Abstract
This study aimed to assess the occurrence of toxigenic fungi and mycotoxin contamination in stored wheat grains by using advanced molecular and analytical techniques. A multiplex polymerase chain reaction (PCR) strategy was established for rapid identification of mycotoxigenic fungi, and an improved analytical method was developed for simultaneous multi-mycotoxin determination in wheat grains by liquid chromatography-tandem mass spectrometry (LC/MS/MS) without the need for any clean-up. The optimized multiplex PCR method was highly specific in detecting fungal species containing species-specific and mycotoxin metabolic pathway genes. The method was applied for evaluation of 34 wheat grain samples collected from storage warehouses for the presence of mycotoxin-producing fungi, and a few samples were found positive for Fusarium and Aspergillus species. Further chemical analysis revealed that 17 samples contained mycotoxins above the level of detection, but only six samples were found to be contaminated over the EU regulatory limits with at least one mycotoxin. Aflatoxin B1, fumonisins, and deoxynivalenol were the most common toxins found in these samples. The results showed a strong correlation between the presence of mycotoxin biosynthesis genes as analyzed by multiplex PCR and mycotoxin detection by LC/MS/MS. The present findings indicate that a combined approach might provide rapid, accurate, and sensitive detection of mycotoxigenic species and mycotoxins in wheat grains.
Keywords:
toxigenic fungi; aflatoxins; fumonisins; deoxynivalenol; wheat grains; multiplex PCR; LC/MS/MS 1. Introduction
Wheat grain and associated by-products are important sources of energy and protein for humans and all classes of farm animals. When grains are colonized by moulds there is a significant risk of contamination with mycotoxins, which are toxic chemical products, formed as secondary metabolites by these fungi. Many species of Fusarium, Aspergillus, Penicillium, and Alternaria are not only recognised as plant pathogens but are also sources of important mycotoxins of concern in relation to animal and human health [1]. Traditionally, toxigenic fungi in crops have been divided into two distinct classes: ‘field fungi’ (or plant pathogens), which invade and produce their toxins before harvest; and ‘storage’ (or saprophytic) fungi, which become a problem after harvest. However, the original source of both of these classes of fungi is in the field [2]. Moreover, some fungi might belong to both classes and colonize grains before and after harvest. Aspergillus flavus, for example, is associated with Aspergillus infections of grain crops in the field; it also contaminates stored grains when prevailing abiotic factors (such as temperature and water activity) are favourable [3,4]. Interactions between environmental stress factors, such as water activity and temperature, may have an influence on growth, expression of biosynthetic regulatory genes and mycotoxin production by mycotoxigenic fungal species [5,6]. Good postharvest practices avoiding high temperatures and rapid drying of grain can avoid a decrease in the grain quality and reduce the health risk due to mould growth and potential toxin contamination [6]. Poor postharvest management can lead to rapid deterioration in quality, with severe decreases in germinability and nutritional value of stored grain, possibly accompanied by undesirable fungal contamination and, consequently, toxin production [7]. The most frequently detected mycotoxins in wheat grain are deoxynivalenol (DON), fumonisins (Fs), and zearalenone (ZEN), produced by Fusarium species, and aflatoxins (AFs) and ochratoxin A (OTA) produced by Aspergillus and Penicillium species, respectively [8]. Due to their wide range of physical and chemical properties, mycotoxins are stable chemical compounds which cannot be destroyed during most food processing operations. The Food and Agricultural Organization (FAO) of the United Nations estimated that each year approximately 25% of the world’s crops are contaminated by mycotoxins, which cause annual losses of around one billion metric tons of food products. Timely assessment of these contaminants and identification of the main toxicogenic fungal species are important, not only for assessing food quality, but also for the development of control strategies for ensuring food safety [9]. Surveillance and monitoring for mycotoxigenic fungi and mycotoxins becomes critical for maintaining a high quality of grains and grain products in indoor storage facilities.
Molecular approaches, such as PCR, can serve as good alternatives to conventional methods for detection of mycotoxigenic fungi. Multiplex PCR assays that simultaneously amplify numbers of species-specific genes, and/or structural or regulatory genes involved in mycotoxin biosynthesis pathways have been successfully applied to the detection of mycotoxigenic fungi in a variety of foods and feeds [10,11,12,13]. However, there have been only a few examples of differentiation of mycotoxin-producing fungi in wheat grains by means of multiplex PCR. Wang and co-workers developed a multiplex PCR assay for simultaneous detection of genes involved in biosynthesis of type B trichothecene mycotoxins that are found in wheat grain [14], with emphasis on the genetic identification of certain mycotoxin chemotypes of Fusarium species. Further work is now needed in order to develop a sensitive PCR assays for detection of a wide range of mycotoxigenic fungi in cereal grains.
Traditionally, mycotoxin analyses are mainly performed by means of high-performance liquid chromatography after clean-up in a solid phase extraction/immunoaffinity column [15,16]. These methods normally enable the determination of only single classes of mycotoxins, including a limited number of target analytes, increasing the cost and time of the analysis due to labour-intensive sample preparation. In order to improve the sensitivity of mycotoxin detection and to reduce the cost of the analyses it would be preferable to determine as many mycotoxins as possible by routine analysis in different types of matrices in one single extraction. Furthermore, multi-mycotoxin determination methods are required because of the co-occurrence of several mycotoxins in various commodities [17]. The complexity of the wheat grain matrix, as well as the wide range of physical and chemical properties of mycotoxins, necessitate selective and sensitive detection techniques for co-occurring toxins. Liquid chromatography/mass spectrometry (LC/MS), and particularly LC coupled to tandem mass spectrometry (LC/MS/MS) have become very popular in recent years for mycotoxin analysis. However, a multi-toxin sample extraction and preparation can be challenging due to matrix effects and chemical diversity of the analytes. Solid phase extraction methodology has been developed for analysis of a number of mycotoxins in foodstuffs [18,19,20]. The major drawback of this procedure is that it is time consuming, especially when large numbers of samples need to be analysed [21]. Immunoaffinity columns, for clean-up purposes, have become increasingly popular in recent years due to the high degree of selectivity and specificity [22,23,24]. The fact that these columns are only used once and their relative high costs are major disadvantages. The use of various modifications of the QuEChERS methodology (quick, easy, cheap, effective, rugged, and safe approach) has also been recently introduced in multi-mycotoxin analysis [25]; however, the efficiency of the method for eliminating matrix effects has yet to be determined. In the current study we developed a new low-cost, rapid, and simple extraction and analysis method for multi-toxin detection in stored wheat grains. Considering the chemical diversity of the analytes, crude sample extracts were injected into the LC/MS/MS system without further purification. The method was successfully applied for rapid and simultaneous determination of 10 mycotoxins in stored wheat grains. To the best of our knowledge, this is the first report on evaluation and optimization of a reliable multiplex PCR assay and LC/MS/MS multi-method for simultaneous detection and accurate determination of the critically important mycotoxigenic fungi and mycotoxins, respectively, in wheat grains collected from storage warehouses in Israel. This combined approach will enable to evaluate the mycotoxicological risk of stored wheat grains, to minimize economic losses and reduce the hazard to animal and human health.
