The Influence of Resiniferatoxin (RTX) and Tetrodotoxin (TTX) on the Distribution, Relative Frequency, and Chemical Coding of Noradrenergic and Cholinergic Nerve Fibers Supplying the Porcine Urinary Bladder Wall
Abstract
1. Introduction
2. Results
2.1. The Distribution and Relative Frequency of VAChT-Immunoreactive Nerve Fibers
2.1.1. Control Animals
2.1.2. RTX-Treated Pigs
2.1.3. TTX-Treated Pigs
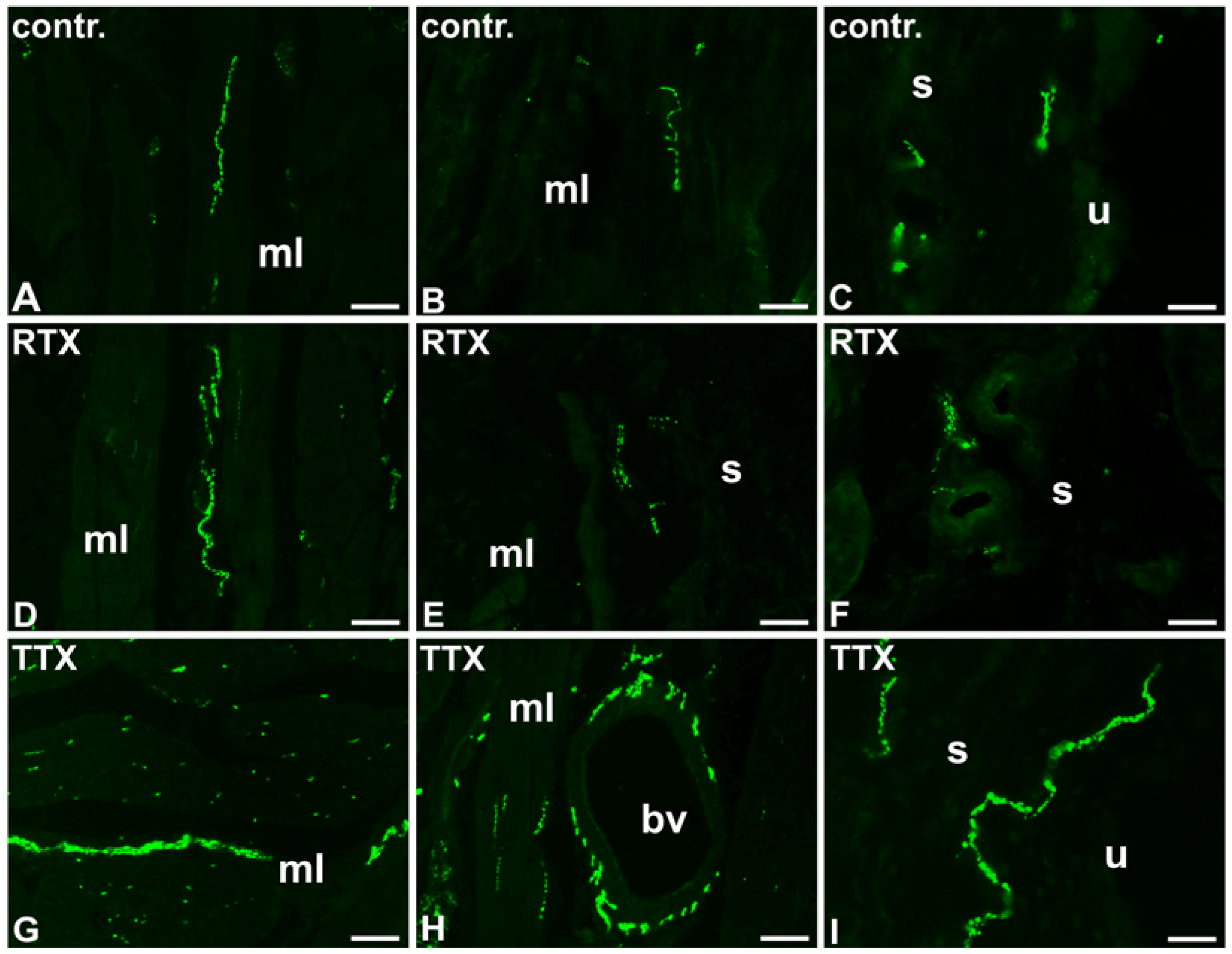
2.2. The Distribution and Relative Frequency of DβH-Immunoreactive Nerve Fibers
2.2.1. Control Animals
2.2.2. RTX-Treated Pigs
2.2.3. TTX-Treated Pigs
3. Discussion
4. Materials and Methods
4.1. Laboratory Animals
4.2. Sectioning of the Urinary Bladder Wall Samples and Immunohistochemical Procedure
4.3. Control of Specificity of the Immunohistochemical Procedures
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haylen, B.T.; de Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol. Urodyn. 2010, 29, 4–20. [Google Scholar] [CrossRef] [PubMed]
- De Groat, W.C.; Griffiths, D.; Yoshimura, N. Neural control of the lower urinary tract. Compr. Physiol. 2015, 5, 327–396. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.M.; Meinberg, M.F.; Monteiro, M.V.; Roque, M.; Haddad, J.M.; Castro, R.A. The Effectiveness of Anticholinergic Therapy for Overactive Bladders: Systematic Review and Meta-Analysis. Rev. Bras. Ginecol. Ostet. 2016, 38, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.; Dinis, P. Resiniferatoxin and botulinum toxin type A for treatment of lower urinary tract symptoms. Neurourol. Urodyn. 