Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas aeruginosa
Abstract
:1. Introduction
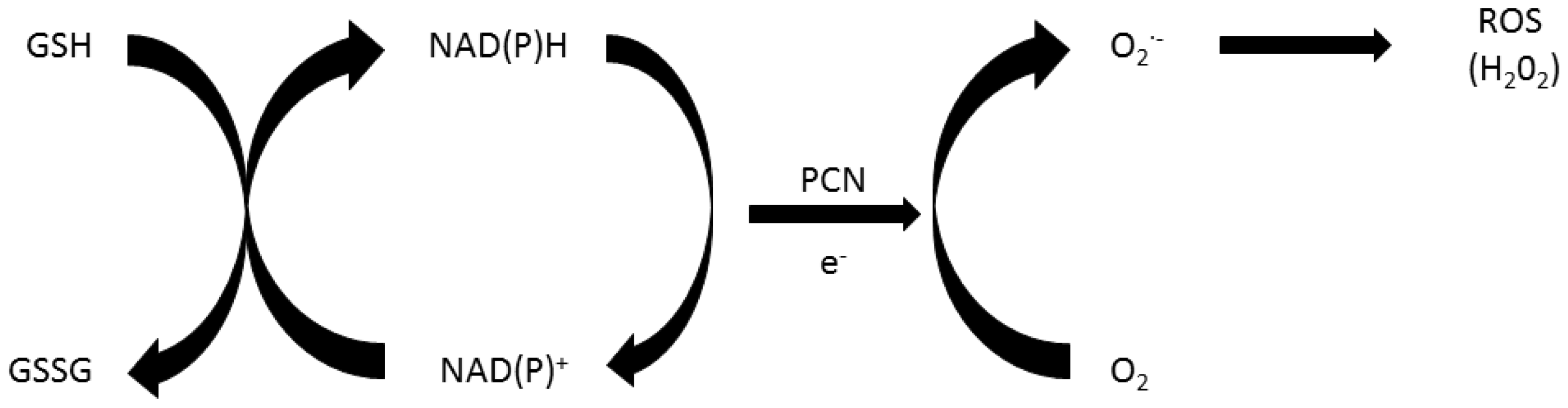
2. Role of Oxidative Stress in Pyocyanin’s Toxicity
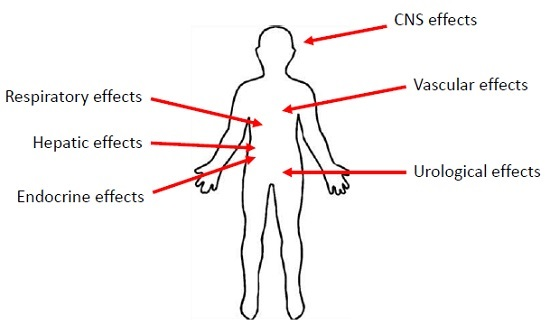
3. Manifestations of PCN Exposure
3.1. Inflammatory Effects of PCN
3.2. The Effects of PCN on the Respiratory System
3.3. The Effects of PCN on the Urological Systems
3.4. The Effects of PCN on the Central Nervous System
3.5. The Effects of PCN on the Vascular System
3.6. The Effects of PCN on the Hepatic System
3.7. Endocrine Effects of PCN
3.8. Role of PCN in Normal Monitored Cellular Activities
4. Future Directions
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Establishment of Pseudomonas aeruginosa infection: Lessons from a versatile opportunist. Microbes Infect. 2000, 2, 1051–1060. [Google Scholar] [CrossRef]
- Mittal, R.; Aggarwal, S.; Sharma, S.; Chhibber, S.; Harjai, K. Urinary tract infections caused by Pseudomonas aeruginosa: A minireview. J. Infect. Pub. Health 2009, 2, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Polovina, M.; Potpara, T.; Milosevic, I.; Stepanovic, J.; Jovanovic, M.; Pavlovic, M. Mitral valve endocarditis caused by Pseudomonas aeruginosa: A case report. J. Infect. Dev. Ctries. 2014, 8, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Seyman, D.; Inan, D.; Sepin Ozen, N.; Ogunc, D. An outbreak of Pseudomonas aeruginosa infective endocarditis subsequent to coronary angiography. Rev. Chil. Infectol. 2014, 31, 268–273. [Google Scholar]
- Setoguchi, M.; Iwasawa, E.; Hashimoto, Y.; Isobe, M. A patient with infective endocarditis caused by community-acquired Pseudomonas aeruginosa infection. Intern. Med. 2013, 52, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Archer, G.; Fekety, F.R.; Supena, R. Pseudomonas aeruginosa endocarditis in drug addicts. Am. Heart J. 1974, 88, 570–578. [Google Scholar] [CrossRef] [Green Version]
- Chuang, Y.; Chang, W.; Lu, C.; Wu, H.; Chang, H. Pseudomonas aeruginosa central nervous system infections: Analysis of clinical features of 16 adult patients. Zhonghua Yi Xue Za Zhi 1999, 62, 300–307. [Google Scholar] [PubMed]
- Turner, J.M.; Messenger, A.J. Occurrence, biochemistry and physiology of phenazine pigment production. Adv. Microb. Physiol. 1986, 27, 211–275. [Google Scholar] [PubMed]
- Oliver, A.; Cantón, R.; Campo, P.; Baquero, F.; Blázquez, J. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 2000, 288, 1251–1253. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, Y.Q.; Reszka, K.J.; Britigan, B.E. Direct oxidation of 2′,7′-dichlorodihydrofluorescein by pyocyanin and other redox-active compounds independent of reactive oxygen species production. Free Radic. Biol. Med. 2004, 36, 90–100. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, Y.Q.; Reszka, K.J.; Spitz, D.R.; Denning, G.M.; Britigan, B.E. Pseudomonas aeruginosa pyocyanin directly oxidizes glutathione and decreases its levels in airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L94–L103. [Google Scholar] [CrossRef] [PubMed]