2. Results and Discussion
2.1. Specificity and Sensitivity of Multiplex PCR
In this study, a multiplex PCR assay was used to facilitate simultaneous detection of a wide range of potentially mycotoxin-producing fungi in wheat grain storage warehouses in Israel. Five sets of four or five species-specific primer pairs were assembled for the molecular identification of the fungal species most frequently found in stored wheat grain [26]. They comprised seven Aspergillus spp., nine Fusarium spp., and five Penicillium spp. (Table 1).

Table 1.
Sets of species-specific primers used in this study 1.
The specificity of all the species-specific primers was assessed by performing multiplex PCR on the genomic DNA of standard fungal isolates, which resulted in complete amplification of all target genes (Figure 1A). In addition, three sets of four to six primers were assembled, to amplify the genes associated with mycotoxin biosynthesis, in order to detect mycotoxigenic fungi (Table 2). The annealing temperature of each primer group for amplification of the target genes was set by using temperature gradient conditions. The specificity of the developed multiplex PCR assay was confirmed; it provided good discrimination among the tested species, as well as between mycotoxin-producing and non-producing strains. In addition, none of the primers showed cross-reactions with the species amplified by the respective other primers.
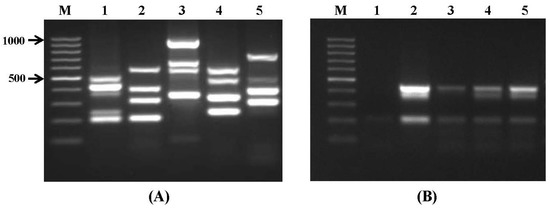
Figure 1.
Specificity and sensitivity of multiplex PCR assays using species specific primers. (A) Specificity test. Lanes: M-100 bp DNA ladder; 1: primer set I; 2: primer set II; 3: primer set III; 4: primer set IV; 5: primer set V; and (B) sensitivity test. Lanes: 1: negative control; 2: positive control (wheat sample inoculated by A. parasiticus NRRL 6111, A. fumigatus NRRL 62427, A. carbonarius NRRL 368 and incubated for 48 hrs); 3–5 (represent samples inoculated by A. parasiticus NRRL 6111, A.fumigatus NRRL 62427, A. carbonarius NRRL 368 without incubation): 3: 104 spores/g; 4: 105 spores/g; and 5: 106 spores/g.

Table 2.
Primer sets for genes involved in mycotoxin biosynthesis 1.
The PCR-based detection of microorganisms in food and feed, as well as in biological samples, is always challenging because of the variety of species that need to be determined, or the presence of substances that inhibit the PCR or reduce its amplification efficiency, either of which can cause complete reaction failure, leading to false negative results or reduced sensitivity of specific detection of the mycotoxin-producing moulds [27]. However, in the present study, the newly-developed assays have advantages over previously-reported multiplex PCRs in terms of the broad range of mycotoxigenic fungi detected in a few single assays, which could detect all target fungal species with higher specificity (Figure 1A). To determine the minimum amount of fungal template necessary to obtain visible amplification products, the multiplex PCR assays were carried out with serial dilutions of fungal genomic DNA. The detection limit for purified genomic DNA to produce a visible band on an ethidium bromide-stained agarose gel was determined to be 100 pg for all strains tested. When the sensitivity of the multiplex PCR was evaluated by artificial inoculation of wheat grain samples with known amounts of Aspergillus spp. conidia, the detection limit for DNA of each species, extracted directly from grains, was 1 × 104 spores/g in samples with no incubation (Figure 1B). The sensitivity of the PCR reaction might be influenced by various components of biological matrices, such as fats, or phenolic and polysaccharide compounds, which can reduce the purity of the extracted DNA [10,28,29]. In some previous studies, an enrichment step was performed that involved incubation of contaminated food samples for a certain period of time, to improve the sensitivity of fungal-specific PCR assays [8,12,30]. Nevertheless, in the present study, the higher efficacy of the assay was achieved by use of an efficient DNA extraction method and optimized PCR conditions, and it resulted in an improvement of at least one log of sensitivity, compared with that reported in previous studies [10,31].
2.2. Application to Stored Wheat Grain Samples
In light of the high importance of wheat grain storage within the marketing, distribution, and food security system, the high quality and safety of this product need to be ensured. In order to characterize mycotoxin-producing fungi by multiplex PCR, sets of the primers directed to the structural and regulatory genes involved in biosynthesis of aflatoxins (aflR1, nor1, avfA, ver1), Fusarium toxins (fum1, fum13, tri5, tri6, zea), and OTA (pks, otanps) were tested by using DNA extracted from various species of Aspergillus, Fusarium, and Penicillium, respectively (Figure 2A–C). The obtained results clearly show the presence of specific fragments amplified from DNA of isolates with potential mycotoxin-producing abilities. Some Aspergillus strains (A. flavus NRRL 3518, A. flavus SS2, A. carbonarius NRRL 368, and A. ochraceus NRRL 35018) did not show amplification in any target genes (Figure 2A,C). The probability of a particular toxin being produced can be predicted according to the presence or absence of an amplification product, but the effective biosynthesis of the toxin remains to be confirmed by analytical chemistry analysis [32,33].
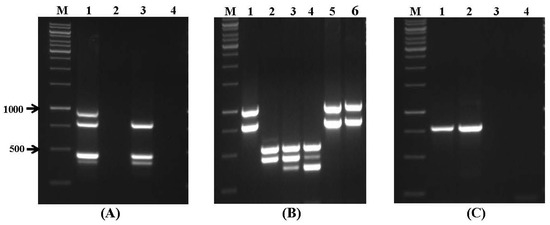
Figure 2.