2007, 26, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Lepiarczyk, E.; Bossowska, A.; Kaleczyc, J.; Majewski, M. The influence of botulinum toxin type A (BTX) on the immunohistochemical characteristics of noradrenergic and cholinergic nerve fibers supplying the porcine urinary bladder wall. Pol. J. Vet. Sci. 2011, 14, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.C. Resiniferatoxin: The evolution of the "molecular scalpel" for chronic pain relief. Pharmaceuticals 2016, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.; Guimaräes, M.; Silva, C.; Reis, M. Suppression of bladder hyperreflexia by intravesical resiniferatoxin. Lancet 1997, 350, 640–641. [Google Scholar] [CrossRef]
- Lago, J.; Rodríguez, L.P.; Blanco, L.; Vieites, J.M.; Cabado, A.G. Tetrodotoxin, an extremely potent marine neurotoxin: Distribution, toxicity, origin and therapeutical uses. Mar. Drugs 2015, 13, 6384–6406. [Google Scholar] [CrossRef] [PubMed]
- Magarlamov, T.Y.; Melnikova, D.I.; Chernyshev, A.V. Tetrodotoxin-producing bacteria: Detection, distribution and migration of the toxin in aquatic systems. Toxins 2017, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.M.; McGeown, J.G.; McMurray, G.; McCloskey, K.D. Functional innervation of Guinea-pig bladder interstitial cells of cajal subtypes: Neurogenic stimulation evokes in situ calcium transients. PLoS ONE 2013, 8, e53423. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Filho, A.C.; Shah, A.; Augusto, T.M.; Barbosa, G.O.; Leiria, L.O.; de Carvalho, H.F.; Antunes, E.; Grant, A.D. Menthol inhibits detrusor contractility independently of TRPM8 activation. PLoS ONE 2014, 9, e111616. [Google Scholar] [CrossRef]