- Reszka, K.J.; O’Malley, Y.; McCormick, M.L.; Denning, G.M.; Britigan, B.E. Oxidation of pyocyanin, a cytotoxic product from Pseudomonas aeruginosa, by microperoxidase 11 and hydrogen peroxide. Free Radic. Biol. Med. 2004, 36, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Dowling, R.B. Pseudomonas aeruginosa and other related species. Thorax 1998, 53, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.W.; Hassett, D.J.; Ran, H.; Kong, F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 2004, 10, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Fothergill, J.L.; Panagea, S.; Hart, C.A.; Walshaw, M.J.; Pitt, T.L.; Winstanley, C. Widespread pyocyanin over-production among isolates of a cystic fibrosis epidemic strain. BMC Microbiol. 2007, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.S.; Rowe, J.J. Antibiotic action of pyocyanin. Antimicrob. Agents Chemother. 1981, 20, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Gardner, P.R. Superoxide production by the mycobacterial and pseudomonad quinoid pigments phthiocol and pyocyanine in human lung cells. Arch. Biochem. Biophys. 1996, 333, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Friedheim, E.A. Pyocyanine, an accessory respiratory enzyme. J. Exp. Med. 1931, 54, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Van Delden, C.; Iglewski, B.H. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 1998, 4, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Reimer, A.; Edvaller, B.; Johansson, B. Concentrations of the Pseudomonas aeruginosa toxin pyocyanin in human ear secretions. Acta Otolaryngol. Suppl. 2000, 543, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Sykes, D.A.; Watson, D.; Rutman, A.; Taylor, G.W.; Cole, P.J. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect. Immun 1988, 56, 2515–2517. [Google Scholar] [PubMed]
- Cruickshank, C.N.; Lowbury, E.J. The effect of pyocyanin on human skin cells and leucocytes. Br. J. Exp. Pathol. 1953, 34, 583–587. [Google Scholar] [PubMed]
- Lau, G.W.; Ran, H.; Kong, F.; Hassett, D.J.; Mavrodi, D. Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect. Immun 2004, 72, 4275–4278. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Bu, Y.; Suga, H. Induction and inhibition of Pseudomonas aeruginosa quorum sensing by synthetic autoinducer analogs. Chem. Biol. 2003, 10, 81–89. [Google Scholar] [CrossRef]
- Britigan, B.E.; Roeder, T.L.; Rasmussen, G.T.; Shasby, D.M.; McCormick, M.L.; Cox, C.D. Interaction of the Pseudomonas aeruginosa secretory products pyocyanin and pyochelin generates hydroxyl radical and causes synergistic damage to endothelial cells. Implications for Pseudomonas-associated tissue injury. J. Clin. Investig. 1992, 90, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Denning, G.M.; Wollenweber, L.A.; Railsback, M.A.; Cox, C.D.; Stoll, L.L.; Britigan, B.E. Pseudomonas pyocyanin increases interleukin-8 expression by human airway epithelial cells. Infect. Immun 1998, 66, 5777–5784. [Google Scholar] [PubMed]
- McDermott, C.; Chess-Williams, R.; Mills, K.A.; Kang, S.H.; Farr, S.E.; Grant, G.D.; Perkins, A.V.; Davey, A.K.; Anoopkumar-Dukie, S. Alterations in acetylcholine, PGE2 and IL6 release from urothelial cells following treatment with pyocyanin and lipopolysaccharide. Toxicol. Vitro Intern. J. Publ. Assoc. BIBRA 2013, 27, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- McDermott, C.; Chess-Williams, R.; Grant, G.D.; Perkins, A.V.; McFarland, A.J.; Davey, A.K.; Anoopkumar-Dukie, S. Effects of Pseudomonas aeruginosa virulence factor pyocyanin on human urothelial cell function and viability. J. Urol. 2012, 187, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Hempenstall, A.; Grant, G.D.; Anoopkumar-Dukie, S.; Johnson, P.J. Pyocyanin inhibits both nitric oxide-dependent and independent relaxation in porcine coronary arteries. Clin. Exp. Pharmacol. Physiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- McFarland, A.J.; Anoopkumar-Dukie, S.; Perkins, A.V.; Davey, A.K.; Grant, G.D. Inhibition of autophagy by 3-methyladenine protects 1321N1 astrocytoma cells against pyocyanin- and 1-hydroxyphenazine-induced toxicity. Arch. Toxicol. 2012, 86, 275–284. [Google Scholar] [CrossRef] [PubMed]
