Essential Oils Modulate Gene Expression and Ochratoxin A Production in Aspergillus carbonarius
Abstract
:1. Introduction
2. Results
2.1. Essential Oils Effect on Growth of A. carbonarius S402 and Its OTA Production
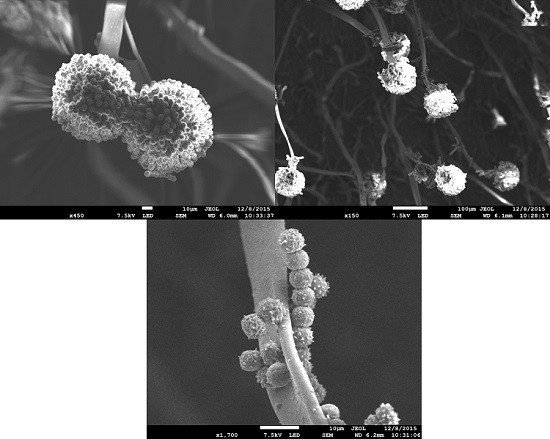
2.2. Microscopic Morphology Evaluation
2.3. Analysis of Genes Expression Involved in OTA Biosynthesis by A. carbonarius
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Essential Oils
5.2. Strain and Culture Conditions
5.3. Scanning Electron Microscopy (SEM) for Morphologic Study and Spores Count
5.4. OTA Extraction and HPLC Analysis
5.5. RNA Extraction, cDNA Synthesis and qRT-PCR
5.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| A. carbonarius | Aspergillus carbonarius |
| qRT-PCR | Quantitative Reverse Transcription-Polymerase Chain Reaction |
| OTA | Ochratoxin A |
| IARC | International Agency for Research on Cancer |
| E.O | Essential oil |
| SGM | Synthetic Grape Medium |
| HPLC | High Performance Liquid Chromatography |
| mM | Milliomolar |
| N.D | Not detectable |
| r.p.m | Round per minute |
References
- Reverberi, M.; Ricelli, A.; Zjalic, S.; Fabbri, A.A.; Fanelli, C. Natural functions of mycotoxins and control of their biosynthesis in fungi. Appl. Microbiol. Biotechnol. 2010, 87, 899–911. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, A.; Rizk, T.; Lteif, R.; Azouri, H.; Delia, M.-L.; Lebrihi, A. Occurrence of ochratoxin A- and aflatoxin B1-producing fungi in lebanese grapes and ochratoxin A content in musts and finished wines during 2004. J. Agric. Food Chem. 2006, 54, 8977–8982. [Google Scholar] [CrossRef] [PubMed]
- Ponsone, M.L.; Chiotta, M.L.; Combina, M.; Torres, A.; Knass, P.; Dalcero, A.; Chulze, S. Natural occurrence of ochratoxin A in musts, wines and grape vine fruits from grapes harvested in argentina. Toxins 2010, 2, 1984–1996. [Google Scholar] [CrossRef] [PubMed]
- IARC. Some Naturally Occuring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC: Lyon, France, 1993; Volume 56, pp. 489–521. [Google Scholar]
- Abouzied, M.; Horvath, A.; Podlesny, P.; Regina, N.; Metodiev, V.; Kamenova-Tozeva, R.; Niagolova, N.; Stein, A.; Petropoulos, E.; Ganev, V. Ochratoxin A concentrations in food and feed from a region with balkan endemic nephropathy. Food Addit. Contam. 2002, 19, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Mantle, P.G.; Faucet-Marquis, V.; Manderville, R.A.; Squillaci, B.; Pfohl-Leszkowicz, A. Structures of covalent adducts between DNA and ochratoxin A: A new factor in debate about genotoxicity and human risk assessment. Chem. Res. Toxicol. 2009, 23, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Abrunhosa, L.; Serra, R.; Venâncio, A. Biodegradation of ochratoxin A by fungi isolated from grapes. J. Agric. Food Chem. 2002, 50, 7493–7496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, K.; Badeaa, R. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem. Toxicol. 2002, 40, 1669–1675. [Google Scholar] [CrossRef]
- Basílico, M.Z.; Basílico, J.C. Inhibitory effects of some spice essential oils on Aspergillus ochraceus nrrl 3174 growth and ochratoxin A production. Lett. Appl. Microbiol. 1999, 29, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Perrone, G.; Solfrizzo, M.; Epifani, F.; Abbas, A.; Dobson, A.D.; Mule, G. Characterisation of a pks gene which is expressed during ochratoxin A production by Aspergillus carbonarius. Int. J. Food Microbiol. 2009, 129, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Knox, B.P.; Bruno, K.S.; Solfrizzo, M.; Baker, S.E.; Perrone, G. Identification and characterization of the polyketide synthase involved in ochratoxin A biosynthesis in Aspergillus carbonarius. Int. J. Food Microbiol. 