Specificity of multiplex PCR assays using sets of primers for mycotoxin biosynthetic genes. (A) Primer set VI. Lanes: M: 1 kb DNA ladder; 1: A. parasiticus NRRL 6111; 2: A. flavus NRRL 3518; 3: A. flavus SS1; 4: A. flavus SS2; (B) primer set VII. Lanes: 1: F. verticillioides NRRL 25457; 2: F. sporotrichioides NRRL 3299; 3: F. culmorum NRRL 13320; 4: F. graminearum NRRL 3376; 5: F. verticillioides SS4; 6: F. verticillioides SS5; and (C) primer set VIII. Lanes: 1: P. verrucosum NRRL 965; 2: P. viridicatum NRRL 5571; 3: A. carbonarius NRRL 368; and 4: A. ochraceus NRRL 35018.
Primer sets for genes related to aflatoxin and Fusarium toxin biosynthesis were also tested on DNA samples obtained from wheat grain artificially infected with A. parasiticus NRRL 6111 (Figure 3A) and Fusarium spp. (Figure 3B). These primers enabled us to identify potential aflatoxigenic, fumonisin- and trichothecene-producing isolates in wheat grains. Primer sets for genes related to aflatoxin and Fusarium toxin biosynthesis were also tested on DNA samples obtained from wheat grain artificially infected with A. parasiticus NRRL 6111 (Figure 3A) and Fusarium spp. (Figure 3B). These primers enabled us to identify potential aflatoxigenic, fumonisin-, and trichothecene-producing isolates in wheat grains.
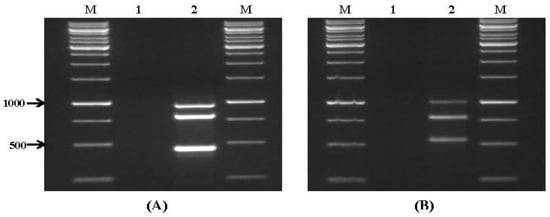
Figure 3.
Specificity of multiplex PCR assays in artificially contaminated wheat grain samples. (A) Primer set VI. Lanes: M: 1 kb DNA ladder; 1: negative control (no inoculation); 2: wheat sample artificially inoculated with A. parasiticus NRRL 6111; and (B) primer set VII. Lanes: 1: negative control (no inoculation); 2: wheat sample artificially inoculated with Fusarium spp.
Since wheat grains could easily be contaminated with a variety of mycobiota before harvest, and during postharvest handling and storage, multiplex PCR assays were applied; a total of 34 wheat grain samples collected from eight storage warehouses were tested for the presence of genes involved in the biosynthesis of mycotoxins. The samples were examined directly by using this method, without a fungus isolation and incubation step. Among the 34 grain samples 22 showed positive signals when mycotoxin gene-based primer sets were used (Table 3).

Table 3.
Detection of mycotoxin biosynthetic pathway genes by multiplex PCR in stored wheat grain samples.
Figure 4 shows some of the results obtained when a multiplex PCR was applied to DNA extracted from wheat samples collected from two storage warehouses in northern Israel; in this case amplification products were obtained, indicating the presence of potentially aflatoxigenic Aspergillus isolates (Figure 4A) and toxigenic Fusarium spp. (Figure 4B). There was no amplification in the clean, uncontaminated sample that was used as a negative control. As noted above, relatively moderate amounts of fungal contamination (at least 104 spores/g) could be detected by multiplex PCR. Yet, these results suggest that this method can be used as a diagnostic tool, which may enable the rapid and cost-effective detection of potential mycotoxigenic fungi in stored wheat grain.
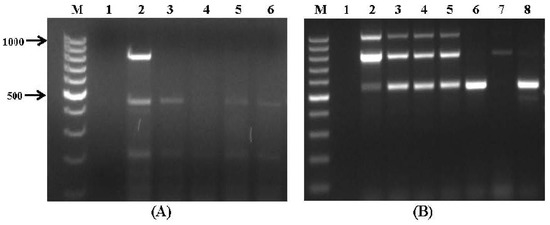
Figure 4.
Multiplex PCR using DNA isolated from stored wheat grain. (A) Primer set VI. Lanes: 1: negative control; 2–6: wheat grain naturally contaminated by aflatoxicgenic Aspergillus spp.; and (B) primer set VII. Lanes: M: 100 bp DNA ladder; 1: negative control; 2–8: wheat grain naturally contaminated by toxicgenic Fusarium spp.
2.3. Development of the Analytical Method
The LC/MS/MS multi-toxin method was optimized for the detection and quantification of AFB1, AFB2, AFG1, AFG2, OTA, ZEN, DON, FB1, FB2, and T-2 toxins. Due to the large number and the chemical diversity of the analytes, the application of an appropriate combination of solvents is required for extraction procedure during the development of a multi-toxin method. Different mixtures of water and organic solvents (such as methanol and acetonitrile) with or without the addition of acetic acid were tested. The best recovery and specificity values were obtained using a water/methanol (25:75 v/v) mixture for the extraction of the 10 mycotoxins from wheat grain samples. In contrast to the majority of the studies that have described an additional clean-up procedure [34,35,36,37], in the current study the developed multi-toxin method allows direct injection of the crude extracts to LC/MS/MS system with no further clean-up and with sufficient sensitivity and selectivity to detect and accurately quantify mycotoxin contents in wheat grains at levels lower than the maximum residue limit (MRL, value fixed by the legislation). The mycotoxins of interest in this study belong to different groups in terms of chemical properties. Therefore, the mobile phase composition was also an important factor which influenced the signal intensity and, thus, the sensitivity of each compound. Several combinations of mobile phase were tested including formic acid, acetic acid, ammonium formate, ammonium acetate, methanol, and acetonitrile. Finally, ammonium acetate acidified with acetic acid in combination with methanol was chosen as the best compromise for multi-mycotoxin analyses. The gradient rate was optimized to avoid co-eluting compounds from the matrix (especially for AFG2 qualifier ion). Mycotoxin validation results fulfilled the acceptance criteria in terms of accuracy, linearity, selectivity, and precision. The calibration levels, calculated concentration (for accuracy), precision (RSD%), limit of quantification (LOQ) and limit of detection (LOD) are summarized in Table 4. Calibration curves were linear (R2 > 0.995) for all compounds and the selectivity was acceptable for all compounds (interfering peaks were <30% of LOQ).

Table 4.
Summary of LC/MS/MS validation results.