- McFarland, A.J.; Grant, G.D.; Perkins, A.V.; Flegg, C.; Davey, A.K.; Allsopp, T.J.; Renshaw, G.; Kavanagh, J.; McDermott, C.M.; Anoopkumar-Dukie, S. Paradoxical role of 3-methyladenine in pyocyanin-induced toxicity in 1321N1 astrocytoma and SH-SY5Y neuroblastoma cells. Intern. J. Toxicol. 2013, 32, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Muller, M. Pyocyanin induces oxidative stress in human endothelial cells and modulates the glutathione redox cycle. Free Radic. Biol. Med. 2002, 33, 1527–1533. [Google Scholar] [CrossRef]
- Cheluvappa, R.; Jamieson, H.A.; Hilmer, S.N.; Muller, M.; Le Couteur, D.G. The effect of Pseudomonas aeruginosa virulence factor, pyocyanin, on the liver sinusoidal endothelial cell. J. Gastroenterol. Hepatol. 2007, 22, 1350–1351. [Google Scholar] [CrossRef] [PubMed]
- Muller, M. Premature cellular senescence induced by pyocyanin, a redox-active Pseudomonas aeruginosa toxin. Free Radic. Biol. Med. 2006, 41, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.; Thornalley, P.J. Free radical production from the aerobic oxidation of reduced pyridine nucleotides catalysed by phenazine derivatives. Biochim. Biophys. Acta 1983, 724, 456–464. [Google Scholar] [CrossRef]
- Britigan, B.E.; Railsback, M.A.; Cox, C.D. The Pseudomonas aeruginosa secretory product pyocyanin inactivates alpha1 protease inhibitor: Implications for the pathogenesis of cystic fibrosis lung disease. Infect. Immun 1999, 67, 1207–1212. [Google Scholar] [PubMed]
- Ran, H.; Hassett, D.J.; Lau, G.W. Human targets of Pseudomonas aeruginosa pyocyanin. Proc. Natl. Acad. Sci. USA 2003, 100, 14315–14320. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, Y.Q.; Abdalla, M.Y.; McCormick, M.L.; Reszka, K.J.; Denning, G.M.; Britigan, B.E. Subcellular localization of Pseudomonas pyocyanin cytotoxicity in human lung epithelial cells. Am. J. Phys. Lung Cell. Mol. Phys. 2003, 284, L420–L430. [Google Scholar]
- Rada, B.; Leto, T.L. Pyocyanin effects on respiratory epithelium: Relevance in Pseudomonas aeruginosa airway infections. Trends Microbiol. 2013, 21, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Manefield, M. Pyocyanin promotes extracellular DNA release in Pseudomonas aeruginosa. PLoS ONE 2012, 7, e46718. [Google Scholar] [CrossRef] [PubMed]
- Suntres, Z.E.; Omri, A.; Shek, P.N. Pseudomonas aeruginosa-induced lung injury: Role of oxidative stress. Microbial Pathog. 2002, 32, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Rada, B.; Gardina, P.; Myers, T.G.; Leto, T.L. Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal Immunol. 2011, 4, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Bannister, J.V.; Bannister, W.H.; Rotilio, G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit. Rev. Biochem. 1987, 22, 111–180. [Google Scholar] [CrossRef] [PubMed]
- Heffner, J.E.; Repine, J.E. Pulmonary strategies of antioxidant defense. Am. Rev. Respir. Dis. 1989, 140, 531–554. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, D.A.; Forman, H.J. Cellular glutathione and thiols metabolism. Biochem. Pharmacol. 2002, 64, 1019–1026. [Google Scholar] [CrossRef]