2014, 179, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Bruno, K.S.; Solfrizzo, M.; Perrone, G.; Mule, G.; Visconti, A.; Baker, S.E. New insight into the ochratoxin A biosynthetic pathway through deletion of a nonribosomal peptide synthetase gene in Aspergillus carbonarius. Appl. Environ. Microbiol. 2012, 78, 8208–8218. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Sempere, A.; Marin, S.; Sanchis, V.; Ramos, A.J. Vea and laea transcriptional factors regulate ochratoxin A biosynthesis in Aspergillus carbonarius. Int. J. Food Microbiol. 2013, 166, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Xing, F.; Selvaraj, J.N.; Wang, Y.; Zhao, Y.; Zhou, L.; Liu, X.; Liu, Y. Inhibitory effect of essential oils on Aspergillus ochraceus growth and ochratoxin A production. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Holt, D.L. Growth inhibition of Penicillium expansum by several commonly used food ingredients. J. Food Prot. 1993, 56, 862–867. [Google Scholar]
- Sinha, K.; Sinha, A.; Prasad, G. The effect of clove and cinnamon oils on growth of and aflatoxin production by Aspergillus flavus. Lett. Appl. Microbiol. 1993, 16, 114–117. [Google Scholar] [CrossRef]
- Patkar, K.; Usha, C.; Shetty, H.S.; Paster, N.; Lacey, J. Effect of spice essential oils on growth and aflatoxin b1 production by Aspergillus flavus. Lett. Appl. Microbiol. 1993, 17, 49–51. [Google Scholar] [CrossRef]
- Daouk, R.K.; Dagher, S.M.; Sattout, E.J. Antifungal activity of the essential oil of Origanum syriacum L. J. Food Prot. 1995, 58, 1147–1149. [Google Scholar]
- Lukas, B.; Schmiderer, C.; Franz, C.; Novak, J. Composition of essential oil compounds from different syrian populations of Origanum syriacum l. (lamiaceae). J. Agric. Food Chem. 2009, 57, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.; Tzakou, O.; Pitarokili, D.; Couladis, M. Antifungal activities of selected aromatic plants growing wild in greece. Food/Nahrung 2002, 46, 317–320. [Google Scholar] [CrossRef]
- da Cruz Cabral, L.; Fernandez Pinto, V.; Patriarca, A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013, 166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Omidbeygi, M.; Barzegar, M.; Hamidi, Z.; Naghdibadi, H. Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food Control 2007, 18, 1518–1523. [Google Scholar] [CrossRef]
- Rasooli, I.; Abyaneh, M.R. Inhibitory effects of thyme oils on growth and aflatoxin production by Aspergillus parasiticus. Food Control 2004, 15, 479–483. [Google Scholar] [CrossRef]
- Dambolena, J.S.; Lopez, A.G.; Canepa, M.C.; Theumer, M.G.; Zygadlo, J.A.; Rubinstein, H.R. Inhibitory effect of cyclic terpenes (limonene, menthol, menthone and thymol) on Fusarium verticillioides mrc 826 growth and fumonisin b1 biosynthesis. Toxicon Off. J. Int. Soc. Toxinol. 2008, 51, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bansod, S.; Rai, M. Antifungal activity of essential oils from indian medicinal plants against human pathogenic Aspergillus fumigatus and A. Niger. World J. Med. Sci. 2008, 3, 81–88. [Google Scholar]
- Marín, S.; Ramos, A.J.; Sanchis, V. Comparison of methods for the assessment of growth of food spoilage moulds in solid substrates. Int. J. Food Microbiol. 2005, 99, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Baydar, H.; Sağdiç, O.; Özkan, G.; Karadoğan, T. Antibacterial activity and composition of essential oils from origanum, thymbra and satureja species with commercial importance in turkey. Food Control 2004, 15, 169–172. [Google Scholar] [CrossRef]
