Antifungal and Antiaflatoxigenic Methylenedioxy-Containing Compounds and Piperine-Like Synthetic Compounds
Abstract
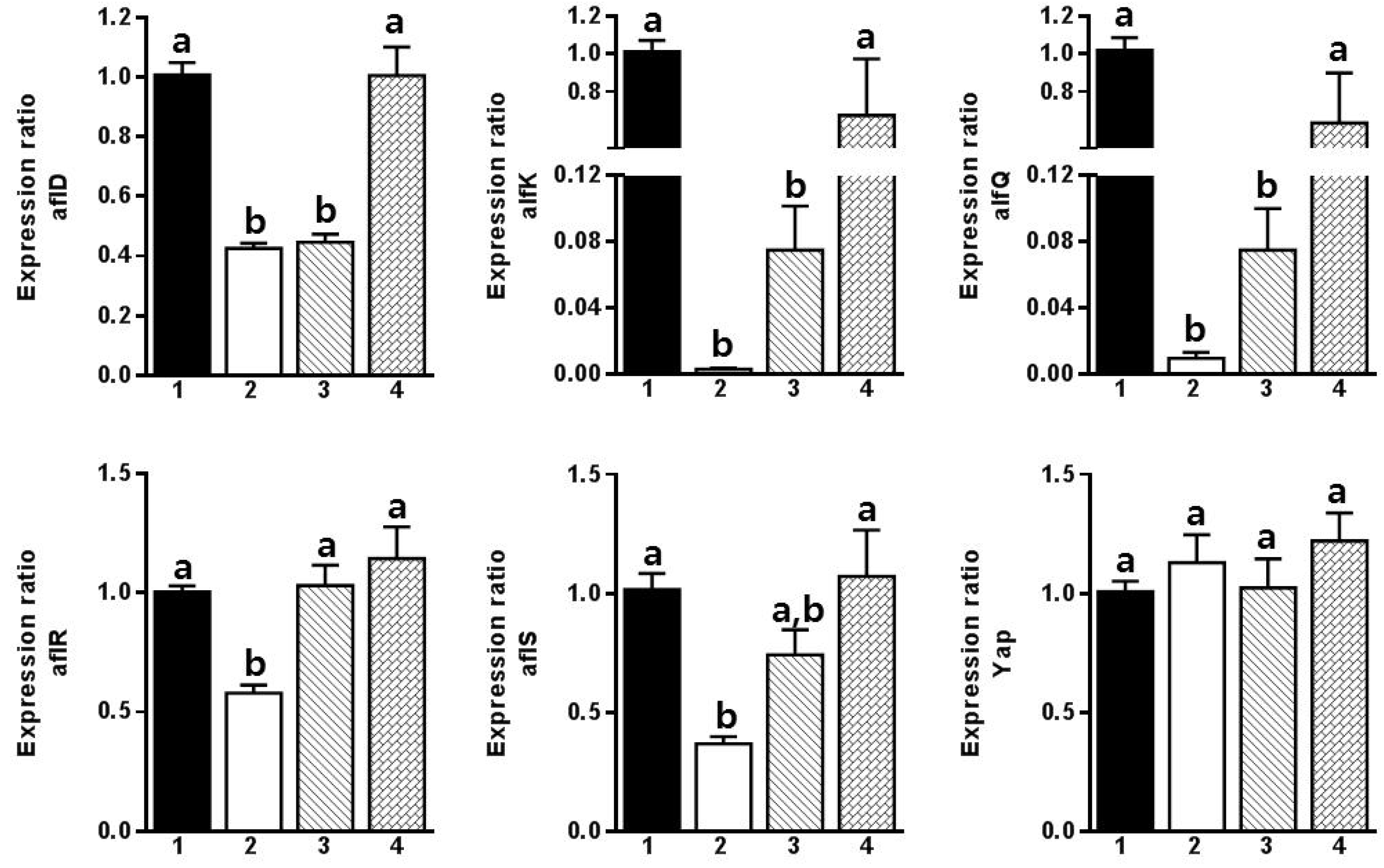
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Aflatoxin Analysis by High-Performance Liquid Chromatography (HPLC)
3.3. Total RNA Isolation and Quantitative Reverse Transcription-PCR (RT-qPCR)
3.4. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, F.; Guclu, H. Aflatoxin regulations in a network of global maize trade. PLoS ONE 2012, 7, e45151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F. Global impacts of aflatoxin in maize: Trade and human health. World Mycotoxin J. 2015, 8, 137–142. [Google Scholar] [CrossRef]
- Bui-Klimke, T.R.; Guclu, H.; Kensler, T.W.; Yuan, J.M.; Wu, F. Aflatoxin regulations and global pistachio trade: Insights from social network analysis. PLoS ONE 2014, 9, e92149. [Google Scholar]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin contmination in the EU feed supply chain: A focus on cereal byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1470985026757&uri=CELEX:32006R1881 (accessed on 26 July 2016).