- Powis, G.; Montfort, W.R. Properties and biological activities of thioredoxins. Ann. Rev. Biophys. Biomol. Struct. 2001, 30, 421–455. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Gloyne, L.S.; Grant, G.D.; Perkins, A.V.; Powell, K.L.; McDermott, C.M.; Johnson, P.V.; Anderson, G.J.; Kiefel, M.; Anoopkumar-Dukie, S. Pyocyanin-induced toxicity in A549 respiratory cells is causally linked to oxidative stress. Toxicol. Vitro Intern. J. Publ. Assoc. BIBRA 2011, 25, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.; Davey, A.K.; Perkins, A.V.; Grant, G.D.; McFarland, A.J.; McDermott, C.M.; Anoopkumar-Dukie, S. ERK1/2 activation modulates pyocyanin-induced toxicity in A549 respiratory epithelial cells. Chem-Biol. Interact. 2014, 208, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Lauredo, I.T.; Sabater, J.R.; Ahmed, A.; Botvinnikova, Y.; Abraham, W.M. Mechanism of pyocyanin- and 1-hydroxyphenazine-induced lung neutrophilia in sheep airways. J. Appl. Physiol. 1998, 85, 2298–2304. [Google Scholar] [PubMed]
- Cheluvappa, R.; Cogger, V.C.; Kwun, S.Y.; O’Reilly, J.N.; Le Couteur, D.G.; Hilmer, S.N. Liver sinusoidal endothelial cells and acute non-oxidative hepatic injury induced by Pseudomonas aeruginosa pyocyanin. Intern. J. Exp. Pathol. 2008, 89, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Leidal, K.G.; Munson, K.L.; Denning, G.M. Small molecular weight secretory factors from Pseudomonas aeruginosa have opposite effects on IL-8 and rantes expression by human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2001, 25, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Nutman, J.; Berger, M.; Chase, P.A.; Dearborn, D.G.; Miller, K.M.; Waller, R.L.; Sorensen, R.U. Studies on the mechanism of t cell inhibition by the Pseudomonas aeruginosa phenazine pigment pyocyanine. J. Immunol. 1987, 138, 3481–3487. [Google Scholar] [PubMed]
- Ulmer, A.J.; Pryjma, J.; Tarnok, Z.; Ernst, M.; Flad, H.D. Inhibitory and stimulatory effects of Pseudomonas aeruginosa pyocyanine on human T and B lymphocytes and human monocytes. Infect. Immun. 1990, 58, 808–815. [Google Scholar] [PubMed]
- Prince, L.R.; Bianchi, S.M.; Vaughan, K.M.; Bewley, M.A.; Marriott, H.M.; Walmsley, S.R.; Taylor, G.W.; Buttle, D.J.; Sabroe, I.; Dockrell, D.H.; et al. Subversion of a lysosomal pathway regulating neutrophil apoptosis by a major bacterial toxin, pyocyanin. J. Immunol. 2008, 180, 3502–3511. [Google Scholar] [PubMed]
- Gibson, J.; Sood, A.; Hogan, D.A. Pseudomonas aeruginosa-Candida albicans interactions: Localization and fungal toxicity of a phenazine derivative. Appl. Environ. Microbiol. 2009, 75, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Mavrodi, D.V.; Bonsall, R.F.; Delaney, S.M.; Soule, M.J.; Phillips, G.; Thomashow, L.S. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J. Bacteriol. 2001, 183, 6454–6465. [Google Scholar] [PubMed]
- Chai, W.; Zhang, J.; Zhu, Z.; Liu, W.; Pan, D.; Li, Y.; Chen, B. Pyocyanin from Pseudomonas induces IL-8 production through the PKC and NF-κB pathways in U937 cells. Mol. Med. Rep. 2013, 8, 1404–1410. [Google Scholar] [PubMed]
- Chai, W.; Zhang, J.; Duan, Y.; Pan, D.; Liu, W.; Li, Y.; Yan, X.; Chen, B. Pseudomonas pyocyanin stimulates IL-8 expression through MAPK and NF-κB pathways in differentiated U937 cells. BMC Microbiol. 2014, 14, 1. [Google Scholar]
- Usher, L.R.; Lawson, R.A.; Geary, I.; Taylor, C.J.; Bingle, C.D.; Taylor, G.W.; Whyte, M.K. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: A potential mechanism of persistent infection. J. Immunol. 2002, 168, 1861–1868. [Google Scholar] [PubMed]