- Pawar, V.; Thaker, V. In vitro efficacy of 75 essential oils against Aspergillus niger. Mycoses 2006, 49, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Helal, G.; Sarhan, M.; Abu Shahla, A.; Abou El-Khair, E. Effects of cymbopogon citratus l. Essential oil on the growth, morphogenesis and aflatoxin production of Aspergillus flavus mL2-strain. J. Basic Microbiol. 2007, 47, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Rasooli, I.; Owlia, P. Chemoprevention by thyme oils of Aspergillus parasiticus growth and aflatoxin production. Phytochemistry 2005, 66, 2851–2856. [Google Scholar] [CrossRef] [PubMed]
- Esper, R.H.; Gonçalez, E.; Felicio, R.C.; Felicio, J.D. Fungicidal activity and constituents of Ageratum conyzoides essential oil from three regions in são paulo state, brazil. Arq. Inst. Biol. 2015, 82, 1–4. [Google Scholar] [CrossRef]
- Murthy, P.S.; Borse, B.B.; Khanum, H.; Srinivas, P. Inhibitory effects of ajowan (trachyspermum ammi) ethanolic extract on A. ochraceus growth and ochratoxin production. Turk. J. Biol. 2009, 33, 211–217. [Google Scholar]
- Caceres, I.; El Khoury, R.; Medina, A.; Lippi, Y.; Naylies, C.; Atoui, A.; El Khoury, A.; Oswald, I.P.; Bailly, J.D.; Puel, O. Deciphering the anti-aflatoxinogenic properties of eugenol using a large-scale q-PCR approach. Toxins 2016, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, M.; Gallo, A.; Scalzo, R.L.; Haidukowski, M.; Picchi, V.; Perrone, G. Inhibition of ochratoxin a production in Aspergillus carbonarius by hydroxycinnamic acids from grapes. World Mycotoxin J. 2015, 8, 283–289. [Google Scholar] [CrossRef]
- Boutigny, A.L.; Barreau, C.; Atanasova-Penichon, V.; Verdal-Bonnin, M.N.; Pinson-Gadais, L.; Richard-Forget, F. Ferulic acid, an efficient inhibitor of type b trichothecene biosynthesis and Tri gene expression in Fusarium liquid cultures. Mycol. Res. 2009, 113, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yukl, E.T.; Moënne-Loccoz, P.; Ortiz de Montellano, P.R. Fungal heme oxygenases: Functional expression and characterization of Hmx1 from Saccharomyces cerevisiae and Cahmx1 from candida albicans. Biochemistry 2006, 45, 14772–14780. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, T.; Yaguchi, A.; Takahashi-Ando, N.; Kimura, M.; Takahashi, H.; Nakajima, T.; Sugita-Konishi, Y.; Nagasawa, H.; Sakuda, S. Spiroethers of german chamomile inhibit production of aflatoxin G1 and trichothecene mycotoxin by inhibiting cytochrome p450 monooxygenases involved in their biosynthesis. FEMS Microbiol. Lett. 2008, 284, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Carmo, E.S.; Lima, E.D.O.; Souza, E.L.D.; Sousa, F.B.D. Effect of Cinnamomum zeylanicum blume essential oil on the rowth and morphogenesis of some potentially pathogenic Aspergillus species. Braz. J. Microbiol. 2008, 39, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Tripathi, A. Effects of citrus sinensis (L.) osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) van tieghem. Microbiol. Res. 2008, 163, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, H.; Mathieu, F.; Taillandier, P.; Lebrihi, A. Ochratoxin A removal in synthetic and natural grape juices by selected oenological saccharomyces strains. J. Appl. Microbiol. 2004, 97, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. Qbase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8. [Google Scholar] [CrossRef] [PubMed]
- Cabañes, F.J.; Bragulat, M.R.; Castellá, G. Characterization of nonochratoxigenic strains of Aspergillus carbonarius from grapes. Food Microbiol. 2013, 36, 135–141. [Google Scholar] [CrossRef] [PubMed]
- The National Center for Biotechnology Information advances science and health by providing access to biomedical and genomic information. Available online: http://www.ncbi.nlm.nih.gov/ (accessed on 30 January 2014).
