Fungal Ribotoxins: A Review of Potential Biotechnological Applications
Abstract
:1. Introduction
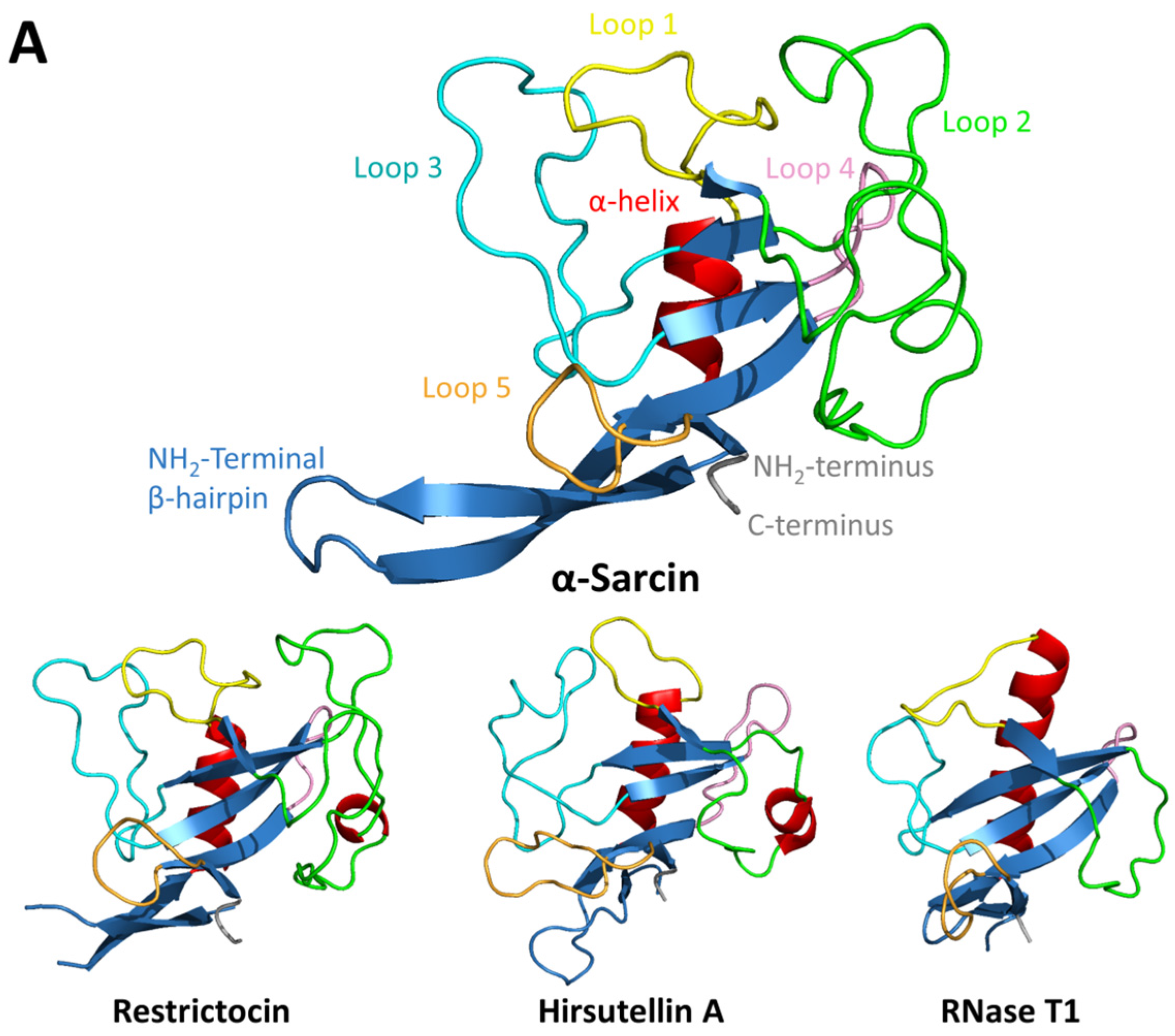
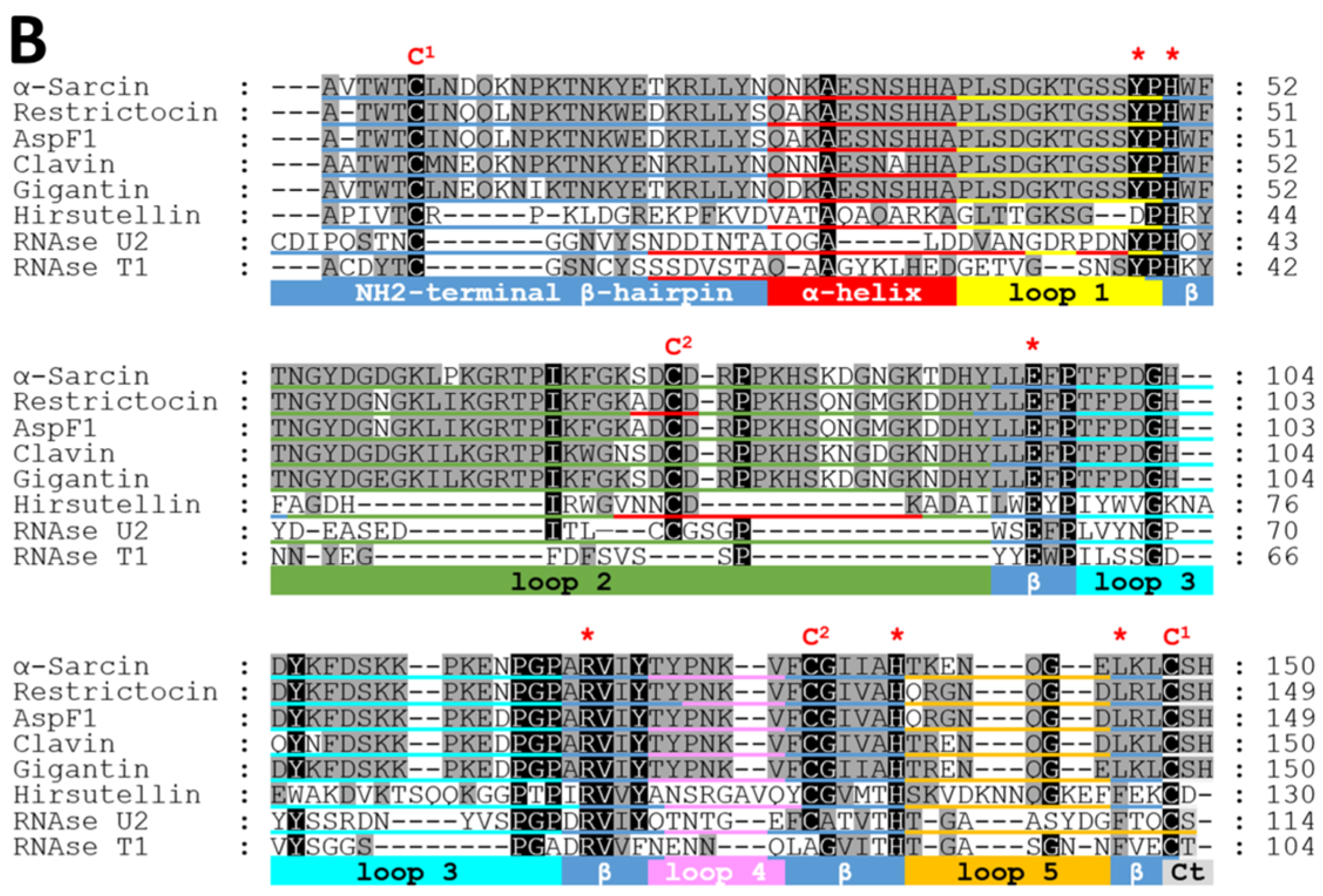
2. Fungal Ribotoxins
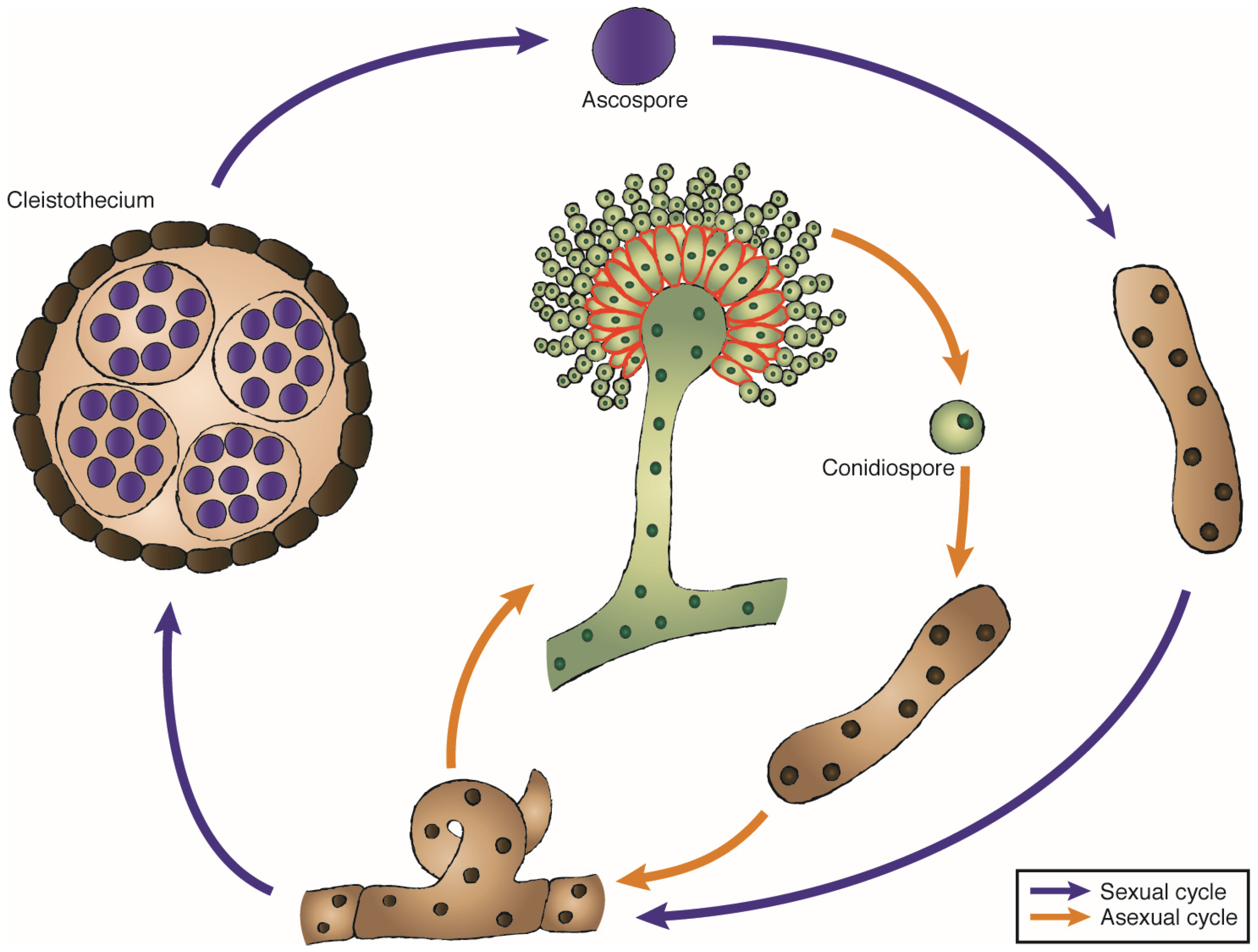
3. The Genus Aspergillus and Other Ribotoxin-Producer Fungi
4. The Potential Biotechnological Uses of Fungal Ribotoxins
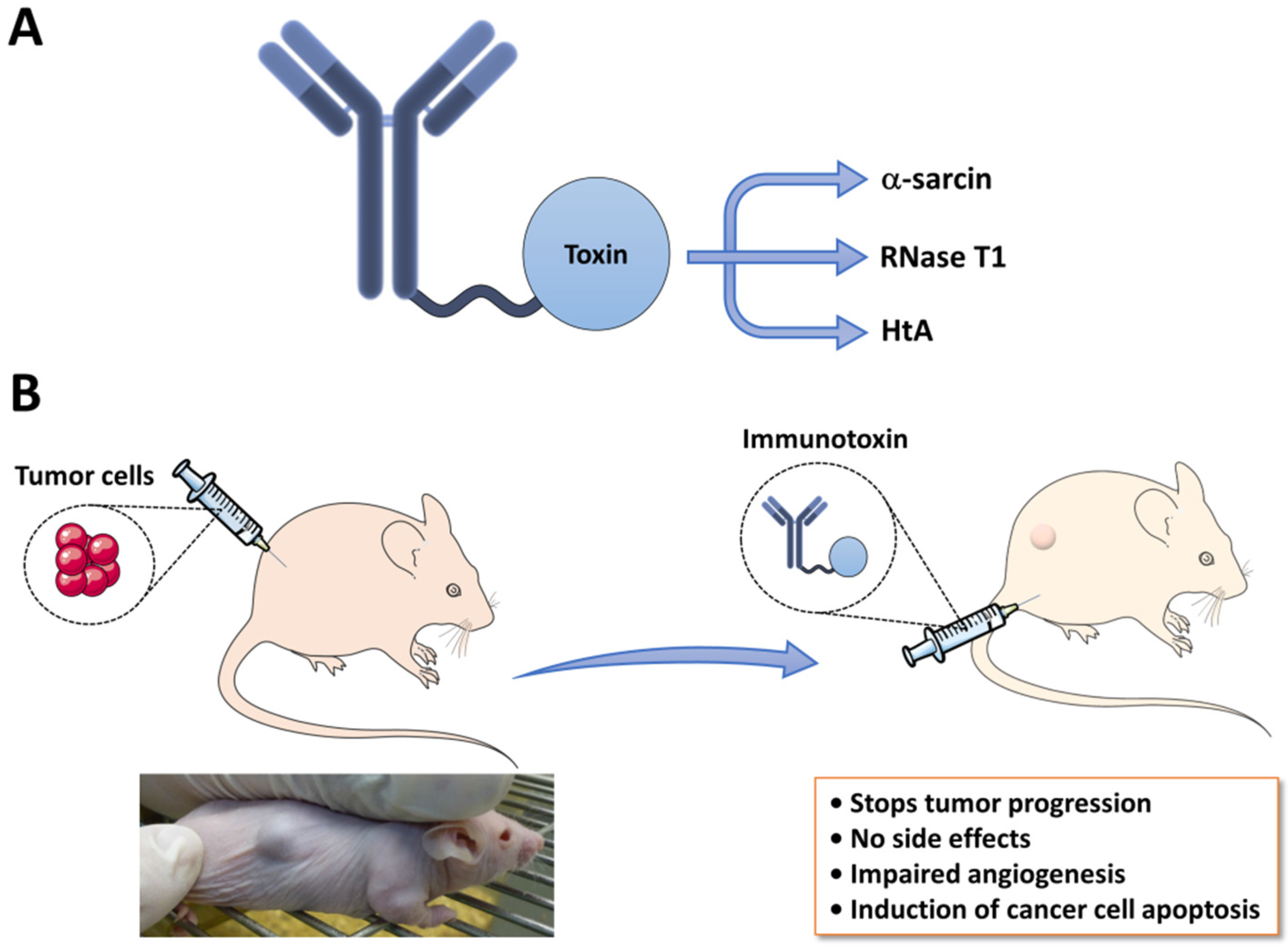
4.1. Immunotoxins
4.2. Role in Allergy and Aspergillus Infection
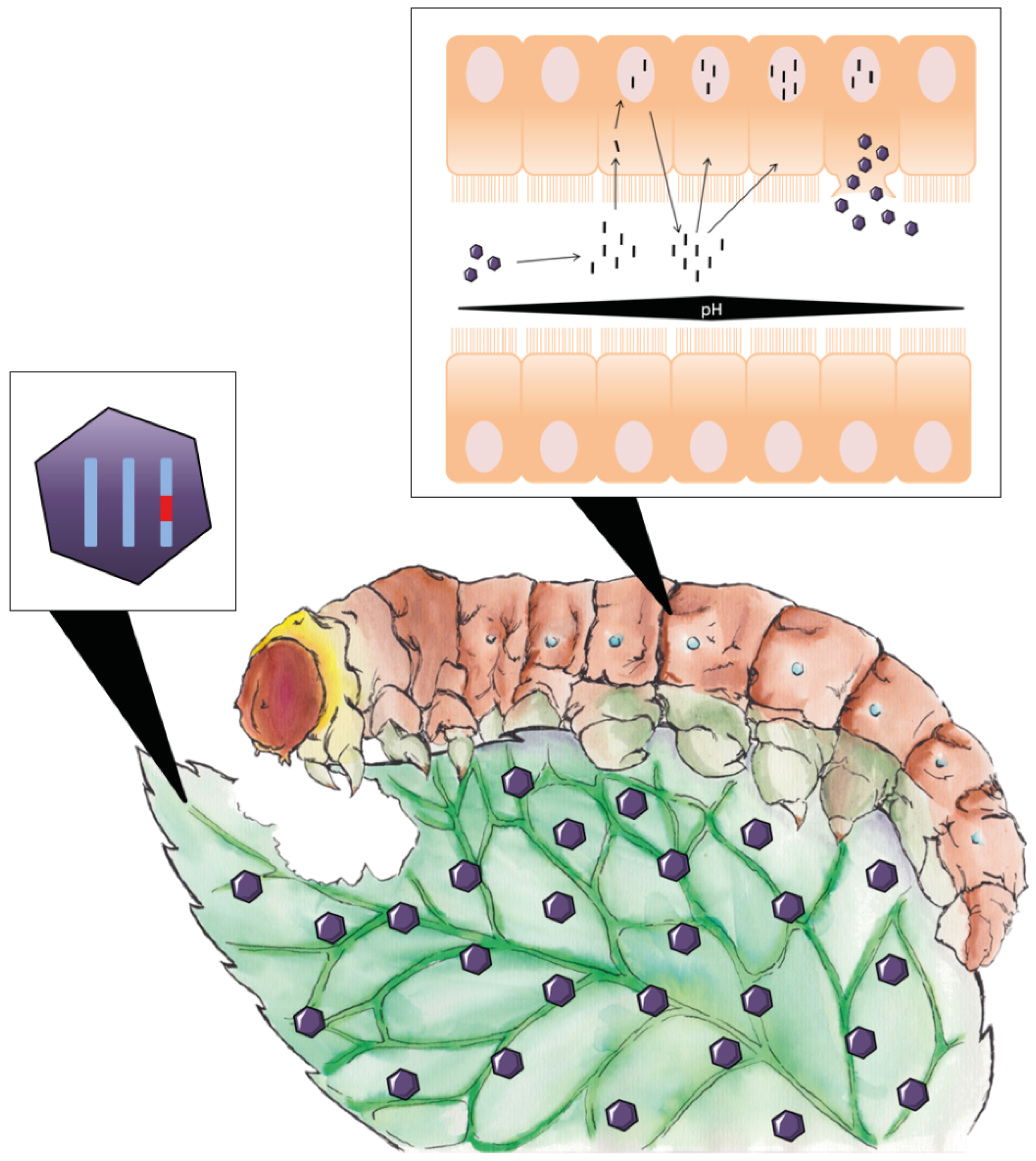
4.3. Ribotoxins as Pest Control Agents
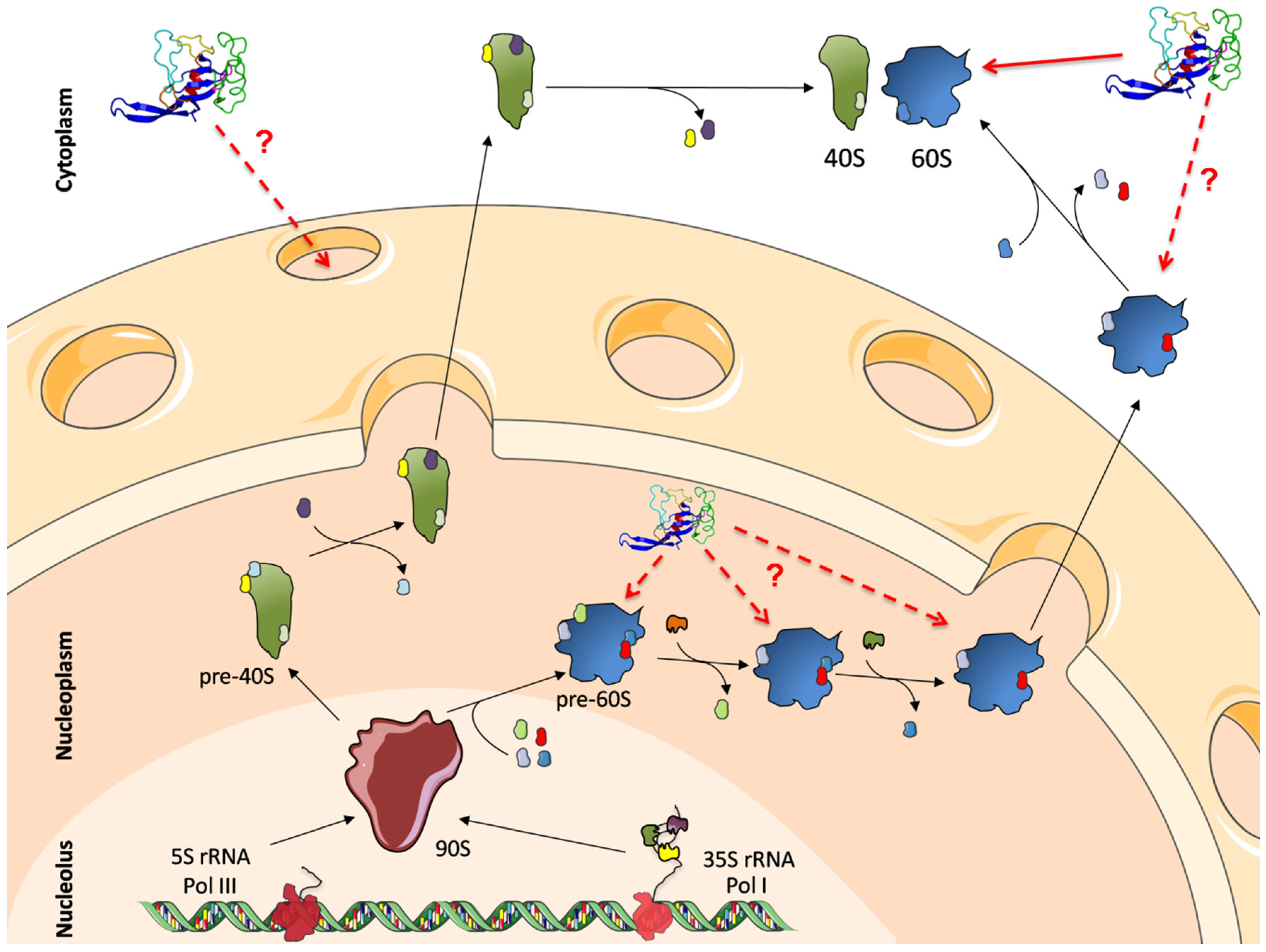
4.4. The Study of Ribosome-Related Diseases. Ribosome Biogenesis as an Additional Target of Ribotoxins
5. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berenbaum, M.R.; Eisner, T. Ecology. Bugs’ bugs. Science 2008, 322, 52–53. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ruiz, A.; Kao, R.; Davies, J.; Martínez-del-Pozo, A. Ribotoxins are a more widespread group of proteins within the filamentous fungi than previously believed. Toxicon 1999, 37, 1549–1563. [Google Scholar] [CrossRef]
- Martínez-Ruiz, A.; Martínez-del-Pozo, A.; Lacadena, J.; Oñaderra, M.; Gavilanes, J.G. Hirsutellin A displays significant homology to microbial extracellular ribonucleases. J. Invertebr. Pathol. 1999, 74, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Lacadena, J.; Álvarez-García, E.; Carreras-Sangrà, N.; Herrero-Galán, E.; Alegre-Cebollada, J.; García-Ortega, L.; Oñaderra, M.; Gavilanes, J.G.; Martínez-del-Pozo, A. Fungal ribotoxins: Molecular dissection of a family of natural killers. FEMS Microbiol. Rev. 2007, 31, 212–237. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Galán, E.; Lacadena, J.; Martínez-del-Pozo, A.; Boucias, D.G.; Olmo, N.; Oñaderra, M.; Gavilanes, J.G. The insecticidal protein hirsutellin A from the mite fungal pathogen Hirsutella thompsonii is a ribotoxin. Proteins 2008, 72, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Olombrada, M.; Medina, P.; Budia, F.; Gavilanes, J.G.; Martínez-Del-Pozo, A.; García-Ortega, L. Characterization of a new toxin from the entomopathogenic fungus Metarhizium anisopliae: The ribotoxin anisoplin. Biol. Chem. 2017, 398, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Schrot, J.; Weng, A.; Melzig, M.F. Ribosome-inactivating and related proteins. Toxins 2015, 7, 1556–1615. [Google Scholar] [CrossRef] [PubMed]
- Olombrada, M.; Rodríguez-Mateos, M.; Prieto, D.; Pla, J.; Remacha, M.; Martínez-del-Pozo, A.; Gavilanes, J.G.; Ballesta, J.P.; García-Ortega, L. The acidic ribosomal stalk proteins are not required for the highly specific inactivation exerted by α-sarcin of the eukaryotic ribosome. Biochemistry 2014, 53, 1545–1547. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, T.; Dowd, P.F.; Kenealy, W.R. The ribosome-inactivating protein restrictocin deters insect feeding on Aspergillus restrictus. Microbiology 1996, 142 Pt 6, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, T.; Dowd, P.F.; Kenealy, W.R. The effect of fungal ribosome inactivating proteins upon feeding choice in C. freemani, and indications of a mutualistic relationship with A. restrictus. Environmental mycology. Mycopathologia 2001, 152, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Olombrada, M.; Herrero-Galán, E.; Tello, D.; Oñaderra, M.; Gavilanes, J.G.; Martínez-del-Pozo, A.; García-Ortega, L. Fungal extracellular ribotoxins as insecticidal agents. Insect Biochem. Mol. Biol. 2013, 43, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Olombrada, M.; Martínez-del-Pozo, A.; Medina, P.; Budia, F.; Gavilanes, J.G.; García-Ortega, L. Fungal ribotoxins: Natural protein-based weapons against insects. Toxicon 2014, 83, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Amat, J.; Herrero-Galán, E.; Oñaderra, M.; Martínez-Del-Pozo, A.; Gavilanes, J.G.; Lacadena, J. Preparation of an engineered safer immunotoxin against colon carcinoma based on the ribotoxin hirsutellin A. FEBS J. 2015, 282, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Amat, J.; Olombrada, M.; Ruiz-de-la-Herran, J.; Pérez-Gómez, E.; Andradas, C.; Sánchez, C.; Martínez, L.; Martínez-del-Pozo, A.; Gavilanes, J.G.; Lacadena, J. Efficient in vivo antitumor effect of an immunotoxin based on ribotoxin α-sarcin in nude mice bearing human colorectal cancer xenografts. Springerplus 2015, 4, 168. [Google Scholar] [CrossRef] [PubMed]
- Narla, A.; Ebert, B.L. Ribosomopathies: Human disorders of ribosome dysfunction. Blood 2010, 115, 3196–3205. [Google Scholar] [CrossRef] [PubMed]
- Nakhoul, H.; Ke, J.; Zhou, X.; Liao, W.; Zeng, S.X.; Lu, H. Ribosomopathies: Mechanisms of disease. Clin. Med. Insights Blood Disord. 2014, 7, 7–16. [Google Scholar] [PubMed]
- Martínez-del-Pozo, A.; Gasset, M.; Oñaderra, M.; Gavilanes, J.G. Conformational study of the antitumor protein α-sarcin. Biochim. Biophys. Acta 1988, 953, 280–288. [Google Scholar] [CrossRef]