2.4. Mycotoxin Detection in Stored Wheat Grain
The results of the multiplex PCR assays were found to be in good agreement with those obtained by chemical analysis of the samples (Table 5), which were subjected to mycotoxin screening by LC/MS/MS. The LODs values obtained in the present study ranged from 0.1 ppb for aflatoxins to 3.2 ppb for DON. Overall, 17 wheat samples were positive for mycotoxins above their LODs; however, only six analysed samples were contaminated over the EU regulatory limits with at least one mycotoxin [38]. AFB1 was detected in four postharvest wheat samples at levels above the EU regulatory limit of 2 ppb in grains for human consumption (Table 5); these samples were found to be contaminated with A. flavus possessing aflatoxin biosynthesis genes (Figure 2A) and A. flavus-specific aspergillopepsin pepO gene (Figure S1A).

Table 5.
Mycotoxin contamination in stored wheat grain samples (ppb).
Molecular analysis of the grain samples revealed amplification of the ver1 and/or aflR1 genes (Figure 4A), which are associated with aflatoxin biosynthesis. A PCR-based assay of Fusarium mycotoxin biosynthesis genes showed the amplification of fum1 and fum13 genes associated with fumonisin biosynthesis in four wheat samples (Figure 4B, lanes 2–5; samples #18–21). Among the strains isolated from the samples F. verticillioides was identified by morphological and molecular characterization as the predominant species, harbouring the fum1 and fum13 genes (Figure 2B). The LC/MS/MS analysis clearly indicated the presence of FB1 and FB2 toxins in the samples with high FB1 contamination (2.34 ppm) in stored grain sample #18 (Table 5). Another two wheat DNA samples (#22 and #24) showed amplification of tri6 gene involved in trichothecene biosynthesis, according to the results of the multiplex PCR assay (Figure 4B; lanes 6 and 8). When these DNA samples were tested against Fusarium species-specific sets of primers the 570-bp amplification fragment was obtained, which indicated the presence of F. culmorum (Figure S1B). The grain samples were cultured to confirm this identification and were found to be contaminated predominantly with F. culmorum that exhibited DON-producing abilities. Type B trichothecene DON, also known as vomitoxin, was detected and quantified by LC/MS/MS in both samples; its concentration exceeded the regulatory limit in sample #24 (1.74 ppm; Table 5). It has been reported previously that F. verticillioides and F. culmorum were isolated from various corn growing areas in Israel [39,40]; because of the rise in average temperatures caused by climate change, these species are now frequently reported as the main agents of Fusarium diseases of cereals in the Mediterranean region, especially in wet years [41,42,43,44]. Moreover, F. verticillioides and F. culmorum are also known as postharvest pathogens, especially on freshly-harvested grain that has not been dried or stored properly [7,45]. Further research is needed to study the relationship between interacting environmental stress factors (such as temperature and water availability) and the activity of key mycotoxigenic fungi, occurring at postharvest stage of wheat grains, for better understanding the conditions which represent higher risks from mycotoxin production.
A strong correlation was found between LC/MS/MS analysis of three stored grain samples (#4–#6), in which FB1 and FB2 were present at values below the regulatory guidelines (Table 5), and an established multiplex PCR assay of DNAs extracted from the same wheat samples, resulted in specific amplification of target genes fum1 and fum13. However, some differences were observed between molecular and analytical chemistry results: in particular, samples #18–#21, in addition to fum1 and fum13 fragments, showed amplification of the tri6 gene by multiplex PCR (Figure 4B), whereas the production of only fumonisins was observed by multi-mycotoxin LC/MS analysis. This could be because a lack of proper environmental conditions might have inhibited the expression of specific toxin metabolic pathway genes [11]. Furthermore, the PCR assay might yield false positive results because of difficulties in detecting mutations outside the primers’ targeted gene sequence region [46]. Nevertheless, some researchers have pointed out that, for diagnostic purposes of screening food and feed samples, false positive results in the detection of mycotoxigenic fungi are more acceptable than false negatives [10,47]. The use of internal amplification controls can prevent false negatives that might be caused by PCR inhibitors; these controls are often used to rule out failure of amplification in cases where the target sequence is not detected [48]. In the present study the nucleic acid amplification tests did not include internal controls because of the high efficacy of the assays and the positive correlation between the multiplex PCR and LC/MS/MS results (correlation coefficient of 0.99). The need to use internal controls should be determined on a case-by-case basis, because development and implementation of such controls can be difficult, and they can impair assay sensitivity [49]. Moreover, if the reaction failure rate is found to be ≤2% during test verification, routine use of an internal control is not necessary [50].
In conclusion, a relatively small number of stored wheat grain samples were found to be contaminated, mainly by Fusarium and Aspergillus spp. Co-occurrence of different mycotoxins produced by these fungi was observed, although most of the toxins were detected at very low levels (Table 5), as mandated by international regulatory standards. Nevertheless, the potential presence of opportunistic human pathogens, such as A. fumigatus, in stored wheat grain may represent a high risk of lung infection (pulmonary aspergillosis) to farm workers who have a compromised immune system. The current study might have important implications for keeping mycotoxin-producing fungi and mycotoxins out of the food-supply chain and reducing the hazard to human health. The strong correlation between multiplex PCR and LC/MS/MS results obtained in the present study demonstrated a rapid, accurate, and sensitive means for detection of mycotoxigenic species and mycotoxins in wheat grains. Combinations of molecular and analytical procedures in food safety laboratories might serve as a reliable diagnostic means for the rapid screening of large numbers of samples. In addition, such an approach could be very useful for obtaining information about the potential toxigenicity of fungal species and their concomitant mycotoxins that might contaminate wide ranges of agricultural and food products.
3. Materials and Methods
3.1. Strains and Media
Standard fungal strains (Table S2) were obtained from the USDA Agricultural Research Service Culture Collection (Northern Regional Research Laboratory, Peoria, IL, USA); they were stored in 25% glycerol at −80 °C until use and then were maintained on PD (0.4% potato starch, 2% dextrose) agar plates at 30 °C. The strains were grown in YPD liquid medium (1% yeast extract, 2% peptone, 2% dextrose) for genomic DNA extraction.
3.2. Wheat Grain Samples
Samples were taken from eight wheat grain storage warehouses in various parts of Israel, 3–6 months after harvest. In each warehouse 1-kg aliquots of grain, destined for human consumption, were collected from the front face and the centre, at points located 1 m in horizontal depth within the grain mass, and from areas close to the walls. Grain temperature and moisture content were in the ranges of 27–33 °C and 10.5–12.9%, respectively. The collected samples were kept in sterile plastic bags during transport to the laboratory and on the same day a 100-g aliquot from each thoroughly-mixed sample was frozen in liquid nitrogen, lyophilized, and milled into a fine powder with a grain grinder. The samples were stored at 4 °C pending analysis.