- Baquião, A.C.; de Oliveira, M.M.; Reis, T.A.; Zorzete, P.; Atayde, D.D.; Corrêa, B. Monitoring and determination of fungi and mycotoxins in stored Brazil nuts. J. Food Prot. 2013, 76, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Asghar, M.A.; Iqbal, J.; Ahmed, A.; Shamsuddin, Z.A. Aflatoxins contamination and prevention in red chillies (Capsicum annuum L.) in Pakistan. Food Addit. Contam. Part B Surveill. 2014, 7, 1–6. [Google Scholar] [CrossRef]
- Lee, J.; Her, J.Y.; Lee, K.G. Reductions of aflatoxins (B1, B2, G1, and G2) in soybean-based model systems. Food Chem. 2015, 189, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Aiko, V.; Edamana, P.; Mehta, A. Decomposition and detoxification of aflatoxin B1 by lactic acid. J. Sci. Food Agric. 2015. [Google Scholar] [CrossRef]
- Yehia, R.S. Aflatoxin detoxification by manganese peroxidase purified from Pleurotus ostreatus. Braz. J. Microbiol. 2014, 45, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Zeinvand-Lorestani, H.; Sabzevari, O.; Setayesh, N.; Amini, M.; Nili-Ahmadabadi, A.; Faramarzi, M.A. Comparative study of in vitro prooxidative properties and genotoxicity induced by aflatoxin B1 and its laccase-mediated detoxification products. Chemosphere 2015, 135, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Pitonzo, R.; Cicero, N.; D’Oca, M.C. Mycotoxin contamination of animal feeding stuff: detoxification by gamma-irradiation and reduction of aflatoxins and ochratoxin A concentration. Food Addit. Contam. Part A Chem. Anal. Expo. Risk Assess. 2014, 31, 2034–2039. [Google Scholar] [CrossRef]
- Luo, X.; Wang, R.; Wang, L.; Li, Y.; Wang, Y.; Chen, Z. Detoxification of aflatoxin in corn flour by ozone. J. Sci. Food Agric. 2014, 94, 2253–2258. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Zhou, T.; Yin, Y.; Xie, M.; Ruan, Z.; Young, J.C. Microbial strategies to control aflatoxins in food and feed. World Mycotoxin J. 2011, 4, 413–424. [Google Scholar] [CrossRef]
- Shetty, P.H.; Jespersen, L. Saccharomyces cerevisiae and lactic acid bacteria as potential decontamination agents. Trends Food Sci. Technol. 2006, 17, 48–55. [Google Scholar] [CrossRef]
- Kachouri, F.; Ksontini, H.; Hamdi, M. Removal of aflatoxin B1 and inhibition of Aspergillus flavus growth by the use of Lactobacillus plantarum on olives. J. Food Prot. 2014, 77, 1760–1767. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.S.; Hernlem, B.J.; Yokoyama, W.; Sarreal, S.B. Intracellular trehalose and sorbitol synergistically promoting cell viability of a biocontrol yeast, Pichia anomala, for aflatoxin reduction. World J. Microbiol. Biotechnol. 2015, 31, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Diao, E.; Shan, C.; Hou, H.; Wang, S.; Li, M.; Dong, H. Structures of the ozonolysis products and ozonolysis pathway of aflatoxin B1 in acetonitrile solution. J. Agric. Food Chem. 2012, 60, 9364–9370. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, J.P.; Macdonald, A.M.C.; Postel, D.; Dijksma, W.T.P.; Dujardin, A.; Placinta, C.M. Pesticide use and mycotoxin production in Fusarium and Aspergillus phytopathogens. Euro. J. Plant Pathol. 1998, 104, 741–751. [Google Scholar]