- Verheecke, C.; Liboz, T.; Anson, P.; Diaz, R.; Mathieu, F. Reduction of aflatoxin production by Aspergillus flavus and Aspergillus parasiticus in interaction with streptomyces. Microbiology 2015, 161, 967–972. [Google Scholar] [CrossRef] [PubMed]

| Essential Oils | Dry Weight (g) | Radial Growth (cm) | OTA Concentration (ng/mL*g of Dry Weight) | |||
|---|---|---|---|---|---|---|
| Control | 0.36 ± 0.05 a | 5.1 ± 0.01 c | 50.58 ± 0.79 | |||
| 5 µL/mL | 5 µL/mL | 1 µL/mL | 5 µL/mL | 1 µL/mL | 5 µL/mL | |
| Anise | 0.38 ± 0.01 a | 0.3 ± 0.01 b | 4.5 ± 0.01 c | 3.5 ± 0.05 c,d | 16.66 ± 0.55 * | 10.36 ± 0.27 * |
| Cardamom | 0.36 ± 0.005 a | 0.31 ± 0.005 b | 4.5 ± 0.01 c | 4.4 ± 1 c | 13.15 ± 0.20 * | 8.37 ± 0.15 * |
| Celery | 0.36 ± 0.05 a | 0.34 ± 0.05 a | 4.1 ± 0.05 c | 3.8 ± 0.00 d | 16.43 ± 0.24 * | 15.60 ± 0.24 * |
| Chamomile | 0.36 ± 0.005 a | 0.31 ± 0.005 b | 5.1 ± 0.05 c | 4.5 ± 0.01 c | 16.54 ± 0.26 * | 10.66 ± 0.19 * |
| Cinnamon | No growth | No growth | N.D | N.D | N.D | N.D |
| Fennel | 0.32 ± 0.05 b | 0.31 ± 0.005 b | 4.1 ± 0.05 c | 3.4 ± 0.01 c | 6.8 ± 0.12 * | 5.6 ± 0.10 * |
| Oregano | No growth | No growth | N.D | N.D | N.D | N.D |
| Rosemary | 0.36 ± 0.01 a | 0.33 ± 0.001 a | 4.5 ± 0.00 c | 4.5 ± 0.00 c | 23.62 ± 0.65 * | 11.03 ± 2.80 * |
| Taramira | No growth | No growth | N.D | N.D | N.D | N.D |
| Thyme | 0.2 ± 0.01 * | No growth | 2.1 ± 0.01 * | N.D | 3.56 ± 0.17 * | N.D |
| Essential Oils | Fennel | Cardamom | Chamomile | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 µL/mL | 5 µL/mL | 1 µL/mL | 5 µL/mL | 1 µL/mL | 5 µL/mL | |||||||
| Genes | Relative expression | % of OTA reduction | Relative expression | % of OTA reduction | Relative expression | % of OTA reduction | Relative expression | % of OTA reduction | Relative expression | % of OTA reduction | Relative expression | % of OTA reduction |
| acpks | 0.04 * | 86.90% | 0.008 * | 88.60% | 0.24 * | 74.20% | 0.15 * | 83.50% | 0.2 * | 67.50% | 0.02 * | 79.10% |
| acOTApks | 0.009 * | 0.001 * | 0.14 * | 0.02 * | 0.12 * | 0.05 * | ||||||
| acOTAnrps | 0.7 | 0.02 * | 0.2 * | 0.18 * | 0.2 * | 0.16 * | ||||||
| veA | 0.09 * | 0.07 * | 0.45 * | 0.29 * | 0.37 * | 0.06 * | ||||||
| laeA | 0.08 * | 0.06 * | 0.9 | 0.3 * | 0.29 * | 0.04 * | ||||||
| Essential Oils | Rosemary | Anise | Celery | |||||||||
| 1 µL/mL | 5 µL/mL | 1 µL/mL | 5 µL/mL | 1 µL/mL | 5 µL/mL | |||||||
| Genes | Relative expression | % of OTA reduction | Relative expression | % of OTA reduction | Relative expression | % of OTA reduction | Relative expression | % of OTA reduction | Relative expression | % of OTA reduction | % of OTA reduction | Relative expression |