- Varga, J.; Samson, R.A. Ribotoxin genes in isolates of Aspergillus section Clavati. Antonie Van Leeuwenhoek 2008, 94, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-Z.; Boucias, D.G.; McCoy, C.W. Extraction and characterization of the insecticidal toxin hirsutellin A produced by Hirsutella thompsonii var. thompsonii. Exp. Mycol. 1995, 19, 254–262. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W.L. The PyMOL Molecular Graphics System; DeLano Scientific: San Diego, CA, USA, 2008. [Google Scholar]
- García-Ortega, L.; Álvarez-García, E.; Gavilanes, J.G.; Martínez-del-Pozo, A.; Joseph, S. Cleavage of the sarcin-ricin loop of 23S rRNA differentially affects EF-G and EF-Tu binding. Nucleic Acids Res. 2010, 38, 4108–4119. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, R.M.; Schmeing, T.M.; Kelley, A.C.; Ramakrishnan, V. The mechanism for activation of GTP hydrolysis on the ribosome. Science 2010, 330, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Lacadena, J.; Martínez-del-Pozo, A.; Lacadena, V.; Martínez-Ruiz, A.; Mancheño, J.M.; Oñaderra, M.; Gavilanes, J.G. The cytotoxin α-sarcin behaves as a cyclizing ribonuclease. FEBS Lett. 1998, 424, 46–48. [Google Scholar] [CrossRef]
- Olmo, N.; Turnay, J.; González de Buitrago, G.; López de Silanes, I.; Gavilanes, J.G.; Lizarbe, M.A. Cytotoxic mechanism of the ribotoxin α-sarcin. Induction of cell death via apoptosis. Eur. J. Biochem. 2001, 268, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Moffat, K. Insights into specificity of cleavage and mechanism of cell entry from the crystal structure of the highly specific Aspergillus ribotoxin, restrictocin. Structure 1996, 4, 837–852. [Google Scholar] [CrossRef]
- Pérez-Cañadillas, J.M.; Santoro, J.; Campos-Olivas, R.; Lacadena, J.; Martínez-del-Pozo, A.; Gavilanes, J.G.; Rico, M.; Bruix, M. The highly refined solution structure of the cytotoxic ribonuclease α-sarcin reveals the structural requirements for substrate recognition and ribonucleolytic activity. J. Mol. Biol. 2000, 299, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Viegas, A.; Herrero-Galán, E.; Oñaderra, M.; Macedo, A.L.; Bruix, M. Solution structure of hirsutellin A. New insights into the active site and interacting interfaces of ribotoxins. FEBS J. 2009, 276, 2381–2390. [Google Scholar] [CrossRef] [PubMed]
- Masip, M.; Lacadena, J.; Mancheño, J.M.; Oñaderra, M.; Martínez-Ruiz, A.; Martínez-del-Pozo, A.; Gavilanes, J.G. Arginine 121 is a crucial residue for the specific cytotoxic activity of the ribotoxin α-sarcin. Eur. J. Biochem. 2001, 268, 6190–6196. [Google Scholar] [CrossRef] [PubMed]
- Masip, M.; García-Ortega, L.; Olmo, N.; García-Mayoral, M.F.; Pérez-Cañadillas, J.M.; Bruix, M.; Oñaderra, M.; Martínez-del-Pozo, A.; Gavilanes, J.G. Leucine 145 of the ribotoxin α-sarcin plays a key role for determining the specificity of the ribosome-inactivating activity of the protein. Protein Sci. 2003, 12, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-García, E.; García-Ortega, L.; Verdun, Y.; Bruix, M.; Martínez-del-Pozo, A.; Gavilanes, J.G. Tyr-48, a conserved residue in ribotoxins, is involved in the RNA-degrading activity of α-sarcin. Biol. Chem. 2006, 387, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Galán, E.; García-Ortega, L.; Lacadena, J.; Martínez-Del-Pozo, A.; Olmo, N.; Gavilanes, J.G.; Oñaderra, M. Implication of an Asp residue in the ribonucleolytic activity of hirsutellin A reveals new electrostatic interactions at the active site of ribotoxins. Biochimie 2012, 94, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Galán, E.; García-Ortega, L.; Lacadena, J.; Martínez-Del-Pozo, A.; Olmo, N.; Gavilanes, J.G.; Oñaderra, M. A non-cytotoxic but ribonucleolytically specific ribotoxin variant: Implication of tryptophan residues in the cytotoxicity of hirsutellin A. Biol. Chem. 2012, 393, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Olombrada, M.; García-Ortega, L.; Lacadena, J.; Oñaderra, M.; Gavilanes, J.G.; Martínez-Del-Pozo, A. Involvement of loop 5 lysine residues and the N-terminal β-hairpin of the ribotoxin hirsutellin A on its insecticidal activity. Biol. Chem. 2016, 397, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Turnay, J.; Olmo, N.; Jiménez, A.; Lizarbe, M.A.; Gavilanes, J.G. Kinetic study of the cytotoxic effect of α-sarcin, a ribosome inactivating protein from Aspergillus giganteus, on tumour cell lines—Protein biosynthesis inhibition and cell binding. Mol. Cell. Biochem. 1993, 122, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Marheineke, K.; Grunewald, S.; Christie, W.; Reilander, H. Lipid composition of Spodoptera frugiperda (Sf9) and Trichoplusia ni (Tn) insect cells used for baculovirus infection. FEBS Lett. 1998, 441, 49–52. [Google Scholar] [CrossRef]