3.3. Reagents and Solvents
All common chemicals and solvents used for DNA extraction were purchased from Sigma-Aldrich and Bio-Lab (Jerusalem, Israel), respectively, unless otherwise specified.
Mycotoxin standards were purchased from Fermentek (Jerusalem, Israel). Methanol and acetonitrile (both LC gradient grade) were obtained from J.T. Baker (Deventer, The Netherlands); ammonium acetate and acetic acid (MS grade) were obtained from Fisher Scientific (Fair Lawn, NJ, USA). Ultrapure water was obtained using a Micropure purification system (Thermo Scientific, Langenselbold, Germany).
3.4. DNA Extraction
Fungal genomic DNA was isolated from lyophilized mycelial mats grown overnight in YPD medium. The DNA was extracted using a lysis buffer containing hexadecyltrimethylammonium bromide (CTAB). Lyophilized mycelial mats were pulverized with 5 mL of 3-mm-diameter glass beads in a disposable 50-mL conical centrifuge tube. Ten millilitres of DNA extraction buffer (l.0 M Tris/HCl, pH 7.5; 1% (w/v) CTAB; 5 M NaCl; 0.5 M EDTA; 1% (v/v) 2-mercaptoethanol; and proteinase K at 0.3 mg/mL) were added to the powdered mycelia and mixed gently, and the mixture was incubated at 65 °C for 30 min. The extracts were cooled prior to the addition of an equal volume of chloroform, gently mixed and centrifuged at 6000 rpm for 10 min. The aqueous supernatant was recovered and the nucleic acids were precipitated with an equal volume of 2-propanol. Gentle mixing resulted in the formation of high-molecular-mass DNA, which was precipitated by centrifugation at 4800× g for 5 min. The DNA was resuspended in TE buffer solution (Tris-EDTA, pH 8.0) containing RNase A at 10 µg/mL, and further purified by phenol-chloroform extraction (A260/A280 ratio of 1.8–2.0). Finally, the DNA was precipitated with 100% ethanol containing 3 M sodium acetate, rinsed in 70% (v/v) ethanol and resuspended in TE buffer. The DNA extraction from wheat grain samples was performed as described above. The purity of the extracted DNA was assayed with an ND-2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
3.5. Multiplex PCR
In order to optimize the multiplex PCR assay for direct detection of mycotoxigenic fungal species in naturally infected wheat grain samples, primers used previously for species-specific detection and for amplification of genes involved in mycotoxin biosynthesis (Table 1, Table 2 and Table S2) were tested for referenced fungi and confirmed by performing monoplex PCR. The multiplex PCR was standardized by empirically varying critical factors that affect multiplexing, such as primer concentrations, template amount, and annealing temperature. Eight different sets of 4–6 pairs of primers were combined for multiplex PCR, with annealing temperature and amplicon size taken into account. Multiplex PCR was performed in a 25-µL reaction mix containing 20–50 ng of each genomic DNA as a template, 2 × multiplex PCR master-mix with 3 mM MgCl2 (QIAGEN, Hilden, Germany) and each primer at 0.2 µM. The PCR sequence comprised: initial heat activation of DNA polymerase at 95 °C for 15 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55–60 °C (Table 1 and Table 2) for 90 s, extension at 72 °C for 90 s, and a final extension at 72 °C for 10 min. PCR products were electrophoresed on a 1% agarose gel with a 100-bp DNA size marker at 96 V for 1 h.
3.6. Multiplex PCR with Artificially Contaminated Wheat Grain Samples
Validation and sensitivity assessment of the method were carried out by inoculation of 2 g of sterile wheat grain samples with the individual spore suspensions (104–106 spores/g) of A. carbonarius, A. fumigatus, and A. parasiticus; an uninoculated sample was used as a negative control. DNA was isolated from the samples as described above, and subjected to multiplex PCR assay.
3.7. Isolation of Fungal Species from Wheat Grain
The wheat samples were analysed for presence of potentially mycotoxigenic fungi. The fungi were obtained by direct plating of wheat seeds from each sample onto PDA media supplemented with chloramphenicol at 10 µg/mL, incubated at 28 °C for three days, and identified by morphological analysis and sequencing of ribosomal DNA internal transcribed spacers (ITS). The ITS regions 1 and 2 of ribosomal DNA were used to compare the ITS1-ITS2 nucleotide sequences. The universal ITS primers, ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) were used and the ITS regions were amplified selectively by PCR. The sequence of nucleotide alignments obtained was referenced against the GeneBank database [51] with the nucleotide BLAST program.
3.8. Preparation of Mycotoxin Standard Solutions
Individual stock standard solutions (1 mg/mL) of aflatoxins B1, B2, G1, and G2 (AFB1, AFB2, AFG1, AFG2), OTA, ZEN, DON, fumonisins B1 and B2 (FB1, FB2), and T-2 toxin (T-2) were prepared in methanol. Multi-toxin working standard solutions with a series of toxin concentrations were prepared by dilution of the stock solutions of the analytes in methanol. All solutions were stored at –20 °C and were brought to room temperature before use.
3.9. Sample Preparation for Mycotoxin Analysis
Five grams of the ground sample material were mixed with 20 mL of 25:75 (v/v) water/methanol and placed in an orbital shaker for 30 min. After centrifugation at 8500× g for 15 min, 5 mL of the supernatant were transferred to a 15-mL glass tube and evaporated under a stream of nitrogen gas at 50 °C. The dry residue was reconstituted with 0.25 mL of a 95:5 (v/v) water/methanol mixture and centrifuged for 10 min at 17,000× g, at 4 °C; the supernatant was used directly for the analysis. Samples, in which the concentration exceeded the highest level of calibration, were diluted and re-injected.
3.10. Instrumentation for Mycotoxin Analysis
Detection and quantification of mycotoxins were performed with high-performance liquid chromatography coupled with tandem mass-spectrometry (LC/MS/MS). Chromatographic separation was carried out using Nexera X2 UHPLC (Shimadzu, Tokyo, Japan) equipped with 100 × 2.1 mm, 2.6 µm Kinetex C18 column, (Phenomenex, Torrance, CA, USA). The column was maintained at 40 °C and the injection volume was 2 µL. The mobile phase consisted of 2.5 mM ammonium acetate acidified with 0.1% acetic acid (A), and methanol (B). The methanol (B) concentration was raised gradually from 5% to 95% within 8 min, brought back to the initial conditions at 9 min, and allowed to stabilize for 3 min. The mobile phase was delivered at a flow rate of 0.4 mL/min. The LC system was coupled with API 6500 hybrid triple quadrupole/linear ion trap mass spectrometer (Sciex, Concord, ON, Canada), equipped with a turbo-ion electrospray (ESI) ion source. The mass-spectrometer was operated in scheduled multiple reaction monitoring (sMRM) in both positive and negative mode within a single run. Positive polarity was applied for all analytes except for DON and ZEA. Precursor/quantifier/qualifier ions are specified in Table 4. Source temperature was set at 350 °C, ion-spray voltages at −4500 V (negative mode) and 5000 V (positive mode), curtain gas at 35 arbitrary units (au), nebulizer gas at 60 au, and turbo gas at 40 au.