- Allen, L.; Dockrell, D.H.; Pattery, T.; Lee, D.G.; Cornelis, P.; Hellewell, P.G.; Whyte, M.K. Pyocyanin production by Pseudomonas aeruginosa induces neutrophil apoptosis and impairs neutrophil-mediated host defenses in vivo. J. Immunol. 2005, 174, 3643–3649. [Google Scholar] [CrossRef] [PubMed]
- Manago, A.; Becker, K.A.; Carpinteiro, A.; Wilker, B.; Soddemann, M.; Seitz, A.P.; Edwards, M.J.; Grassmé, H.; Szabo, I.; Gulbins, E. Pseudomonas aeruginosa pyocyanin induces neutrophil death via mitochondrial reactive oxygen species and mitochondrial acid sphingomyelinase. Antioxid. Redox signal. 2015, 22, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.C.; Poncz, L.; Klinger, J.D.; Stern, R.C.; Tomashefski, J.F., Jr.; Dearborn, D.G. Biochemical and pathologic evidence for proteolytic destruction of lung connective tissue in cystic fibrosis. Am. Rev. Respir. Dis. 1985, 132, 529–535. [Google Scholar] [PubMed]
- Lyczak, J.B.; Cannon, C.L.; Pier, G.B. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 2002, 15, 194–222. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.; Gibson, R.L.; McNamara, S.; Emerson, J.; Burns, J.L.; Castile, R.; Hiatt, P.; McCoy, K.; Wilson, C.B.; Inglis, A.; et al. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr. Pulmonol. 2001, 32, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.; Fridovich, I. Mechanism of the antibiotic action pyocyanine. J. Bacteriol. 1980, 141, 156–163. [Google Scholar] [PubMed]
- Gibson, R.L.; Burns, J.L.; Ramsey, B.W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Criti. Care Med. 2003, 168, 918–951. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.M.; Prince, L.R.; McPhillips, K.; Allen, L.; Marriott, H.M.; Taylor, G.W.; Hellewell, P.G.; Sabroe, I.; Dockrell, D.H.; Henson, P.W.; et al. Impairment of apoptotic cell engulfment by pyocyanin, a toxic metabolite of Pseudomonas aeruginosa. Am. J. Respir. Criti. care Med. 2008, 177, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Schall, T.J.; Bacon, K.; Toy, K.J.; Goeddel, D.V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature 1990, 347, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.C.; Klepac-Ceraj, V.; Lorenzi, M.M.; Grotzinger, H.; Martin, T.R.; Newman, D.K. Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity. Am. J. Respir. Cell Mol. Biol. 2012, 47, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Look, D.C.; Stoll, L.L.; Romig, S.A.; Humlicek, A.; Britigan, B.E.; Denning, G.M. Pyocyanin and its precursor phenazine-1-carboxylic acid increase IL-8 and intercellular adhesion molecule-1 expression in human airway epithelial cells by oxidant-dependent mechanisms. J. Immunol. 2005, 175, 4017–4023. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Kuang, Z.; Walling, B.E.; Bhatia, S.; Sivaguru, M.; Chen, Y.; Gaskins, H.R.; Lau, G.W. Pseudomonas aeruginosa pyocyanin causes airway goblet cell hyperplasia and metaplasia and mucus hypersecretion by inactivating the transcriptional factor FOXA2. Cell. Microbiol. 2012, 14, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Munro, N.; Barker, A.; Rutman, A.; Taylor, G.; Watson, D.; McDonald-Gibson, W.; Towart, R.; Taylor, W.; Wilson, R.; Cole, P. Effect of pyocyanin and 1-hydroxyphenazine on in vivo tracheal mucus velocity. J. Appl. Physiol. 1989, 67, 316–323. [Google Scholar] [PubMed]