- Gasset, M.; Martínez-del-Pozo, A.; Oñaderra, M.; Gavilanes, J.G. Study of the interaction between the antitumour protein α-sarcin and phospholipid vesicles. Biochem. J. 1989, 258, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Gasset, M.; Oñaderra, M.; Thomas, P.G.; Gavilanes, J.G. Fusion of phospholipid vesicles produced by the anti-tumour protein α-sarcin. Biochem. J. 1990, 265, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Mancheño, J.M.; Gasset, M.; Albar, J.P.; Lacadena, J.; Martinez-del-Pozo, A.; Oñaderra, M.; Gavilanes, J.G. Membrane interaction of a beta-structure-forming synthetic peptide comprising the 116–139th sequence region of the cytotoxic protein α-sarcin. Biophys J 1995, 68, 2387–2395. [Google Scholar] [CrossRef]
- Mancheño, J.M.; Martínez-del-Pozo, A.; Albar, J.P.; Oñaderra, M.; Gavilanes, J.G. A peptide of nine amino acid residues from α-sarcin cytotoxin is a membrane-perturbing structure. J. Pept. Res. 1998, 51, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Rodríguez, C.; Olombrada, M.; Partida-Hanon, A.; Lacadena, J.; Oñaderra, M.; Gavilanes, J.G.; García-Ortega, L.; Martínez-del-Pozo, A. Involvement of loops 2 and 3 of α-sarcin on its ribotoxic activity. Toxicon 2015, 96, 1–9. [Google Scholar] [CrossRef] [PubMed]
- García-Ortega, L.; Masip, M.; Mancheño, J.M.; Oñaderra, M.; Lizarbe, M.A.; García-Mayoral, M.F.; Bruix, M.; Martínez-del-Pozo, A.; Gavilanes, J.G. Deletion of the NH2-terminal β-hairpin of the ribotoxin α-sarcin produces a nontoxic but active ribonuclease. J Biol. Chem. 2002, 277, 18632–18639. [Google Scholar] [CrossRef] [PubMed]
- García-Mayoral, F.; García-Ortega, L.; Álvarez-García, E.; Bruix, M.; Gavilanes, J.G.; Martínez-del-Pozo, A. Modeling the highly specific ribotoxin recognition of ribosomes. FEBS Lett. 2005, 579, 6859–6864. [Google Scholar] [CrossRef] [PubMed]
- Korennykh, A.V.; Piccirilli, J.A.; Correll, C.C. The electrostatic character of the ribosomal surface enables extraordinarily rapid target location by ribotoxins. Nat. Struct. Mol. Biol. 2006, 13, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-García, E.; Martínez-del-Pozo, A.; Gavilanes, J.G. Role of the basic character of α-sarcin’s NH2-terminal β-hairpin in ribosome recognition and phospholipid interaction. Arch. Biochem. Biophys. 2009, 481, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gerczei, T.; Glover, L.; Correll, C.C. Crystal structures of restrictocin-inhibitor complexes with implications for RNA recognition and base flipping. Nat. Struct. Biol. 2001, 8, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Tumer, N.E.; Li, X.P. Interaction of ricin and Shiga toxins with ribosomes. Curr. Top. Microbiol. Immunol. 2012, 357, 1–18. [Google Scholar] [PubMed]
- Ayub, M.J.; Smulski, C.R.; Ma, K.W.; Levin, M.J.; Shaw, P.C.; Wong, K.B. The C-terminal end of P proteins mediates ribosome inactivation by trichosanthin but does not affect the pokeweed antiviral protein activity. Biochem. Biophys. Res. Commun. 2008, 369, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Alford, S.C.; Pearson, J.D.; Carette, A.; Ingham, R.J.; Howard, P.L. α-Sarcin catalytic activity is not required for cytotoxicity. BMC Biochem. 2009, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-García, E.; Diago-Navarro, E.; Herrero-Galán, E.; García-Ortega, L.; López-Villarejo, J.; Olmo, N.; Díaz-Orejas, R.; Gavilanes, J.G.; Martinez-Del-Pozo, A. The ribonucleolytic activity of the ribotoxin α-sarcin is not essential for in vitro protein biosynthesis inhibition. Biochim. Biophys. Acta 2011, 1814, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.H.; Goerner, G.L. α-Sarcin, a new antitumor agent I. Isolation, purification, chemical composition, and the identity of a new amino acid. Appl. Microbiol. 1965, 13, 314–321. [Google Scholar] [PubMed]
- Machida, M.; Asai, K.; Sano, M.; Tanaka, T.; Kumagai, T.; Terai, G.; Kusumoto, K.; Arima, T.; Akita, O.; Kashiwagi, Y.; et al. Genome sequencing and analysis of Aspergillus oryzae. Nature 2005, 438, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Casselton, L.; Zolan, M. The art and design of genetic screens: Filamentous fungi. Nat. Rev. Genet. 2002, 3, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, T.T.; Kenealy, W.R. Production and localization of restrictocin in Aspergillus restrictus. J. Gen. Microbiol. 1992, 138, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Kenealy, W.R. Regulation of restrictocin production in Aspergillus restrictus. J. Gen. Microbiol. 1992, 138, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Boucias, D.G.; Pendland, J.C.; Liu, W.Z.; Maruniak, J. The mode of action of hirsutellin A on eukaryotic cells. J. Invertebr. Pathol. 1996, 67, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.Y.; Zhou, X.; Kaya, H.K. Virulence and site of infection of the fungus, Hirsutella thompsonii, to the honey bee ectoparasitic mite, Varroa destructor. J. Invertebr. Pathol. 2002, 81, 185–195. [Google Scholar] [CrossRef]
- Vey, A.; Quiot, J.M.; Mazet, I.; McCoy, C.W. Toxicity and pathology of crude broth filtarte produced by Hirsutella thompsonii var. thompsonii in shake culture. J. Invertebr. Pathol. 1993, 61, 131–137. [Google Scholar] [CrossRef] [PubMed]