- Kuga, N.; Tanioka, A.; Hagihara, K.; Kawai, T. Modulation of afferent nerve activity by prostaglandin E2 upon urinary bladder distension in rats. Exp. Physiol. 2016, 101, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Dalmose, A.L.; Hvistendahl, J.J.; Olsen, L.H.; Eskild-Jensen, A.; Djurhuus, J.C.; Swindle, M.M. Surgically induced urologic models in swine. J. Invest. Surg. 2000, 13, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Kuzmuk, K.N.; Schook, L.B. Pigs as a model for biomedical sciences. In The Genetics of the Pig, 2nd ed.; Rothschild, M.F., Ruvinsky, A., Eds.; CAB International: Oxford Shire, UK, 2011; pp. 426–444. [Google Scholar]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Bassols, A.; Costa, C.; Eckersall, P.D.; Osada, J.; Sabrià, J.; Tibau, J. The pig as an animal model for human pathologies: A proteomics perspective. Proteom. Clin. Appl. 2014, 8, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Lepiarczyk, E.; Majewski, M.; Bossowska, A. The influence of intravesical administration of resiniferatoxin (RTX) on the chemical coding of sympathetic chain ganglia (SChG) neurons supplying the porcine urinary bladder. Histochem. Cell Biol. 2015, 144, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Lepiarczyk, E.; Dudek, A.; Kaleczyc, J.; Majewski, M.; Markiewicz, W.; Radziszewski, P.; Bossowska, A. The influence of resiniferatoxin on the chemical coding of caudal mesenteric ganglion neurons supplying the urinary bladder in the pig. J. Physiol. Pharmacol. 2016, 67, 625–632. [Google Scholar] [PubMed]