3.11. Validation of Analytical Parameters
Validation of the method was carried out in wheat matrix, according to Commission Regulation (EC) No 401/2006 [52]. Three non-contaminated wheat grain samples were spiked with multi-mycotoxin standard solutions at three concentration levels. The calibration levels are specified in Table 4. Extraction and analysis were performed as described above. The spiking experiments were performed in triplicate at three different time points. Validation parameters, such as precision, accuracy, LOD, LOQ, and specificity, were determined.
Supplementary Materials
The following are available online at www.mdpi.com/2072-6651/9/10/302/s1, Figure S1: Species specific multiplex PCR assays using DNA isolated from stored wheat grain, Table S1: Primer sets used in this study (including references [53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72]), Table S2: Fungal isolates used in this study.
Acknowledgments
This study was supported by a grant (no. 20-14-0021) from the Chief Scientist of the Israeli Ministry of Agriculture and Rural Development.
Author Contributions
S.S., M.B., and E.S. conceived and designed the experiments; S.S., M.B., V.Z., M.K., A.T., and E.Q. performed the experiments; S.S, M.B., M.K., and E.S. analysed the data; and S.S., M.B., and E.S. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Placinta, C.M.; D’Mello, J.P.F.; Macdonald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed. Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Miller, J.D. Fungi and mycotoxins in grain-implications for stored-product research. J. Stored Prod. Res. 1995, 31, 1–16. [Google Scholar] [CrossRef]
- Campbell, K.W.; White, D.G. Evaluation of corn genotypes for resistance to Aspergillus ear rot, kernel infection, and aflatoxin production. Plant Dis. 1995, 79, 1039–1045. [Google Scholar] [CrossRef]
- Smith, J.E. Aflatoxins; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Medina, A.; Schmidt-Heydt, M.; Rodríguez, A.; Parra, R.; Geisen, R.; Magan, N. Impacts of environmental stress on growth, secondary metabolite biosynthetic gene clusters and metabolite production of xerotolerant/xerophilic fungi. Curr. Genet. 2015, 61, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Bernaldez, V.; Cordoba, J.J.; Magan, N.; Peromingo, B.; Rodríguez, A. The influence of ecophysiological factors on growth, aflR gene expression and aflatoxin B1 production by a type strain of Aspergillus flavus. LWT Food Sci. Technol. 2017, 83, 283–291. [Google Scholar] [CrossRef]
- Magan, N.; Hope, R.; Cairns, V.; Aldred, D. Post-harvest fungal ecology: Impact of fungal growth and mycotoxin accumulation in stored grain. Eur. J. Plant Pathol. 2003, 109, 723–730. [Google Scholar] [CrossRef]
- Priyanka, S.R.; Venkataramana, M.; Balakrishna, K.; Murali, H.S.; Batra, H.V. Development and evaluation of a multiplex PCR assay for simultaneous detection of major mycotoxigenic fungi from cereals. J. Food Sci. Technol. 2015, 52, 486–492. [Google Scholar] [CrossRef]
- Suanthie, Y.; Cousin, M.A.; Woloshuk, C.P. Multiplex real-time PCR for detection and quantification of mycotoxigenic Aspergillus, Penicillium and Fusarium. J. Stored Prod. Res. 2009, 45, 139–145. [Google Scholar] [CrossRef]
- Kim, D.M.; Chung, S.H.; Chun, H.S. Multiplex PCR assay for the detection of aflatoxigenic and non-aflatoxigenic fungi in meju, a korean fermented soybean food starter. Food Microbiol. 2011, 28, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Ramana, M.V.; Balakrishna, K.; Murali, H.C.; Batra, H.V. Multiplex PCR-based strategy to detect contamination with mycotoxigenic Fusarium species in rice and fingermillet collected from southern India. J. Sci. Food Agric. 2011, 91, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, R.; Ramana, M.V.; Shylaja, R.; Uppalapati, S.R.; Murali, H.S.; Batra, H.V. Evaluation of a multiplex PCR assay for concurrent detection of four major mycotoxigenic fungi from foods. J. Appl. Microbiol. 2013, 114, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.; Venancio, A.; Kozakiewicz, Z.; Lima, N. A polyphasic approach to the identification of aflatoxigenic and non-aflatoxigenic strains of Aspergillus Section Flavi isolated from Portuguese almonds. Int. J. Food Microbiol. 2009, 129, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Zhang, J.B.; Chen, F.F.; Li, H.P.; Ndoye, M.; Liao, Y.C. A multiplex PCR assay for genetic chemotyping of toxigenic Fusarium graminearum and wheat grains for 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol and nivalenol mycotoxin. J. Food Agric. Environ. 2012, 10, 505–511. [Google Scholar]
- Gilbert, J.; Anklam, E. Validation of analytical methods for determining mycotoxins in foodstuffs. Trac-Trend Anal. Chem. 2002, 21, 468–486. [Google Scholar] [CrossRef]
- Krska, R.; Baumgartner, S.; Josephs, R. The state-of-the-art in the analysis of type-A and -B trichothecene mycotoxins in cereals. Fresenius' J. Anal. Chem. 2001, 371, 285–299. [Google Scholar] [CrossRef]
- Soleimany, F.; Jinap, S.; Rahmani, A.; Khatib, A. Simultaneous detection of 12 mycotoxins in cereals using RP-HPLC-PDA-FLD with PHRED and a post-column derivatization system. Food Addit. Contam. A 2011, 28, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Biselli, S.; Hummert, C. Development of a multicomponent method for Fusarium toxins using LC-MS/MS and its application during a survey for the content of T-2 toxin and deoxynivalenol in various feed and food samples. Food Addit. Contam. 2005, 22, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Samperi, R.; Stampachiacchiere, S.; Ventura, S.; Lagana, A. Multiclass analysis of mycotoxins in biscuits by high performance liquid chromatography-tandem mass spectrometry. Comparison of different extraction procedures. J. Chromatogr. A 2014, 1343, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Takino, M.; Sugita-Konishi, Y.; Tanaka, T. Development of a liquid chromatography/time-of-flight mass spectrometric method for the simultaneous determination of trichothecenes, zearalenone and aflatoxins in foodstuffs. Rapid Commun. Mass Spectrom. 2006, 20, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, N.; Xian, H.; Wei, D.Z.; Shi, L.; Feng, X.Y. A single-step solid phase extraction for the simultaneous determination of 8 mycotoxins in fruits by ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1429, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Desmarchelier, A.; Tessiot, S.; Bessaire, T.; Racault, L.; Fiorese, E.; Urbani, A.; Chan, W.C.; Cheng, P.; Mottier, P. Combining the quick, easy, cheap, effective, rugged and safe approach and clean-up by immunoaffinity column for the analysis of 15 mycotoxins by isotope dilution liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2014, 1337, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Koesukwiwat, U.; Sanguankaew, K.; Leepipatpiboon, N. Evaluation of a modified quechers method for analysis of mycotoxins in rice. Food Chem. 2014, 153, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.M.; Ciasca, B.; Powers, S.; Visconti, A. Improved method for the simultaneous determination of aflatoxins, ochratoxin A and Fusarium toxins in cereals and derived products by liquid chromatography-tandem mass spectrometry after multi-toxin immunoaffinity clean up. J. Chromatogr. A 2014, 1354, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S.; Berthiller, F.; Burdaspal, P.; Crews, C.; Jonker, M.A.; Krska, R.; MacDonald, S.; Malone, B.; Maragos, C.; Sabino, M.; et al. Developments in mycotoxin analysis: An update for 2009–2010. World Mycotoxin J. 2011, 4, 3–28. [Google Scholar] [CrossRef]