- de Bekker, C.; Smith, P.B.; Patterson, A.D.; Hughes, D.P. Metabolomics reveals the heterogeneous secretome of two entomopathogenic fungi to ex vivo cultured insect tissues. PLoS ONE 2013, 8, e70609. [Google Scholar] [CrossRef] [PubMed]
- Mazet, I.; Vey, A. Hirsutellin A, a toxic protein produced in vitro by Hirsutella thompsonii. Microbiology 1995, 141, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Maimala, S.; Tartar, A.; Boucias, D.; Chandrapatya, A. Detection of the toxin Hirsutellin A from Hirsutella thompsonii. J. Invertebr. Pathol. 2002, 80, 112–126. [Google Scholar] [CrossRef]
- Herrero-Galán, E.; García-Ortega, L.; Olombrada, M.; Lacadena, J.; Martínez-del-Pozo, A.; Gavilanes, J.G.; Oñaderra, M. Hirsutellin A: A Paradigmatic example of the insecticidal function of fungal ribotoxins. Insects 2013, 4, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.P.; Bodley, J.W. The ribosomes of Aspergillus giganteus are sensitive to the cytotoxic action of α-sarcin. FEBS Lett. 1988, 229, 388–390. [Google Scholar] [CrossRef]
- Endo, Y.; Tsurugi, K.; Oka, T.; Natori, Y. The biosynthesis of a cytotoxic protein, α-sarcin, in a mold of Aspergillus giganteus. I. Synthesis of pre- and pro-α-sarcin in vitro. Tokushima J. Exp. Med. 1993, 40, 1–6. [Google Scholar] [PubMed]
- Endo, Y.; Oka, T.; Yokota, S.; Tsurugi, K.; Natori, Y. The biosynthesis of a cytotoxic protein, α-sarcin, in a mold of Aspergillus giganteus. II. Maturation of precursor form of α-sarcin in vivo. Tokushima J. Exp. Med. 1993, 40, 7–12. [Google Scholar] [PubMed]
- Martínez-Ruiz, A.; Martínez-del-Pozo, A.; Lacadena, J.; Mancheño, J.M.; Oñaderra, M.; López-Otín, C.; Gavilanes, J.G. Secretion of recombinant pro- and mature fungal α-sarcin ribotoxin by the methylotrophic yeast Pichia pastoris: The Lys-Arg motif is required for maturation. Protein Expr. Purif. 1998, 12, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, T.; Kenealy, W.R. Effects of leader sequences upon the heterologous expression of restrictocin in Aspergillus nidulans and Aspergillus niger. Can. J. Microbiol. 1995, 41, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Lamy, B.; Davies, J. Isolation and nucleotide sequence of the Aspergillus restrictus gene coding for the ribonucleolytic toxin restrictocin and its expression in Aspergillus nidulans: The leader sequence protects producing strains from suicide. Nucleic Acids Res. 1991, 19, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Exploiting the intron-splicing mechanism of insect cells to produce viral vectors harboring toxic genes for suicide gene therapy. Mol. Ther. Nucleic Acids 2012, 1, e57. [Google Scholar] [CrossRef] [PubMed]
- Roga, V.; Hedeman, L.P.; Olson, B.H. Evaluation of mitogillin (NSC-69529) in the treatment of naturally occurring canine neoplasms. Cancer Chemother. Rep. 1971, 55, 101–103. [Google Scholar] [PubMed]
- Reiter, Y.; Pastan, I. Recombinant Fv immunotoxins and Fv fragments as novel agents for cancer therapy and diagnosis. Trends Biotechnol. 1998, 16, 513–520. [Google Scholar] [CrossRef]
- Kreitman, R.J. Toxin-labeled monoclonal antibodies. Curr. Pharm. Biotechnol. 2001, 2, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, R.J. Immunotoxins for targeted cancer therapy. AAPS J. 2006, 8, E532–E551. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Sangrà, N.; Tomé-Amat, J.; García-Ortega, L.; Batt, C.A.; Oñaderra, M.; Martínez-del-Pozo, A.; Gavilanes, J.G.; Lacadena, J. Production and characterization of a colon cancer-specific immunotoxin based on the fungal ribotoxin α-sarcin. Protein Eng. Des. Sel. 2012, 25, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Amat, J.; Menéndez-Méndez, A.; García-Ortega, L.; Batt, C.A.; Oñaderra, M.; Martínez-del-Pozo, A.; Gavilanes, J.G.; Lacadena, J. Production and characterization of scFvA33T1, an immunoRNase targeting colon cancer cells. FEBS J. 2012, 279, 3022–3032. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Amat, J.; Ruiz-de-la-Herran, J.; Martínez-del-Pozo, A.; Gavilanes, J.G.; Lacadena, J. α-Sarcin and RNase T1 based immunoconjugates: The role of intracellular trafficking in cytotoxic efficiency. FEBS J. 2015, 282, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.D.; Hearn, A.R.; Holgate, R.G.; Kozub, D.; Fogg, M.H.; Carr, F.J.; Baker, M.P.; Lacadena, J.; Gehlsen, K.R. A deimmunised form of the ribotoxin, α-sarcin, lacking CD4+ T cell epitopes and its use as an immunotoxin warhead. Protein Eng. Des. Sel. 2016, 29, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Denton, P.W.; Long, J.M.; Wietgrefe, S.W.; Sykes, C.; Spagnuolo, R.A.; Snyder, O.D.; Perkey, K.; Archin, N.M.; Choudhary, S.K.; Yang, K.; et al. Targeted cytotoxic therapy kills persisting HIV infected cells during ART. PLoS Pathog. 2014, 10, e1003872. [Google Scholar] [CrossRef] [PubMed]