- Lepiarczyk, E.; Bossowska, A.; Kaleczyc, J.; Majewska, M.; Gonkowski, S.; Majewski, M. The Influence of Tetrodotoxin (TTX) on the Distribution and Chemical Coding of Caudal Mesenteric Ganglion (CaMG) Neurons Supplying the Porcine Urinary Bladder. Mar. Drugs 2017, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Akino, H.; Kurokawa, T.; Taga, M.; Yokokawa, R.; Tanase, K.; Nagase, K.; Yokoyama, O. Antidiuretic effect of antimuscarinic agents in rat model depends on C-fibre afferent nerves in the bladder. BJU Int. 2013, 112, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.E. On the Site and Mechanism of Action of β3-Adrenoceptor Agonists in the Bladder. Int. Neurourol. J. 2017, 21, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Yoshimura, N.; Inoue, S.; Hikita, K.; Muraoka, K.; Saito, M.; Chancellor, M.B.; Takenaka, A. Inhibitory role of the spinal galanin system in the control of micturition. Urology 2013, 82, 1188.e9–1188.e14. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.E.; Persson, K. Nitric oxide synthase and nitric oxide-mediated effects in lower urinary tract smooth muscles. World J. Urol. 1994, 12, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Bossowska, A.; Majewski, M. Botulinum toxin type A-induced changes in the chemical coding of dorsal root ganglion neurons supplying the porcine urinary bladder. Pol. J. Vet. Sci. 2012, 15, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Kaleczyc, J.; Timmermans, J.P.; Majewski, M.; Lakomy, M.; Scheuermann, D.W. Immunohistochemical properties of nerve fibres supplying accessory male genital glands in the pig. A colocalisation study. Histochem. Cell Biol. 1999, 111, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Kamińska, B.; Landowski, P.; Całka, J. Immunohistochemical distribution of cocaine- and amphetamine-regulated transcript peptide—Like immunoreactive (CART-LI) nerve fibers and various degree of co-localization with other neuronal factors in the circular muscle layer of human descending colon. Histol. Histopathol. 