- Price, C.L.; Parker, J.E.; Warrilow, A.G.; Kelly, D.E.; Kelly, S.L. Azole fungicides-understanding resistance mechanisms in agricultural fungal pathogens. Pest. Manag. Sci. 2015, 71, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Zhang, Y.; Ren, W.; Chen, C. Physiological and biochemical characteristics of laboratory induced mutants of Botrytis cinerea with resistance to fluazinam. Pestic. Biochem. Physiol. 2015, 117, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Kedia, A.; Prakash, B.; Mishra, P.K.; Dubey, N.K. Antifungal and antiaflatoxigenic properties of Cuminum cyminum (L.) seed essential oil and its efficacy as a preservative in stored commodities. Int. J. Food Microbiol. 2014, 168–169, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M. Effect of Carum copticum essential oil on growth and aflatoxin formation by Aspergillus strains. Nat. Prod. Res. 2015, 29, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.M.; Manyes, L.; Luciano, F.; Manes, J.; Meca, G. Influence of the antimicrobial compound allyl isothiocyanate against the Aspergillus parasiticus growth and its aflatoxin production in pizza crust. Food. Chem. Toxicol. 2015, 83, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Mahoney, N.; Campbell, B.C. Inhibition of aflatoxin B1 biosynthesis by piperlongumine isolated from Piper longum L. J. Microbiol. Biotechnol. 2002, 12, 679–682. [Google Scholar]
- Park, E.S.; Bae, I.K.; Kim, H.J.; Lee, S.E. Novel regulation of aflatoxin B1 biosynthesis in Aspergillus flavus by piperonal. Nat. Prod. Res. 2015. [Google Scholar] [CrossRef]
- Reen, R.K.; Singh, J. In vitro and in vivo inhibition of pulmonary cytochrome P450 activities by piperine, a major ingredient of piper species. Indian J. Exp. Biol. 1991, 29, 568–573. [Google Scholar] [PubMed]
- Dinger, J.; Meye, M.R.; Maurer, H.H. In vitro cytochrome P450 inhibition potential of methylene-derived designer drugs studied with a two-cocktail approach. Arch. Toxicol. 2014. [Google Scholar] [CrossRef]
- Guerra, F.Q.; de Araujo, R.S.; de Sousa, J.P.; Pereira Fde, O.; Mendonca-Junior, F.J.; Barbosa-Filho, J.M.; de Oliveira Lima, E. Evaluation of antifungal and mode of action of new coumarin derivative, 7-hydroxy-6-nitro-2H-1-benzopyran-2-one, against Aspergillus spp. Evid. Based Complement. Alternat. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Madhyastha, M.S.; Bhat, R.V. Aspergillus parasiticus growth and aflatoxin production on black and white pepper and the inhibitory action of their chemical constituents. Appl. Environ. Microbiol. 1984, 48, 376–379. [Google Scholar] [PubMed]
- Liu, H.L.; Luo, R.; Chen, X.Q.; Ba, Y.Y.; Zheng, L.; Guo, W.W.; Wu, X. Identification and simultaneous quantification of five alkaloids in Piper longum L. by HPLC-ESI-MS(n) and UPLC-ESI-MS/MS and their application to Piper nigrum L. Food Chem. 2015, 177, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Lv, M.; Xu, H. Piperine: Bioactivities and structural modifications. Mini. Rev. Med. Chem. 2015, 15, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Heydt, M.; Rufer, C.E.; Abdel-Hadi, A.; Magan, N.; Geisen, R. The production of aflatoxin B1 or G1 by Aspergillus parasiticus at various combinations of temperature and water activity is related to the ratio of aflS to aflR expression. Mycotoxin Res. 2010, 26, 241–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Compound | Concentration (μg/mL) | Mycelial Growth Compared with the Control (%) | Aflatoxin Production Compared with the Control (%) | |||
|---|---|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | |||
| Thiabendazole (Positive control) | 10 | 1.3 ± 2.3 | - * | - | - | - |
| 5 | 6.90 ± 11.1 | ND ** | ND | 35.2 ± 2.60 | ND | |
| 1 | 105 ± 26.1 | >150 | >150 | 131 ± 65.7 | ND | |
| 1,3-Benzodioxole | 1000 | 17.0 ± 3.10 | 0.03 ± 0.05 | 0.2 ± 0.4 | 2.0 ± 1.7 | 0.8 ± 1.3 |