- Rask, C.; Holmgren, J.; Fredriksson, M.; Lindblad, M.; Nordstrom, I.; Sun, J.B.; Czerkinsky, C. Prolonged oral treatment with low doses of allergen conjugated to cholera toxin B subunit suppresses immunoglobulin E antibody responses in sensitized mice. Clin. Exp. Allergy 2000, 30, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Wicklein, D.; Stocker, M.; Klockenbring, T.; Huhn, M.; Wodrich, M.; Haas, H.; Becker, W.M.; Barth, S.; Petersen, A. In contrast to specific B cells, human basophils are unaffected by the toxic activity of an allergen toxin due to lack of internalization of immunoglobulin E-bound allergen. Clin. Exp. Allergy 2006, 36, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Slater, J.E.; Boltansky, H.; Kaliner, M. IgE immunotoxins. Effect of an IgE-ricin A chain conjugate on rat skin histamine content. J. Immunol. 1988, 140, 807–811. [Google Scholar] [PubMed]
- Fishman, A.; Prus, D.; Belostotsky, R.; Lorberboum-Galski, H. Targeted Fc2’-3-PE40 chimeric protein abolishes passive cutaneous anaphylaxis in mice. Clin. Exp. Immunol. 2000, 119, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.N.; Broge, L.; Jacobi, H. Allergy immunotherapy: The future of allergy treatment. Drug Discov. Today 2016, 21, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Asturias, J.A.; Ibarrola, I.; Arilla, M.C.; Vidal, C.; Ferrer, A.; Gamboa, P.M.; Vinuela, J.E.; Sanz, M.L.; Andreu, C.; Martinez, A. Engineering of major house dust mite allergens Der p 1 and Der p 2 for allergen-specific immunotherapy. Clin. Exp. Allergy 2009, 39, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.F.; Postigo, I.; Gutierrez-Rodriguez, A.; Sunen, E.; Guisantes, J.A.; Fernandez, J.; Tomaz, C.T.; Martinez, J. Alt a 15 is a new cross-reactive minor allergen of Alternaria alternata. Immunobiology 2016, 221, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.R. House Dust Mite Allergens: New Discoveries and Relevance to the Allergic Patient. Curr. Allergy Asthma Rep. 2016, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Van Rijt, L.; von Richthofen, H.; van Ree, R. Type 2 innate lymphoid cells: At the cross-roads in allergic asthma. Semin. Immunopathol. 2016, 38, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.S.; Flach, M.; Möhle, L.; Rogell, L.; Hoyler, T.; Ebert, K.; Fabiunke, C.; Pfeifer, D.; Sexl, V.; Fonseca-Pereira, D.; et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 2014, 157, 340–356. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Arruda, L.K.; Mann, B.J.; Chapman, M.D. Selective expression of a major allergen and cytotoxin, Asp f I, in Aspergillus fumigatus. Implications for the immunopathogenesis of Aspergillus-related diseases. J. Immunol. 1992, 149, 3354–3359. [Google Scholar] [PubMed]
- Kurup, V.P.; Banerjee, B.; Murali, P.S.; Greenberger, P.A.; Krishnan, M.; Hari, V.; Fink, J.N. Immunodominant peptide epitopes of allergen, Asp f 1 from the fungus Aspergillus fumigatus. Peptides 1998, 19, 1469–1477. [Google Scholar] [CrossRef]
- García-Ortega, L.; Lacadena, J.; Villalba, M.; Rodríguez, R.; Crespo, J.F.; Rodríguez, J.; Pascual, C.; Olmo, N.; Oñaderra, M.; Martínez-del-Pozo, A.; et al. Production and characterization of a noncytotoxic deletion variant of the Aspergillus fumigatus allergen Aspf1 displaying reduced IgE binding. FEBS J. 2005, 272, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-García, E.; Alegre-Cebollada, J.; Batanero, E.; Monedero, V.; Pérez-Martínez, G.; García-Fernández, R.; Gavilanes, J.G.; Martínez-del-Pozo, A. Lactococcus lactis as a vehicle for the heterologous expression of fungal ribotoxin variants with reduced IgE-binding affinity. J. Biotechnol. 2008, 134, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-García, E.; Batanero, E.; García-Fernández, R.; Villalba, M.; Gavilanes, J.G.; Martínez-del-Pozo, A. A deletion variant of the Aspergillus fumigatus ribotoxin Asp f I induces an attenuated airway inflammatory response in a mouse model of sensitization. J. Investig. Allergol. Clin. Immunol. 2010, 20, 69–75. [Google Scholar] [PubMed]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Kohl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Carlini, C.R.; Grossi-de-Sa, M.F. Plant toxic proteins with insecticidal properties. A review on their potentialities as bioinsecticides. Toxicon 2002, 40, 1515–1539. [Google Scholar] [CrossRef]
- Coca, M.; Bortolotti, C.; Rufat, M.; Penas, G.; Eritja, R.; Tharreau, D.; Martínez del Pozo, A.; Messeguer, J.; San Segundo, B. Transgenic Rice Plants Expressing the Antifungal AFP Protein from Aspergillus giganteus Show Enhanced Resistance to the Rice Blast Fungus Magnaporthe grisea. Plant Mol. Biol. 2004, 54, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.E.; Davidson, G.; Clark, S.J.; Ball, B.V.; Pell, J.K.; Chandler, D.; Sunderland, K.D. Laboratory bioassays to assess the pathogenicity of mitosporic fungi to Varroa destructor (Acari: Mesostigmata), an ectoparasitic mite of the honeybee, Apis mellifera. Biol. Control 2002, 24, 266–276. [Google Scholar] [CrossRef]