2013, 28, 851–858. [Google Scholar] [CrossRef] [PubMed]
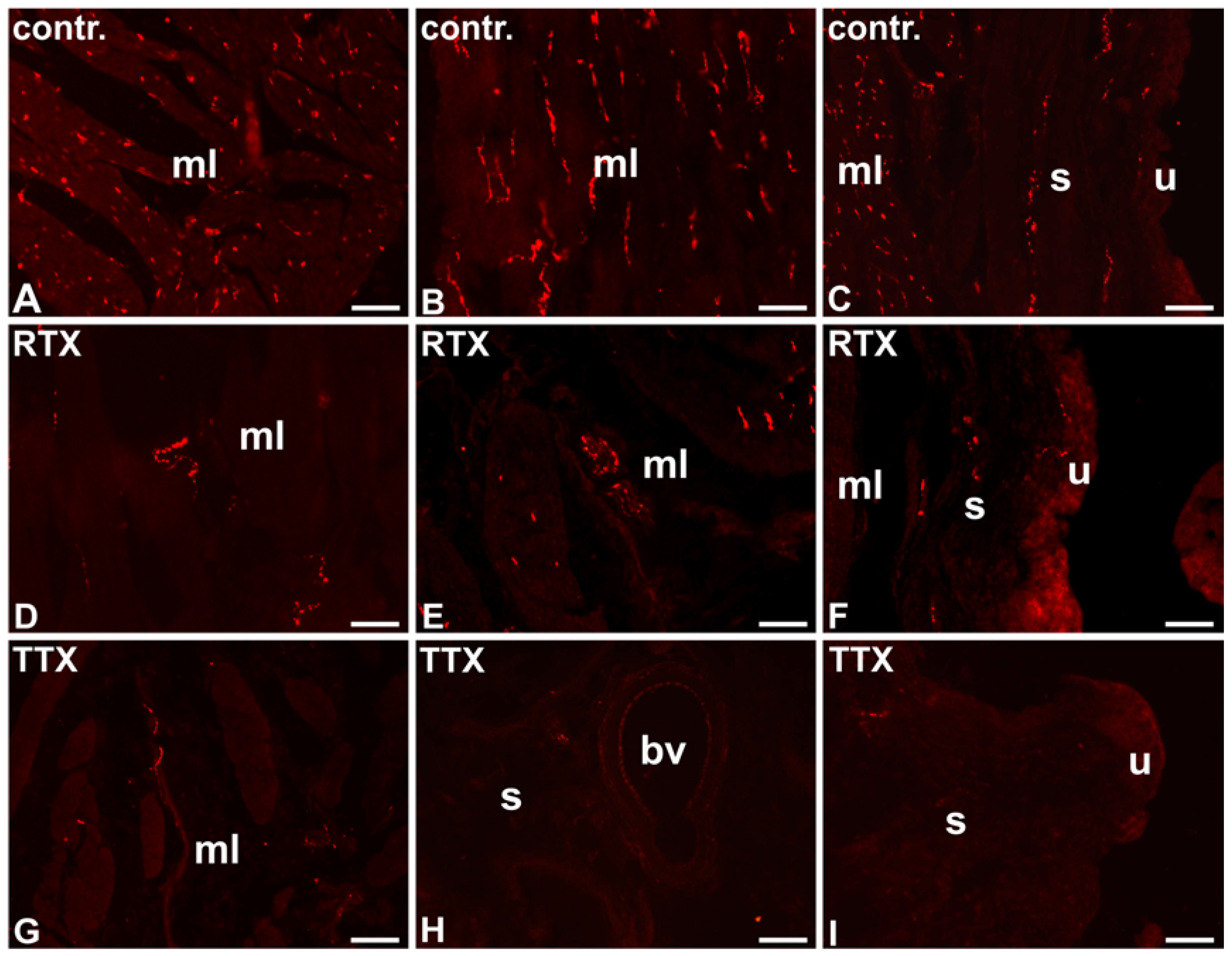
| Part of the Urinary Bladder Wall | Control Pigs | RTX-Treated Pigs | TTX-Treated Pigs |
|---|---|---|---|
| Muscle layer | ++++ | ++ ↓ | ++ ↓ |
| Submucosa | ++ | ++ | +/− ↓ |
| Urothelium | + | + | − ↓ |
| Blood vessels | +++ | + ↓ | +/− ↓ |
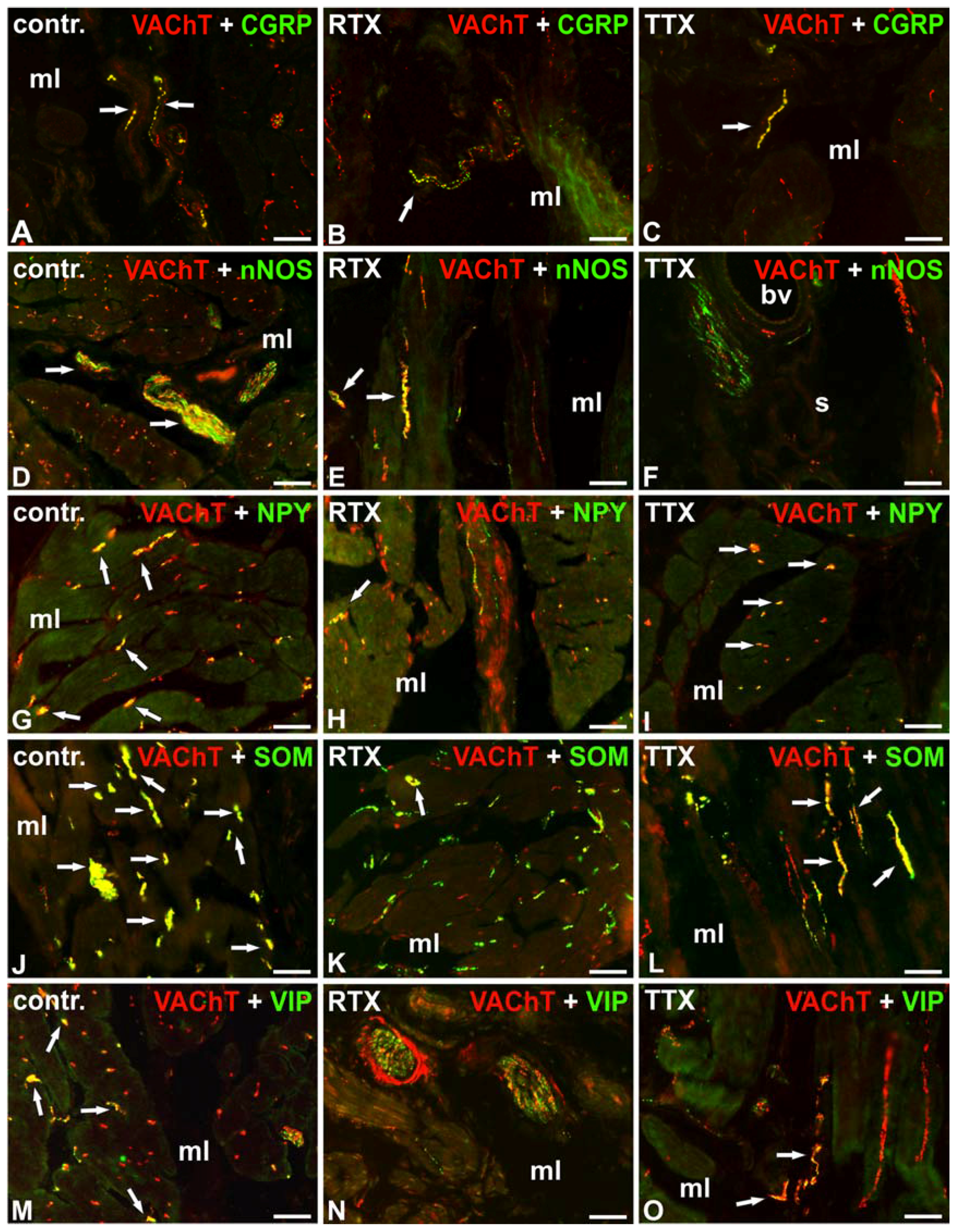
| Substances | Control Pigs | RTX-Treated Pigs | TTX-Treated Pigs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mL | bv | s | u | mL | bv | s | u | mL | bv | s | u | |
| VAChT/CGRP | + | + | + | + | +/− ↓ | - ↓ | − ↓ | − ↓ | + | − ↓ | − ↓ | − ↓ |
| VAChT/GAL | - | - | - | - | - | - | - | - | - | - | - | - |
| VAChT/L-ENK | - | - | - | - | - | - | - | - | - | - | - | - |
| VAChT/nNOS | +++ | + | + | - | + ↓ | +/− ↓ | - | - | − ↓ | − ↓ | − ↓ | - |
| VAChT/NPY | +++ | + | - | - | ++ ↓ | +/− ↓ | - | - | +++ | + | - | - |
| VAChT/PACAP | - | - | - | - | - | - | - | - | - | - | - | - |
| VAChT/SOM | ++++ | ++++ | ++++ | + | ++ ↓ | +/− ↓ | + ↓ | - | ++ ↓ | + ↓ | ++ ↓ | +/− ↓ |
| VAChT/SP | - | - | - | - | - | - | - | - | - | - | - | - |
| VAChT/VIP | + | + | ++ | + | +/− ↓ | − ↓ | − ↓ | − ↓ | + | − ↓ | + ↓ | − ↓ |
| Part of the Urinary Bladder Wall | Control Pigs | RTX-Treated Pigs | TTX-Treated Pigs |
|---|---|---|---|
| Muscle layer | + | + | ++ ↑ |
| Submucosa | ++ | + ↓ | +++ ↑ |
| Urothelium | +/− | − ↓ | + ↑ |