- O’Malley, Y.Q.; Reszka, K.J.; Rasmussen, G.T.; Abdalla, M.Y.; Denning, G.M.; Britigan, B.E. The Pseudomonas secretory product pyocyanin inhibits catalase activity in human lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L1077–L1086. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, F.Y.; Al-Shibib, A.S.; Khammas, K.M.; Taher, R. Pyocyanin preparation from Pseudomonas aeruginosa isolated from heterogeneous clinical materials. Folia Microbiol. 1986, 31, 215–219. [Google Scholar] [CrossRef]
- Muller, M.; Li, Z.; Maitz, P.K.M. Pseudomonas pyocyanin inhibits wound repair by inducing premature cellular senescence: Role for p38 mitogen-activated protein kinase. Burns J. Intern. Soc. Burn Inj. 2009, 35, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Gryglewski, R.J.; Zembowicz, A.; Salvemini, D.; Taylor, G.W.; Vane, J.R. Modulation of the pharmacological actions of nitrovasodilators by methylene blue and pyocyanin. Br. J. Pharmacol. 1992, 106, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Poore, S.; Berry, B.; Eidson, D.; McKie, K.T.; Harris, R.A. Evidence of vascular endothelial dysfunction in young patients with cystic fibrosis. Chest 2013, 143, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Lin, T.Y.; Wang, C.H. Community-acquired Pseudomonas aeruginosa sepsis in previously healthy infants and children: Analysis of forty-three episodes. Pediatr. Infect. Dis. J. 2002, 21, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Boquist, L.; Bostrom, T. Alloxan effects on mitochondria in vitro, studied with regard to inhibition of mitochondrial aconitase. Diabete Metab. 1985, 11, 232–237. [Google Scholar] [PubMed]
- Boquist, L.; Ericsson, I.; Lorentzon, R.; Nelson, L. Alterations in mitochondrial aconitase activity and respiration, and in concentration of citrate in some organs of mice with experimental or genetic diabetes. FEBS Lett. 1985, 183, 173–176. [Google Scholar] [CrossRef]
- Cuestas, R.; Dixit, P.K. Citrate metabolic enzymes in alloxan diabetes. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. 1973, 142, 889–895. [Google Scholar] [CrossRef]
- Cuestas, R.; Dixit, P.K. Oxidation, accumulation, and turnover of citrate in normal and diabetic rats. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. 1974, 147, 181–187. [Google Scholar] [CrossRef]
- Ceriello, A.; Motz, E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Hassett, D.J.; Charniga, L.; Bean, K.; Ohman, D.E.; Cohen, M.S. Response of Pseudomonas aeruginosa to pyocyanin: Mechanisms of resistance, antioxidant defenses, and demonstration of a manganese-cofactored superoxide dismutase. Infect. Immun 1992, 60, 328–336. [Google Scholar] [PubMed]
- Sun-Wada, G.H.; Toyomura, T.; Murata, Y.; Yamamoto, A.; Futai, M.; Wada, Y. The a3 isoform of V-ATPase regulates insulin secretion from pancreatic beta-cells. J. Cell Sci. 2006, 119, 4531–4540. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Young, L.; Chen, Y.; Ran, H.; Meyers, M.; Joseph, P.; Cho, Y.-H.; Hasset, D.J.; Lau, G.W. Pseudomonas aeruginosa pyocyanin inactivates lung epithelial vacuolar ATPase-dependent cystic fibrosis transmembrane conductance regulator expression and localization. Cell. Microbiol. 2006, 8, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Mahajan-Miklos, S.; Tan, M.-W.; Rahme, L.G.; Ausubel, F.M. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa–Caenorhabditis elegans pathogenesis model. Cell 1999, 96, 47–56. [Google Scholar] [CrossRef]