- Whetstone, P.A.; Hammock, B.D. Delivery methods for peptide and protein toxins in insect control. Toxicon 2007, 49, 576–596. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Lee, J.B.; Kim, B.S.; Nam, Y.H.; Shin, K.S.; Kim, J.W.; Kim, J.E.; Kwon, G.S. A technique for the prevention of greenhouse whitefly (Trialeurodes vaporariorum) using the entomopathogenic fungus Beauveria bassiana M130. J. Microbiol. Biotechn. 2014, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kanga, L.H.; James, R.R.; Boucias, D.G. Hirsutella thompsonii and Metarhizium anisopliae as potential microbial control agents of Varroa destructor, a honey bee parasite. J. Invertebr. Pathol. 2002, 81, 175–184. [Google Scholar] [CrossRef]
- Rossi-Zalaf, L.S.; Alves, S.B. Susceptibility of Brevipalpus phoenicis to entomopathogenic fungi. Exp. Appl. Acarol. 2006, 40, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Mccoy, C.W.; Couch, T.L. Microbial control of the citrus rust mite with the Mycoacaricide, Mycar®. Fla. Entomol. 1982, 65, 116–126. [Google Scholar] [CrossRef]
- Sreerama Kumar, P.; Singh, L. Enabling mycelial application of Hirsutella thompsonii for managing the coconut mite. Exp. Appl. Acarol. 2008, 46, 169–182. [Google Scholar] [CrossRef] [PubMed]
- St Leger, R.J.; Wang, C.S. Genetic engineering of fungal biocontrol agents to achieve greater efficacy against insect pests. Appl. Microbiol. Biotechnol. 2010, 85, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Cory, J.S.; Hirst, M.L.; Williams, T.; Hails, R.S.; Goulson, D.; Green, B.M.; Carty, T.M.; Possee, R.D.; Cayley, P.J.; Bishop, D.H.L. Field trial of a genetically improved baculovirus insecticide. Nature 1994, 370, 138–140. [Google Scholar] [CrossRef]
- Inceoglu, A.B.; Kamita, S.G.; Hinton, A.C.; Huang, Q.H.; Severson, T.F.; Kang, K.D.; Hammock, B.D. Recombinant baculoviruses for insect control. Pest Manag. Sci. 2001, 57, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Gramkow, A.W.; Perecmanis, S.; Sousa, R.L.; Noronha, E.F.; Felix, C.R.; Nagata, T.; Ribeiro, B.M. Insecticidal activity of two proteases against Spodoptera frugiperda larvae infected with recombinant baculoviruses. Virol. J. 2010, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.J.; Choi, J.Y.; Wang, Y.; Tao, X.Y.; Liu, Q.; Roh, J.Y.; Kim, J.S.; Kim, W.J.; Woo, S.D.; Jin, B.R.; Je, Y.H. NeuroBactrus, a novel, highly effective, and environmentally friendly recombinant baculovirus insecticide. Appl. Environ. Microbiol. 2013, 79, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Ruggero, D.; Shimamura, A. Marrow failure: A window into ribosome biology. Blood 2014, 124, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.R. Twenty years of ribosome assembly and ribosomopathies. RNA 2015, 21, 758–759. [Google Scholar] [CrossRef] [PubMed]
- McCann, K.L.; Baserga, S.J. Genetics. Mysterious ribosomopathies. Science 2013, 341, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Menne, T.F.; Goyenechea, B.; Sanchez-Puig, N.; Wong, C.C.; Tonkin, L.M.; Ancliff, P.J.; Brost, R.L.; Costanzo, M.; Boone, C.; Warren, A.J. The Shwachman-Bodian-Diamond syndrome protein mediates translational activation of ribosomes in yeast. Nat. Genet. 2007, 39, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Woolford, J.L., Jr.; Baserga, S.J. Ribosome biogenesis in the yeast Saccharomyces cerevisiae. Genetics 2013, 195, 643–681. [Google Scholar] [CrossRef] [PubMed]
- Freed, E.F.; Bleichert, F.; Dutca, L.M.; Baserga, S.J. When ribosomes go bad: Diseases of ribosome biogenesis. Mol. Biosyst. 2010, 6, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Draptchinskaia, N.; Gustavsson, P.; Andersson, B.; Pettersson, M.; Willig, T.N.; Dianzani, I.; Ball, S.; Tchernia, G.; Klar, J.; Matsson, H.; et al. The gene encoding ribosomal protein S19 is mutated in Diamond-Blackfan anaemia. Nat. Genet. 1999, 21, 169–175. [Google Scholar] [PubMed]
- Cmejla, R.; Cmejlova, J.; Handrkova, H.; Petrak, J.; Petrtylova, K.; Mihal, V.; Stary, J.; Cerna, Z.; Jabali, Y.; Pospisilova, D. Identification of mutations in the ribosomal protein L5 (RPL5) and ribosomal protein L11 (RPL11) genes in Czech patients with Diamond-Blackfan anemia. Hum. Mutat. 2009, 30, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.E.; Nater, M.; Caywood, E.; McDevitt, M.A.; Kowalski, J.; Takemoto, C.M.; Talbot, C.C., Jr.; Meltzer, P.; Esposito, D.; Beggs, A.H.; et al. Abnormalities of the large ribosomal subunit protein, Rpl35a, in Diamond-Blackfan anemia. Blood 2008, 112, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Gazda, H.T.; Sieff, C.A. Recent insights into the pathogenesis of Diamond-Blackfan anaemia. Br. J. Haematol. 2006, 135, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Gazda, H.T.; Sheen, M.R.; Vlachos, A.; Choesmel, V.; O’Donohue, M.F.; Schneider, H.; Darras, N.; Hasman, C.; Sieff, C.A.; Newburger, P.E.; et al. Ribosomal protein L5 and L11 mutations are associated with cleft palate and abnormal thumbs in Diamond-Blackfan anemia patients. Am. J. Hum. Genet. 