| Blood vessels | ++++ | ++++ | ++++ |
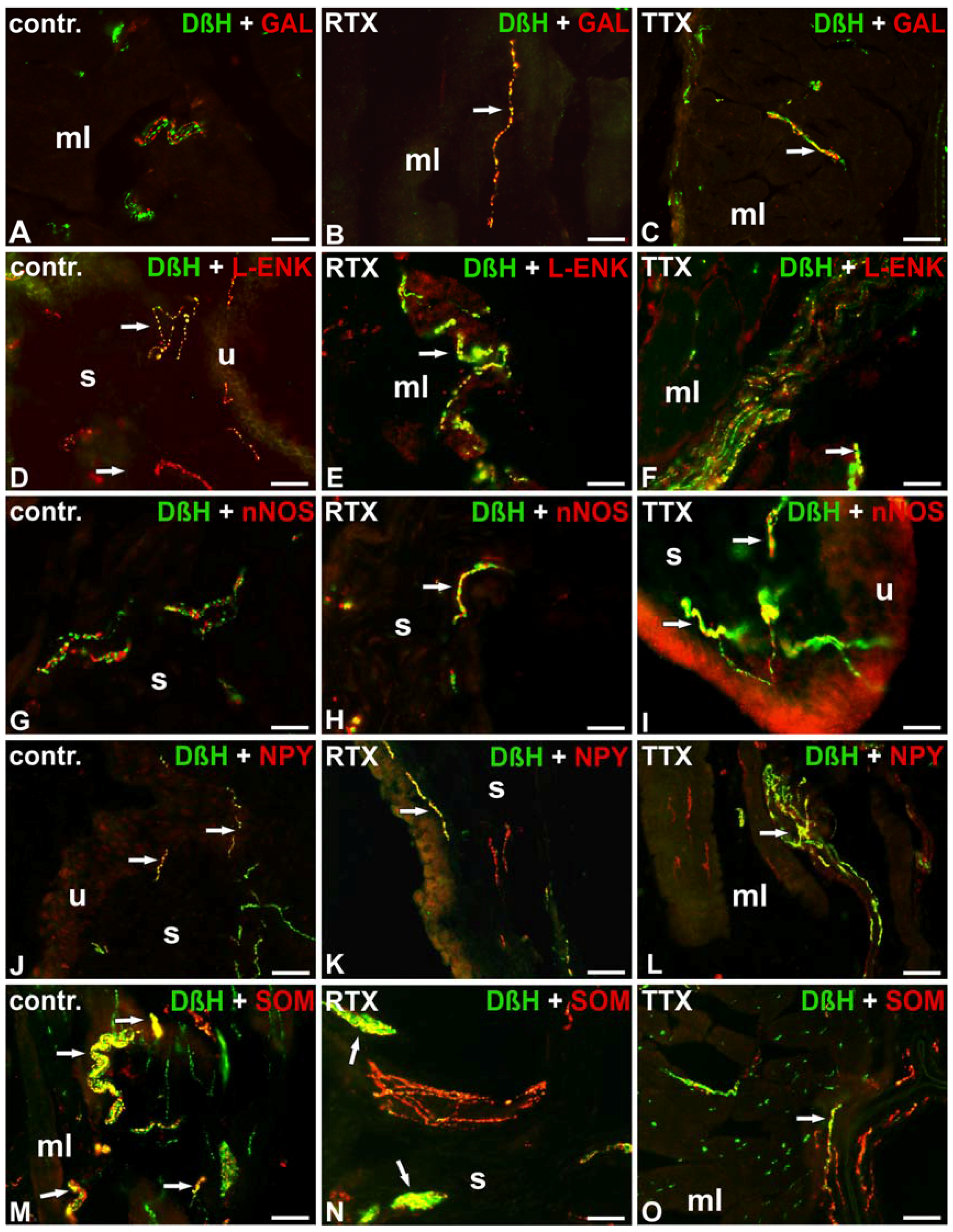
| Substances | Control Pigs | RTX-Treated Pigs | TTX-Treated Pigs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mL | bv | s | u | mL | bv | s | u | mL | bv | s | u | |
| DβH/CGRP | + | - | - | - | + | - | - | - | + | - | - | - |
| DβH/GAL | - | - | - | - | + ↑ | + ↑ | - | - | + ↑ | - | - | - |
| DβH/L-ENK | ++ | +/− | + | + | ++ | +/− | + | + | +++ ↑ | ++ ↑ | + | + |
| DβH/nNOS | - | - | - | - | - | - | + ↑ | + ↑ | - | - | ++ ↑ | ++ ↑ |
| DβH/NPY | ++++ | ++++ | + | + | ++ ↓ | + ↓ | +/− ↓ | − ↓ | ++ ↓ | ++ ↓ | +/− ↓ | − ↓ |
| DβH/PACAP | - | - | - | - | - | - | - | - | - | - | - | - |
| DβH/SOM | ++ | +/− | + | + | ++ | +/− | + | + | +++ ↑ | + ↑ | + | + |
| DβH/SP | - | - | - | - | - | - | - | - | - | - | - | - |
| DβH/VIP | - | - | - | - | - | - | - | - | - | - | - | - |
| Antigen | Code | Dilution | Species | Supplier |
|---|---|---|---|---|
| Primary antibodies | ||||
| CGRP | T-5027 | 1:400 | Guinea pig | Peninsula; San Carlos; CA; USA |
| AB5920 | 1:8000 | Rabbit | Millipore; Temecula; CA; USA | |
| DβH | MAB 308 | 1:300 | Mouse | Millipore; Temecula; CA; USA |
| D9010-07A.50 | 1:4000 | Rabbit | Biomol; Hamburg; Germany | |
| GAL | T-5036 | 1:1000 | Guinea pig | Peninsula; San Carlos; CA; USA |
| AB 5909 | 1:4000 | Rabbit | Millipore; Temecula; CA; USA | |
| L-ENK | 4140-0355 | 1:800 | Mouse | Bio-Rad; Kidlington; UK |
| AB5024 | 1:600 | Rabbit | Merck; Darmstadt; Germany | |
| nNOS | N2280 | 1:400 | Mouse | Sigma; MSU; USA |
| AB 5380 | 1:17000 | Rabbit | Millipore; Temecula; CA; USA | |
