 | 0.9673 | 0.31 | 12.24 |
| DON + FX | 2.72 | 0.29 | 0.9742 | 0.15 | 50.51 |
| DON + NIV | 0.73 | 0.41 | 0.9781 | 0.09 | 5.76 |
| 15-ADON + FX | 4.79 | 0.40 | 0.9546 | 0.58 | 39.84 |
| 15-ADON + NIV | 3.23 | 0.49 | 0.9757 | 0.57 | 18.20 |
| FX + IV | 0.18 | 0.35 | 0.9557 | 0.02 | 2.03 |
| Mycotoxin | Combination Ratio | 10% Cytotoxicity | 30% Cytotoxicity | 50% Cytotoxicity | 70% Cytotoxicity | 90% Cytotoxicity | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CI | DRI | CI | DRI | CI | DRI | CI | DRI | CI | DRI | ||
| DON | 1:1 | 0.12 | 14.59 | 0.29 | 5.61 | 0.52 | 3.08 | 0.95 | 1.69 | 2.42 | 0.64 |
| 15-ADON | 21.29 | 8.65 | 4.91 | 2.79 | 1.13 | ||||||
| DON | 3:1 | 0.06 | 70.23 | 0.42 | 6.51 | 1.48 | 1.46 | 5.47 | 0.33 | 47.19 | 0.03 |
| FX | 22.56 | 3.82 | 1.25 | 0.41 | 0.07 | ||||||
| DON | 3:1 | 0.3 | 33.47 | 0.39 | 10.98 | 0.5 | 5.45 | 0.71 | 2.71 | 1.5 | 0.89 |
| NIV | 3.8 | 3.4 | 4.62 | 2.95 | 2.64 | ||||||
| 15-ADON | 3:1 | 0.73 | 7.62 | 1.39 | 2.6 | 2.16 | 1.33 | 3.43 | 0.6 | 7.63 | 0.23 |
| FX | 1.67 | 0.99 | 0.71 | 0.51 | 0.3 | ||||||
| 15-ADON | 3:1 | 3.38 | 4.1 | 2.29 | 2.61 | 1.9 | 1.97 | 1.7 | 1.48 | 1.69 | 0.94 |
| NIV | 0.32 | 0.52 | 0.71 | 0.98 | 1.6 | ||||||
| FX | 1:1 | 0.07 | 51.46 | 0.16 | 18.3 | 0.26 | 9.56 | 0.43 | 4.99 | 1 | 1.78 |
| NIV | 18.18 | 9.63 | 6.47 | 4.34 | 2.3 | ||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Yu, S.; Tan, Y.; Liu, N.; Wu , A. Individual and Combined Cytotoxic Effects of Co‐Occurring Deoxynivalenol Family Mycotoxins on Human Gastric Epithelial Cells. Toxins 2017, 9, 96. https://doi.org/10.3390/toxins9030096
Yang Y, Yu S, Tan Y, Liu N, Wu A. Individual and Combined Cytotoxic Effects of Co‐Occurring Deoxynivalenol Family Mycotoxins on Human Gastric Epithelial Cells. Toxins. 2017; 9(3):96. https://doi.org/10.3390/toxins9030096
Chicago/Turabian StyleYang, Yunxia, Song Yu, Yanglan Tan, Na Liu, and Aibo Wu . 2017. "Individual and Combined Cytotoxic Effects of Co‐Occurring Deoxynivalenol Family Mycotoxins on Human Gastric Epithelial Cells" Toxins 9, no. 3: 96. https://doi.org/10.3390/toxins9030096
APA StyleYang, Y., Yu, S., Tan, Y., Liu, N., & Wu , A. (2017). Individual and Combined Cytotoxic Effects of Co‐Occurring Deoxynivalenol Family Mycotoxins on Human Gastric Epithelial Cells. Toxins, 9(3), 96. https://doi.org/10.3390/toxins9030096