2008, 83, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Horos, R.; von Lindern, M. Molecular mechanisms of pathology and treatment in Diamond Blackfan Anaemia. Br. J. Haematol. 2012, 159, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Lipton, J.M.; Ellis, S.R. Diamond-Blackfan anemia: Diagnosis, treatment, and molecular pathogenesis. Hematol. Oncol. Clin. N. Am. 2009, 23, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Ebert, B.L.; Pretz, J.; Bosco, J.; Chang, C.Y.; Tamayo, P.; Galili, N.; Raza, A.; Root, D.E.; Attar, E.; Ellis, S.R.; Golub, T.R. Identification of RPS14 as a 5q- syndrome gene by RNA interference screen. Nature 2008, 451, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Padron, E.; Komrokji, R.; List, A.F. The 5q- syndrome: Biology and treatment. Curr. Treat. Options Oncol. 2011, 12, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Boocock, G.R.; Morrison, J.A.; Popovic, M.; Richards, N.; Ellis, L.; Durie, P.R.; Rommens, J.M. Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nat. Genet. 2003, 33, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Myers, K.C.; Davies, S.M.; Shimamura, A. Clinical and molecular pathophysiology of Shwachman-Diamond syndrome: An update. Hematol. Oncol. Clin. N. Am. 2013, 27, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Rujkijyanont, P.; Adams, S.L.; Beyene, J.; Dror, Y. Bone marrow cells from patients with Shwachman-Diamond syndrome abnormally express genes involved in ribosome biogenesis and RNA processing. Br. J. Haematol. 2009, 145, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Heiss, N.S.; Knight, S.W.; Vulliamy, T.J.; Klauck, S.M.; Wiemann, S.; Mason, P.J.; Poustka, A.; Dokal, I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat. Genet. 1998, 19, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.J.; Bessler, M. The genetics of dyskeratosis congenita. Cancer Genet. 2011, 204, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Walne, A.J.; Dokal, I. Advances in the understanding of dyskeratosis congenita. Br. J. Haematol. 2009, 145, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.N.; Li, Y. RNase MRP RNA and human genetic diseases. Cell Res. 2007, 17, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ridanpaa, M.; van Eenennaam, H.; Pelin, K.; Chadwick, R.; Johnson, C.; Yuan, B.; vanVenrooij, W.; Pruijn, G.; Salmela, R.; Rockas, S.; et al. Mutations in the RNA component of RNase MRP cause a pleiotropic human disease, cartilage-hair hypoplasia. Cell 2001, 104, 195–203. [Google Scholar] [CrossRef]
- Gonzales, B.; Henning, D.; So, R.B.; Dixon, J.; Dixon, M.J.; Valdez, B.C. The Treacher Collins syndrome (TCOF1) gene product is involved in pre-rRNA methylation. Hum. Mol. Genet. 2005, 14, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Schlump, J.U.; Stein, A.; Hehr, U.; Karen, T.; Moller-Hartmann, C.; Elcioglu, N.H.; Bogdanova, N.; Woike, H.F.; Lohmann, D.R.; Felderhoff-Mueser, U.; et al. Treacher Collins syndrome: Clinical implications for the paediatrician--a new mutation in a severely affected newborn and comparison with three further patients with the same mutation, and review of the literature. Eur. J. Pediatr. 2012, 171, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- The Treacher Collins Syndrome Collaborative Group. Positional cloning of a gene involved in the pathogenesis of Treacher Collins syndrome. Nat. Genet. 1996, 12, 130–136. [Google Scholar]
- Armistead, J.; Khatkar, S.; Meyer, B.; Mark, B.L.; Patel, N.; Coghlan, G.; Lamont, R.E.; Liu, S.; Wiechert, J.; Cattini, P.A.; et al. Mutation of a gene essential for ribosome biogenesis, EMG1, causes Bowen-Conradi syndrome. Am. J. Hum. Genet. 2009, 84, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Wurm, J.P.; Kotter, P.; Leisegang, M.S.; Schilling, V.; Buchhaupt, M.; Held, M.; Bahr, U.; Karas, M.; Heckel, A.; et al. The Bowen-Conradi syndrome protein Nep1 (Emg1) has a dual role in eukaryotic ribosome biogenesis, as an essential assembly factor and in the methylation of Ψ1191 in yeast 18S rRNA. Nucleic Acids Res. 2011, 39, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Wurm, J.P.; Meyer, B.; Bahr, U.; Held, M.; Frolow, O.; Kotter, P.; Engels, J.W.; Heckel, A.; Karas, M.; Entian, K.D.; et al. The ribosome assembly factor Nep1 responsible for Bowen-Conradi syndrome is a pseudouridine-N1-specific methyltransferase. Nucleic Acids Res. 2010, 38, 2387–2398. [Google Scholar] [CrossRef] [PubMed]
- Chagnon, P.; Michaud, J.; Mitchell, G.; Mercier, J.; Marion, J.F.; Drouin, E.; Rasquin-Weber, A.; Hudson, T.J.; Richter, A. A missense mutation (R565W) in cirhin (FLJ14728) in North American Indian childhood cirrhosis. Am. J. Hum. Genet. 2002, 71, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Freed, E.F.; Prieto, J.L.; McCann, K.L.; McStay, B.; Baserga, S.J. NOL11, implicated in the pathogenesis of North American Indian childhood cirrhosis, is required for pre-rRNA transcription and processing. PLoS Genet. 2012, 8, e1002892. [Google Scholar] [CrossRef] [PubMed]