- Birck, N.M.; Lorini, I.; Scussel, V.M. Fungus and Mycotoxins in Wheat Grain at Post Harvest. In Proceedings of the 9th International Working Conference on Stored Product Protection, Campinas, SP, Brazil, 15–18 October 2006; pp. 198–205. [Google Scholar]
- Mule, G.; Susca, A.; Logrieco, A.; Stea, G.; Visconti, A. Development of a quantitative real-time PCR assay for the detection of Aspergillus carbonarius in grapes. Int. J. Food Microbiol. 2006, 111, S28–S34. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Rodriguez, M.; Andrade, M.J.; Cordoba, J.J. Development of a multiplex real-time PCR to quantify aflatoxin, ochratoxin a and patulin producing molds in foods. Int. J. Food Microbiol. 2012, 155, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.; Wiseman, G.; Knight, A.; Bramley, P.; Foster, L.; Rollinson, S.; Damant, A.; Primrose, S. Measurement issues associated with quantitative molecular biology analysis of complex food matrices for the detection of food fraud. Analyst 2016, 141, 45–61. [Google Scholar] [CrossRef] [PubMed]
- González-Salgado, A.; González-Jaén, M.T.; Vázquez, C.; Patiño, B. Highly sensitive PCR-based detection method specific for Aspergillus flavus in wheat flour. Food Addit. Contam. 2008, 25, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Sardinas, N.; Vazquez, C.; Gil-Serna, J.; Gonzalez-Jaen, M.T.; Patino, B. Specific detection of Aspergillus parasiticus in wheat flour using a highly sensitive PCR assay. Food Addit. Contam. A 2010, 27, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Lenart, A.M.; Klimek-Kopyra, A.; Mateusz Boroń, P. Morphological and molecular identification and PCR amplification to determine the toxigenic potential of Fusarium spp. isolated from maize ears in southern Poland. Phytoparasitica 2013, 41, 241–248. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Zhou, Y.; Du, L.; Wang, Q. Fumonisin detection and analysis of potential fumonisin-producing Fusarium spp. in asparagus (Asparagus officinalis L.) in Zhejiang province of China. J. Sci. Food Agric. 2010, 90, 836–842. [Google Scholar] [PubMed]
- Vaclavikova, M.; MacMahon, S.; Zhang, K.; Begley, T.H. Application of single immunoaffinity clean-up for simultaneous determination of regulated mycotoxins in cereals and nuts. Talanta 2013, 117, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Hong, S.Y.; Kang, J.W.; Cho, S.M.; Lee, K.R.; An, T.K.; Lee, C.; Chung, S.H. Simultaneous determination of multi-mycotoxins in cereal grains collected from South Korea by LC/MS/MS. Toxins 2017, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, W.; Zhang, Y.; Hu, X.; Wu, L.; Wang, B. QuEChERS purification combined with ultrahigh-performance liquid chromatography tandem mass spectrometry for simultaneous quantification of 25 mycotoxins in cereals. Toxins 2016, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Krska, R.; Schubert-Ullrich, P.; Molinelli, A.; Sulyok, M.; Macdonald, S.; Crews, C. Mycotoxin analysis: An update. Food Addit. Contam. 2008, 25, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin contamination in the EU feed supply chain: A focus on cereal byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Galperin, M.; Levy, Y.; Perl-Treves, R. Genetic diversity of Fusarium moniliforme detected by vegetative compatibility groups and random amplified polymorphic DNA markers. Plant Pathol. 1997, 46, 871–881. [Google Scholar] [CrossRef]
- Toth, B.; Mesterhazy, A.; Nicholson, P.; Teren, J.; Varga, J. Mycotoxin production and molecular variability of European and American isolates of Fusarium culmorum. Eur. J. Plant Pathol. 2004, 110, 587–599. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium toxins in cereals: Occurrence, legislation, factors promoting the appearance and their management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, L.G.; Gargouri, S.; Barreau, C.; Richard-Forget, F.; Hajlaoui, M.R. Trichothecene chemotypes of Fusarium culmorum infecting wheat in Tunisia. Int. J. Food Microbiol. 2010, 140, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Pancaldi, D.; Tonti, S.; Prodi, A.; Salomoni, D.; Dal Pra, M.; Nipoti, P.; Alberti, I.; Pisi, A. Survey of the main causal agents of Fusarium head blight of durum wheat around Bologna, northern Italy. Phytopathol. Mediterr. 2010, 49, 258–266. [Google Scholar]
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium culmorum: Causal agent of foot and root rot and head blight on wheat. Mol. Plant Pathol. 2013, 14, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Aldred, D.; Mylona, K.; Lambert, R.J.W. Limiting mycotoxins in stored wheat. Food Addit. Contam. A 2010, 27, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Degola, F.; Berni, E.; Dall’Asta, C.; Spotti, E.; Marchelli, R.; Ferrero, I.; Restivo, F.M. A multiplex RT-PCR approach to detect aflatoxigenic strains of Aspergillus flavus. J. Appl. Microbiol. 2007, 103, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Scherm, B.; Palomba, M.; Serra, D.; Marcello, A.; Migheli, Q. Detection of transcripts of the aflatoxin genes aflD, af1O, and aflP by reverse transcription-polymerase chain reaction allows differentiation of aflatoxin-producing and non-producing isolates of Aspergillus flavus and Aspergillus parasiticus. Int. J. Food Microbiol. 2005, 98, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Hoorfar, J.; Malorny, B.; Abdulmawjood, A.; Cook, N.; Wagner, M.; Fach, P. Practical considerations in design of internal amplification controls for diagnostic PCR assays. J. Clin. Microbiol. 2004, 42, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Molecular Diagnostic Methods for Infectious Diseases, Proposed Guideline, 2nd ed.; CLSI document MM3-P2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2005; Volume 25. [Google Scholar]
- Nolte, F.S. Novel internal controls for real-time PCR assays. Clin. Chem. 2004, 50, 801–802. [Google Scholar] [CrossRef] [PubMed]
- GeneBank Database. Available online: http://www.ncbi.nlm.nih.gov/BLAST (accessed on 20 August 2017).