- Melo, J.A.; Ruvkun, G. Inactivation of conserved C. elegans genes engages pathogen-and xenobiotic-associated defenses. Cell 2012, 149, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, M.W.; Nargund, A.M.; Kirienko, N.V.; Gillis, R.; Fiorese, C.J.; Haynes, C.M. Mitochondrial upr-regulated innate immunity provides resistance to pathogen infection. Nature 2014, 516, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Samuel, B.S.; Breen, P.C.; Ruvkun, G. Caenorhabditis elegans pathways that surveil and defend mitochondria. Nature 2014, 508, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.C.; O’Loughlin, C.T.; Zhang, Z.; Siryaporn, A.; Silpe, J.E.; Bassler, B.L.; Semmelhack, M.F. Development of potent inhibitors of pyocyanin production in Pseudomonas aeruginosa. J. Med. Chem. 2015, 58, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, B.; Galloway, W.R.; Wright, M.; Ibbeson, B.M.; Hodgkinson, J.T.; O’Connell, K.M.; Bartolucci, N.; Della Valle, M.; Welch, M.; Spring, D.R. Inhibition of the production of the Pseudomonas aeruginosa virulence factor pyocyanin in wild-type cells by quorum sensing autoinducer-mimics. Org. Biomol. Chem. 2012, 10, 8452–8464. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Ikeda, T.; Takiguchi, N.; Kuroda, A.; Ohtake, H.; Kato, J. Inhibition of quorum sensing in Pseudomonas aeruginosa by N-acyl cyclopentylamides. Appl. Environ. Microbiol. 2007, 73, 3183–3188. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, C.T.; Miller, L.C.; Siryaporn, A.; Drescher, K.; Semmelhack, M.F.; Bassler, B.L. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. USA 2013, 110, 17981–17986. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, B.; Gal, B.; Galloway, W.R.; Hodgkinson, J.T.; Ibbeson, B.M.; Tan, Y.S.; Welch, M.; Spring, D.R. Discovery of an inhibitor of the production of the Pseudomonas aeruginosa virulence factor pyocyanin in wild-type cells. Beilsten J. Org. Chem. 2016, 12, 1428–1433. [Google Scholar] [CrossRef]
- Ugurlu, A.; Karahasan Yagci, A.; Ulusoy, S.; Aksu, B.; Bosgelmez-Tinaz, G. Phenolic compounds affect production of pyocyanin, swarming motility and biofilm formation of Pseudomonas aeruginosa. Asian Pac. J. Trop. Biomed. 2016, 6, 698–701. [Google Scholar] [CrossRef]
- Ho Sui, S.J.; Lo, R.; Fernandes, A.R.; Caulfield, M.D.G.; Lerman, J.A.; Xie, L.; Bourne, P.E.; Baillie, D.L.; Brinkman, F.S.L. Raloxifene attenuates Pseudomonas aeruginosa pyocyanin production and virulence. Intern. J. Antimicrob. Agents 2012, 40, 246–251. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, S.; McDermott, C.; Anoopkumar-Dukie, S.; McFarland, A.J.; Forbes, A.; Perkins, A.V.; Davey, A.K.; Chess-Williams, R.; Kiefel, M.J.; Arora, D.; et al. Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas aeruginosa. Toxins 2016, 8, 236. https://doi.org/10.3390/toxins8080236
Hall S, McDermott C, Anoopkumar-Dukie S, McFarland AJ, Forbes A, Perkins AV, Davey AK, Chess-Williams R, Kiefel MJ, Arora D, et al. Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas aeruginosa. Toxins. 2016; 8(8):236. https://doi.org/10.3390/toxins8080236
Chicago/Turabian StyleHall, Susan, Catherine McDermott, Shailendra Anoopkumar-Dukie, Amelia J. McFarland, Amanda Forbes, Anthony V. Perkins, Andrew K. Davey, Russ Chess-Williams, Milton J. Kiefel, Devinder Arora, and et al. 2016. "Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas aeruginosa" Toxins 8, no. 8: 236. https://doi.org/10.3390/toxins8080236
APA StyleHall, S., McDermott, C., Anoopkumar-Dukie, S., McFarland, A. J., Forbes, A., Perkins, A. V., Davey, A. K., Chess-Williams, R., Kiefel, M. J., Arora, D., & Grant, G. D. (2016). Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas aeruginosa. Toxins, 8(8), 236. https://doi.org/10.3390/toxins8080236