| acpks | 0.1 * | 53.70% | 0.05 * | 78.30% | 0.2 * | 76.90% | 0.1 * | 83.90% | 0.5 * | 68.50% | 0.3 * | 77.10% |
| acOTApks | 0.9 | 0.04 * | 0.19 * | 0.14 * | 0.24 * | 0.19 * | ||||||
| acOTAnrps | 0.2 * | 0.02 * | 0.8 | 0.01 * | 0.7 | 0.48 * | ||||||
| veA | 0.1 * | 0.05 * | 0.9 | 0.13 * | 0.7 | 0.12 * | ||||||
| laeA | 0.2 * | 0.09 * | 0.7 | 0.2 * | 0.8 | 0.2 * | ||||||
| Primer Name | Primer Sequence (5’→3’) | References | Efficiency |
|---|---|---|---|
| acpks-F | GAGTCTGACCATCGACACGG | [10] | 110.0% |
| acpks-R | GGCGACTGTGACACATCCAT | ||
| acOTApks-F | CGTGTCCGATACTGTCTGTGA | [11] | 102% |
| acOTApks-R | GCATGGAGTCCTCAAGAACC | ||
| acOTAnrps-F | ATCCCCGGAATATTGGCACC | [12] | 82.6% |
| acOTAnrps-R | CCTTCGATCAAGAGCTCCCC | ||
| laeA-F | CACCTATACAACCTCCGAACC | [13] | 104.7% |
| laeA-R | GGTTCGGCCAACCGACGACGC | ||
| veA-F | TCCCGGTTCTCACAGGCGTA | [13] | 101.3% |
| veA-R | GCTGTCCTTGGTCTCCTCGTA | ||
| β-tubulin-F | CGCATGAACGTCTACTTCAACG | [43] | 95% |
| β-tubulin-R | AGTTGTTACCAGCACCGGA | ||
| calmodulin-F | CCAGATCACCACCAAGGAGC | [44] | 105% |
| calmodulin-R | GTTATCGCGGTCGAAGACCT | ||
| 18S-F | GCAAATTACCCAATCCCGAC | [44] | 98% |
| 18S-R | GAATTGCCGCGGCTGCTG | ||
| cox5-F | CCCTGTTCTACGTCATTCACTTGTT | [45] | 87% |
| cox5-R | TCTTCTCGGCCTTGGCATACTC | ||
| actin1-F | TTGACAATGGTTCGGGTATGTG | [45] | 82% |
| actin1-R | TTGACAATGGTTCGGGTATGTG | ||
| gpdA-F | ACGGCAAGCTCACTGGTATGT | [45] | 110% |
| gpdA-R | CAGCCTTGATGGTCTTCTTGATG |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Khoury, R.; Atoui, A.; Verheecke, C.; Maroun, R.; El Khoury, A.; Mathieu, F. Essential Oils Modulate Gene Expression and Ochratoxin A Production in Aspergillus carbonarius. Toxins 2016, 8, 242. https://doi.org/10.3390/toxins8080242
El Khoury R, Atoui A, Verheecke C, Maroun R, El Khoury A, Mathieu F. Essential Oils Modulate Gene Expression and Ochratoxin A Production in Aspergillus carbonarius. Toxins. 2016; 8(8):242. https://doi.org/10.3390/toxins8080242
Chicago/Turabian StyleEl Khoury, Rachelle, Ali Atoui, Carol Verheecke, Richard Maroun, Andre El Khoury, and Florence Mathieu. 2016. "Essential Oils Modulate Gene Expression and Ochratoxin A Production in Aspergillus carbonarius" Toxins 8, no. 8: 242. https://doi.org/10.3390/toxins8080242
APA StyleEl Khoury, R., Atoui, A., Verheecke, C., Maroun, R., El Khoury, A., & Mathieu, F. (2016). Essential Oils Modulate Gene Expression and Ochratoxin A Production in Aspergillus carbonarius. Toxins, 8(8), 242. https://doi.org/10.3390/toxins8080242