- European Commission. Commission regulation (EC) No. 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, L70, 12–34. [Google Scholar]
- Logotheti, M.; Kotsovili-Tseleni, A.; Arsenis, G.; Legakis, N.I. Multiplex PCR for the discrimination of A. fumigatus, A. flavus, A. niger and A. terreus. J. Microbiol. Methods 2009, 76, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Susca, A.; Stea, G.; Mule, G.; Perrone, G. Polymerase chain reaction (PCR) identification of Aspergillus niger and Aspergillus tubingensis based on the calmodulin gene. Food Addit. Contam. A 2007, 24, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Susca, A.; Stea, G.; Mule, G. PCR assay for identification of Aspergillus carbonarius and Aspergillus japonicus. Eur. J. Plant Pathol. 2004, 110, 641–649. [Google Scholar] [CrossRef]
- Nicholson, P.; Simpson, D.R.; Weston, G.; Rezanoor, H.N.; Lees, A.K.; Parry, D.W.; Joyce, D. Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiol. Mol. Plant P. 1998, 53, 17–37. [Google Scholar] [CrossRef]
- Parry, D.W.; Nicholson, P. Development of a PCR assay to detect Fusarium poae in wheat. Plant Pathol. 1996, 45, 383–391. [Google Scholar] [CrossRef]
- Demeke, T.; Clear, R.M.; Patrick, S.K.; Gaba, D. Species-specific PCR-based assays for the detection of Fusarium species and a comparison with the whole seed agar plate method and trichothecene analysis. Int. J. Food Microbiol. 2005, 103, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Mule, G.; Susca, A.; Stea, G.; Moretti, A. A species-specific PCR assay based on the calmodulin partial gene for identification of Fusarium verticillioides, F. proliferatum and F. subglutinans. Eur. J. Plant Pathol. 2004, 110, 495–502. [Google Scholar] [CrossRef]
- Doohan, F.M.; Parry, D.W.; Jenkinson, P.; Nicholson, P. The use of species specific PCR based assays to analyse Fusarium ear blight of wheat. Plant Pathol. 1998, 47, 197–205. [Google Scholar] [CrossRef]
- Casasnovas, F.; Fantini, E.N.; Palazzini, J.M.; Giaj-Merlera, G.; Chulze, S.N.; Reynoso, M.M.; Torres, A.M. Development of amplified fragment length polymorphism (AFLP)-derived specific primer for the detection of Fusarium solani aetiological agent of peanut brown root rot. J. Appl. Microbiol. 2013, 114, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, J.D.; O’Keeffe, T.L. Detection and discrimination of four Aspergillus section Nigri species by PCR. Lett. Appl. Microbiol. 2015, 60, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Fox, R.T.V.; Culham, A. Development of a PCR-based assay for rapid and reliable identification of pathogenic Fusaria. FEMS Microbiol. Lett. 2003, 218, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Dombrink-Kurtzman, M.A.; McGovern, A.E. Species-specific identification of Penicillium linked to patulin contamination. J. Food Protect. 2007, 70, 2646–2650. [Google Scholar] [CrossRef]
- Hamamoto, H.; Hasegawa, K.; Nakaune, R.; Lee, Y.J.; Makizumi, Y.; Akutsu, K.; Hibi, T. Tandem repeat of a transcriptional enhancer upstream of the sterol 14 alpha-demethylase gene (CYP51) in Penicillium digitatum. Appl. Environ. Microbiol. 2000, 66, 3421–3426. [Google Scholar] [CrossRef] [PubMed]
- Bogs, C.; Battilani, P.; Geisen, R. Development of a molecular detection and differentiation system for ochratoxin A producing Penicillium species and its application to analyse the occurrence of Penicillium nordicum in cured meats. Int. J. Food Microbiol. 2006, 107, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.H.; Skouboe, P.; Boysen, M.; Soule, J.; Rossen, L. Detection of Penicillium species in complex food samples using the polymerase chain reaction. Int. J. Food Microbiol. 1997, 35, 169–177. [Google Scholar] [CrossRef]
- Kanbe, T.; Yamaki, K.; Kikuchi, A. Identification of the pathogenic Aspergillus species by nested PCR using a mixture of specific primers to DNA topoisomerase II gene. Microbiol. Immunol. 2002, 46, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Manonmani, H.K.; Anand, S.; Chandrashekar, A.; Rati, E.R. Detection of aflatoxigenic fungi in selected food commodities by PCR. Process Biochem. 2005, 40, 2859–2864. [Google Scholar] [CrossRef]
- Priyanka, S.R.; Venkataramana, M.; Kumar, G.P.; Rao, V.K.; Murali, H.C.S.; Batra, H.V. Occurrence and molecular detection of toxigenic Aspergillus species in food grain samples from India. J. Sci. Food Agric. 2014, 94, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.J.; Woloshuk, C.P.; Bhatnagar, D.; Cleveland, T.E. Cloning and characterization of avfA and omtB genes involved in aflatoxin biosynthesis in three Aspergillus species. Gene 2000, 248, 157–167. [Google Scholar] [CrossRef]
- Skory, C.D.; Chang, P.K.; Cary, J.; Linz, J.E. Isolation and characterization of a gene from Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin aflatoxin biosynthesis. Appl. Environ. Microbiol. 1992, 58, 3527–3537. [Google Scholar] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).