| NPY | NA1233 | 1:8000 | Rabbit | Enzo Life Sciences; Farmingdale; NY; USA |
| sc-133080 | 1:100 | Mouse | Santa Cruz Biotechnology; TX; USA | |
| PACAP | T-5039 | 1:300 | Guinea pig | Peninsula; San Carlos; CA; USA |
| T-4465 | 1:20000 | Rabbit | Peninsula; San Carlos; CA; USA | |
| SOM | 11180 | 1:30 | Rabbit | Icn-Cappel; Aurora; OH; USA |
| T-1608 | 1:30 | Rat | Peninsula; San Carlos; CA; USA | |
| SP | 8450-0505 | 1:100 | Rat | Bio-Rad; Kidlington; UK |
| VAChT | H-V006 | 1:6000 | Rabbit | Phoenix Pharmaceuticals Inc; Burlingame; CA; USA |
| VIP | VA 1285 | 1:6000 | Rabbit | Enzo Life Sciences; Farmingdale; NY; USA |
| T-5030 | 1:1000 | Guinea pig | Peninsula; San Carlos; CA; USA | |
| Secondary reagents | ||||
| Biotinylated anti-rabbit immunoglobulins | E 0432 | 1:800 | Goat | Dako; Hamburg; Germany |
| CY3-conjugated streptavidin | 711-165-152 | 1:8000 | - | Jackson I.R.; West Grove; PA; USA |
| FITC-conjugated anti-mouse IgG | 715-096-151 | 1:400 | Donkey | Jackson I.R.; West Grove; PA; USA |
| FITC-conjugated anti-rat IgG | 712-095-153 | 1:400 | Donkey | Jackson I.R.; West Grove; PA; USA |
| FITC-conjugated anti-guinea pig IgG | 706-095-148 | 1:600 | Donkey | Jackson I.R.; West Grove; PA; USA |
| Antigens Used in Pre-Absorption Test | ||
|---|---|---|
| CGRP | C0292 | Sigma; MSU; USA |
| DβH-blocking peptide | MBS9218238 | MyBioSource; CA; USA |
| GAL | G5773 | Sigma; MSU; USA |
| L-ENK | ab142314 | Abcam; UK |
| nNOS | N3033 | Sigma; MSU; USA |
| NPY | N3266 | Sigma; MSU; USA |
| PACAP | A9808 | Sigma; MSU; USA |
| SOM | S9129 | Sigma; MSU; USA |
| SP | S6883 | Sigma; MSU; USA |
| VAChT | V007 | Phoenix Pharmaceuticals Inc; CA; USA |