| 100 | 84.6 ± 5.90 | 25.2 ± 29.8 | 26.6 ± 17.9 | 0.4 ± 0.3 | 23.5 ± 13.9 | |
| Methylenedioxy phenylacetic acid | 100 | 46.0 ± 19.7 | - | - | - | - |
| 10 | 78.8 ± 18.4 | - | - | - | - | |
| Piperine | 3000 | 133 ± 6.02 | 0.7 ± 0.1 | 1.6 ± 0.2 | 0.3 ± 0.6 | 55.2 ± 16.7 |
| 1000 | 119 ± 6.70 | 39.1 ± 3.10 | 107 ± 27.1 | 21.6 ± 5.54 | 2.4 ± 0.053 | |
| Sesamol | 1000 | 34.9 ± 15.1 | 140 ± 36.0 | >150 | 38.4 ± 1.83 | 40.1 ± 34.7 |
| 100 | 114 ± 8.92 | - | - | - | - | |
| Piperonal | 1000 | 34.8 ± 1.17 | 10.5 ± 1.14 | 100 ± 46.2 | 21.3 ± 1.22 | >150 |
| 100 * | 93.9 ± 5.06 | 45.0 ± 47.1 | 10.5 ± 6.12 | 0.30 ± 0.31 | 23.9 ± 19.8 | |
| Compound | Concentration (μg/mL) | Mycelial Growth Compared with the Control (%) | Aflatoxin Production Compared with the Control (%) | |||
|---|---|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | |||
| Thiabendazole (Positive control) | 10 | 1.3 ± 2.3 | - * | - | - | - |
| 5 | 6.90 ± 11.1 | ND ** | ND | 35.2 ± 2.64 | ND | |
| 1 | 105 ± 26.1 | >150.0 | >150.0 | 131 ± 65.7 | ND | |
| 1-(2-Methylpiperidin-1-yl)-3-phenylprop-2-en-1-one | 1000 | 10.3 ± 17.8 | ND | ND | 35.51 | ND |
| 100 | 64.3 ± 10.1 | ND | ND | 89.18 | ND | |
| 10 | - | 47.0 ± 2.45 | 69.4 ± 5.83 | 47.1 ± 6.08 | ND | |
| 1 | - | 38.0 ± 44.3 | 76.3 ± 55.9 | 122 ± 72.2 | 104 ± 58.0 | |
| 3-(Benzo-1,3-dioxol-5-yl)-1-(2-methylpiperidin-1-yl)prop-2-en-1-one | 1000 | 27.5 ± 5.43 | ND | ND | 64.7 | 55.9 |
| 100 | 84.9 ± 31.8 | 96.4 ± 75.3 | 76.2 ± 55.9 | 122 ± 82.2 | 104 ± 57.9 | |
| 10 | 87.6 ± 18.6 | >150 | >150.00 | >150.00 | >150.00 | |
| Gene | Sequence | |
|---|---|---|
| yap | Forward | 5’ TGCAACCTCTCTACAAGCCG 3’ |
| Reverse | 5’ CCGAAGTCTCGAGAAAGAGCC 3’ | |
| aflR | Forward | 5’ GCACCCTGTCTTCCCTAACA 3’ |
| Reverse | 5’ ACGACCATGCTCAGCAAGTA 3’ | |
| aflS | Forward | 5’ GGAATGGGATGGAGATG 3’ |
| Reverse | 5’ GGAATATGGCTGTAGGAAG 3’ | |
| aflK | Forward | 5’ GAACTGCTTCAGTTGCCGTG 3’ |
| Reverse | 5’ ACGAGGGTTCGTTTCTGGAC 3’ | |
| aflD | Forward | 5’ TCCAGGCACACATGATGGTC 3’ |
| Reverse | 5’ TGTGGATAACGAAGTGCCCC 3’ | |
| aflQ | Forward | 5’ TTAAGGCAGCGGAATACAAG 3’ |
| Reverse | 5’ GACGCCCAAAGCCGAACACAAA 3’ | |
| 18S rRNA | Forward | 5’ ATGGCCGTTCTTAGTTGGTG 3’ |
| Reverse | 5’ GTACAAAGGGCAGGGACGTA 3’ | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, Y.-S.; Choi, W.-S.; Park, E.-S.; Bae, I.K.; Choi, S.-D.; Paek, O.; Kim, S.-H.; Chun, H.S.; Lee, S.-E. Antifungal and Antiaflatoxigenic Methylenedioxy-Containing Compounds and Piperine-Like Synthetic Compounds. Toxins 2016, 8, 240. https://doi.org/10.3390/toxins8080240
Moon Y-S, Choi W-S, Park E-S, Bae IK, Choi S-D, Paek O, Kim S-H, Chun HS, Lee S-E. Antifungal and Antiaflatoxigenic Methylenedioxy-Containing Compounds and Piperine-Like Synthetic Compounds. Toxins. 2016; 8(8):240. https://doi.org/10.3390/toxins8080240
Chicago/Turabian StyleMoon, Young-Sun, Won-Sik Choi, Eun-Sil Park, In Kyung Bae, Sung-Deuk Choi, Ockjin Paek, Sheen-Hee Kim, Hyang Sook Chun, and Sung-Eun Lee. 2016. "Antifungal and Antiaflatoxigenic Methylenedioxy-Containing Compounds and Piperine-Like Synthetic Compounds" Toxins 8, no. 8: 240. https://doi.org/10.3390/toxins8080240
APA StyleMoon, Y.-S., Choi, W.-S., Park, E.-S., Bae, I. K., Choi, S.-D., Paek, O., Kim, S.-H., Chun, H. S., & Lee, S.-E. (2016). Antifungal and Antiaflatoxigenic Methylenedioxy-Containing Compounds and Piperine-Like Synthetic Compounds. Toxins, 8(8), 240. https://doi.org/10.3390/toxins8080240