- Lafontaine, D.L.J. Noncoding RNAs in eukaryotic ribosome biogenesis and function. Nat. Struct. Mol. Biol. 2015, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, S.; Occhipinti, L.; Veisu, M.; Panse, V.G. Yvh1 is required for a late maturation step in the 60S biogenesis pathway. J. Cell Biol. 2009, 186, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.Y.; Li, Z.H.; Wang, F.; Marcotte, E.M.; Johnson, A.W. Ribosome stalk assembly requires the dual-specificity phosphatase Yvh1 for the exchange of Mrt4 with P0. J. Cell Biol. 2009, 186, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Panse, V.G.; Johnson, A.W. Maturation of eukaryotic ribosomes: Acquisition of functionality. Trends Biochem. Sci. 2010, 35, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Gerhardy, S.; Menet, A.M.; Pena, C.; Petkowski, J.J.; Panse, V.G. Assembly and nuclear export of pre-ribosomal particles in budding yeast. Chromosoma 2014, 123, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Brina, D.; Miluzio, A.; Ricciardi, S.; Biffo, S. eIF6 anti-association activity is required for ribosome biogenesis, translational control and tumor progression. Biochim. Biophys. Acta 2015, 1849, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Weis, F.; Giudice, E.; Churcher, M.; Jin, L.; Hilcenko, C.; Wong, C.C.; Traynor, D.; Kay, R.R.; Warren, A.J. Mechanism of eIF6 release from the nascent 60S ribosomal subunit. Nat. Struct. Mol. Biol. 2015, 22, 914–919. [Google Scholar] [CrossRef] [PubMed]

| Gene Defect | Impaired Function | Disease | Clinical Features | Treatment | References |
|---|---|---|---|---|---|
| RPS19, RPS26, RPL5, RPL11 and other RPs | Different steps of pre-rRNA processing | Diamond Blackfan anemia (DBA) | Anemia, bone marrow failure, growth retardation, congenital abnormalities (craniofacial, thumb), cardiac defects, cancer predisposition. | Corticosteroids, blood transfusions, hematopoietic stem cell transplantation | [115,116,117,118,119,120,121] |
| RPS14 | 18S pre-rRNA processing | 5q-syndrome | Severe macrocytic anemia, cancer predisposition | Lenalidomide | [122,123] |
| SBDS | Maturation and export of the 60S ribosomal subunit | Shwachman-Diamond syndrome (SDS) | Exocrine pancreas insufficiency, hematologic defects, skeletal abnormalities, cancer predisposition | Pancreatic enzyme supplementation, hematopoietic stem cell transplantation | [124,125,126] |
| DKC1 | Telomerase deficiency, disease aggravated by box H/ACA snoRNP pseudouridylation defects, involved in pre-rRNA modification. | X-linked dyskeratosis congenita (DC) | Mucocutaneous abnormalities, pulmonary fibrosis, bone marrow failure, cancer predisposition | Oxymetolone, Hematopoietic stem cell transplantation | [127,128,129] |
| RMRP | Maturation of 5.8S rRNA of 60S ribosomal subunit; degradation of cell-cycle regulated RNAs; mitochondrial DNA replication | Cartilage-hair hypoplasia (CHH) | Short stature, hair hypoplasia, bone deformities, cancer predisposition | Symptomatic | [130,131] |
| TCOF1 | rDNA transcription and methylation of 18S rRNA | Treacher-Collins syndrome (TCS) | Craniofacial abnormalities | Symptomatic | [132,133,134] |
| EMG1 | Maturation of 40S ribosomal subunit | Bowen-Conradi syndrome | Severe growth retardation | None | [135,136,137] |
| hUTP4/Cirhin | Maturation of the 18S pre-rRNA | North American Indian childhood cirrhosis | Biliary cirrhosis, lethal by adolescence without liver transplant | Liver transplantation | [114,138,139] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olombrada, M.; Lázaro-Gorines, R.; López-Rodríguez, J.C.; Martínez-del-Pozo, Á.; Oñaderra, M.; Maestro-López, M.; Lacadena, J.; Gavilanes, J.G.; García-Ortega, L. Fungal Ribotoxins: A Review of Potential Biotechnological Applications. Toxins 2017, 9, 71. https://doi.org/10.3390/toxins9020071
Olombrada M, Lázaro-Gorines R, López-Rodríguez JC, Martínez-del-Pozo Á, Oñaderra M, Maestro-López M, Lacadena J, Gavilanes JG, García-Ortega L. Fungal Ribotoxins: A Review of Potential Biotechnological Applications. Toxins. 2017; 9(2):71. https://doi.org/10.3390/toxins9020071
Chicago/Turabian StyleOlombrada, Miriam, Rodrigo Lázaro-Gorines, Juan C. López-Rodríguez, Álvaro Martínez-del-Pozo, Mercedes Oñaderra, Moisés Maestro-López, Javier Lacadena, José G. Gavilanes, and Lucía García-Ortega. 2017. "Fungal Ribotoxins: A Review of Potential Biotechnological Applications" Toxins 9, no. 2: 71. https://doi.org/10.3390/toxins9020071
APA StyleOlombrada, M., Lázaro-Gorines, R., López-Rodríguez, J. C., Martínez-del-Pozo, Á., Oñaderra, M., Maestro-López, M., Lacadena, J., Gavilanes, J. G., & García-Ortega, L. (2017). Fungal Ribotoxins: A Review of Potential Biotechnological Applications. Toxins, 9(2), 71. https://doi.org/10.3390/toxins9020071