| VIP | V6130 | Sigma; MSU; USA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lepiarczyk, E.; Bossowska, A.; Kaleczyc, J.; Skowrońska, A.; Majewska, M.; Majewski, M.; Majewski, M. The Influence of Resiniferatoxin (RTX) and Tetrodotoxin (TTX) on the Distribution, Relative Frequency, and Chemical Coding of Noradrenergic and Cholinergic Nerve Fibers Supplying the Porcine Urinary Bladder Wall. Toxins 2017, 9, 310. https://doi.org/10.3390/toxins9100310
Lepiarczyk E, Bossowska A, Kaleczyc J, Skowrońska A, Majewska M, Majewski M, Majewski M. The Influence of Resiniferatoxin (RTX) and Tetrodotoxin (TTX) on the Distribution, Relative Frequency, and Chemical Coding of Noradrenergic and Cholinergic Nerve Fibers Supplying the Porcine Urinary Bladder Wall. Toxins. 2017; 9(10):310. https://doi.org/10.3390/toxins9100310
Chicago/Turabian StyleLepiarczyk, Ewa, Agnieszka Bossowska, Jerzy Kaleczyc, Agnieszka Skowrońska, Marta Majewska, Michal Majewski, and Mariusz Majewski. 2017. "The Influence of Resiniferatoxin (RTX) and Tetrodotoxin (TTX) on the Distribution, Relative Frequency, and Chemical Coding of Noradrenergic and Cholinergic Nerve Fibers Supplying the Porcine Urinary Bladder Wall" Toxins 9, no. 10: 310. https://doi.org/10.3390/toxins9100310
APA StyleLepiarczyk, E., Bossowska, A., Kaleczyc, J., Skowrońska, A., Majewska, M., Majewski, M., & Majewski, M. (2017). The Influence of Resiniferatoxin (RTX) and Tetrodotoxin (TTX) on the Distribution, Relative Frequency, and Chemical Coding of Noradrenergic and Cholinergic Nerve Fibers Supplying the Porcine Urinary Bladder Wall. Toxins, 9(10), 310. https://doi.org/10.3390/toxins9100310







