Effects of Zinc Chelators on Aflatoxin Production in Aspergillus parasiticus
Abstract
:1. Introduction
2. Results
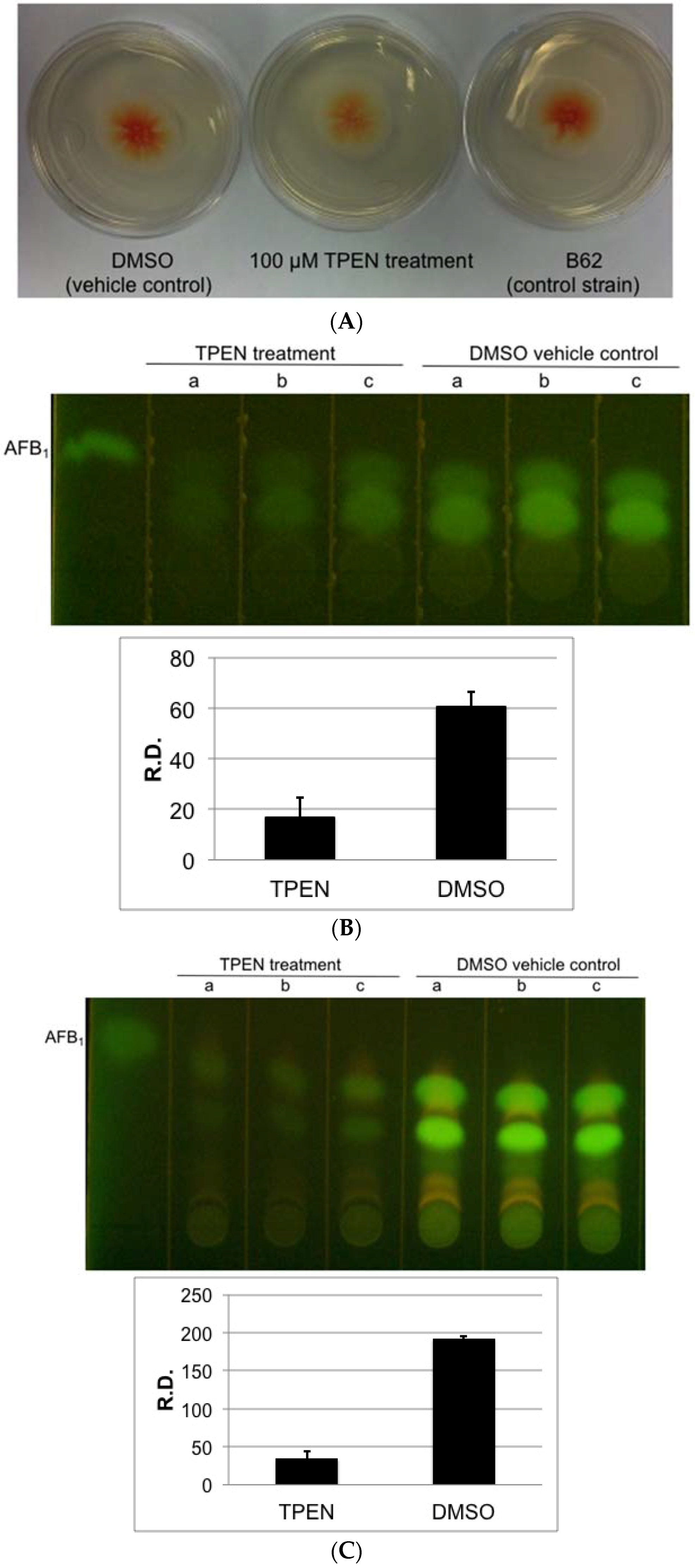
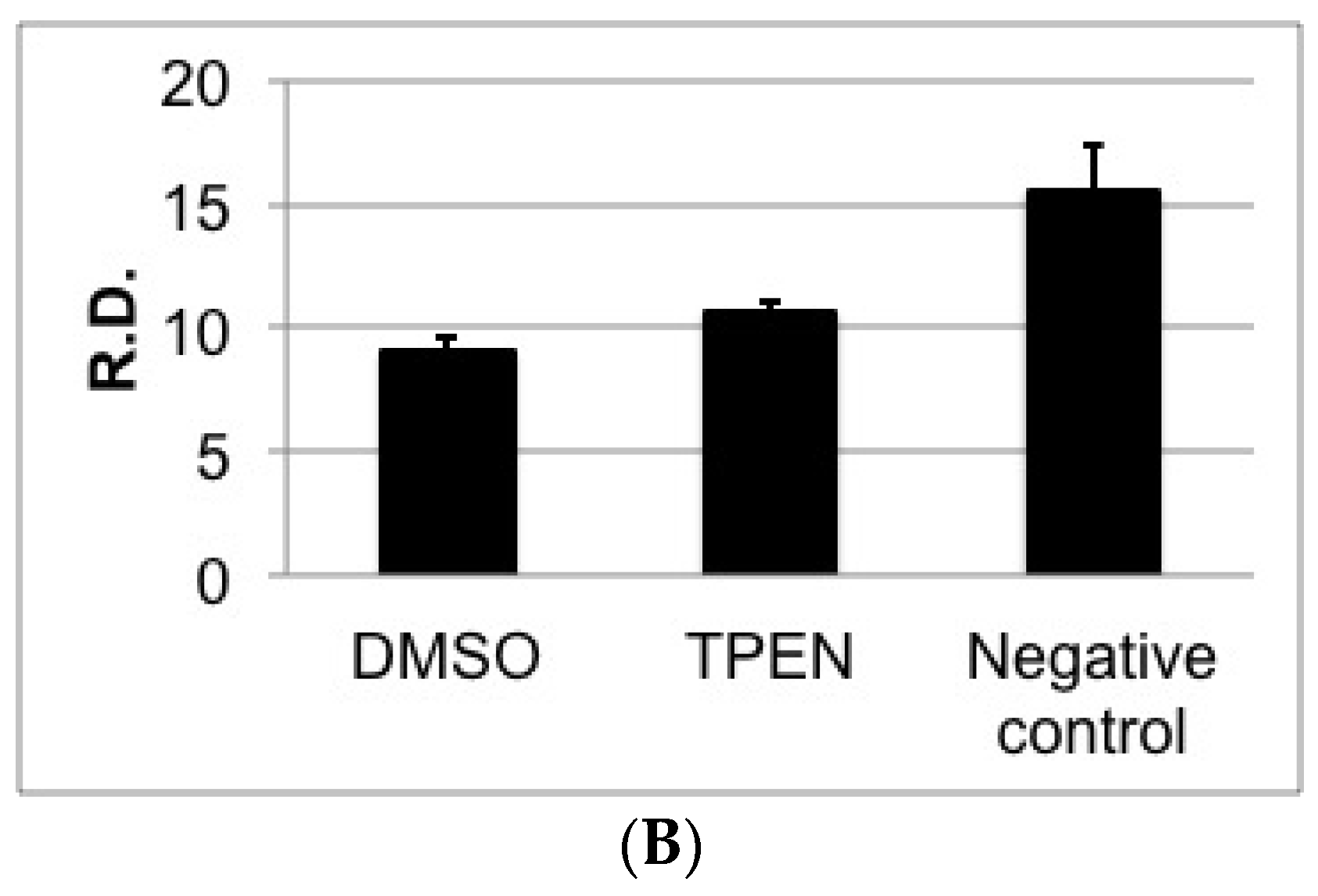
2.1. Effect of Zinc Chelators, TPEN and DMPS on Aflatoxin Accumulation and Growth of A. parasiticus
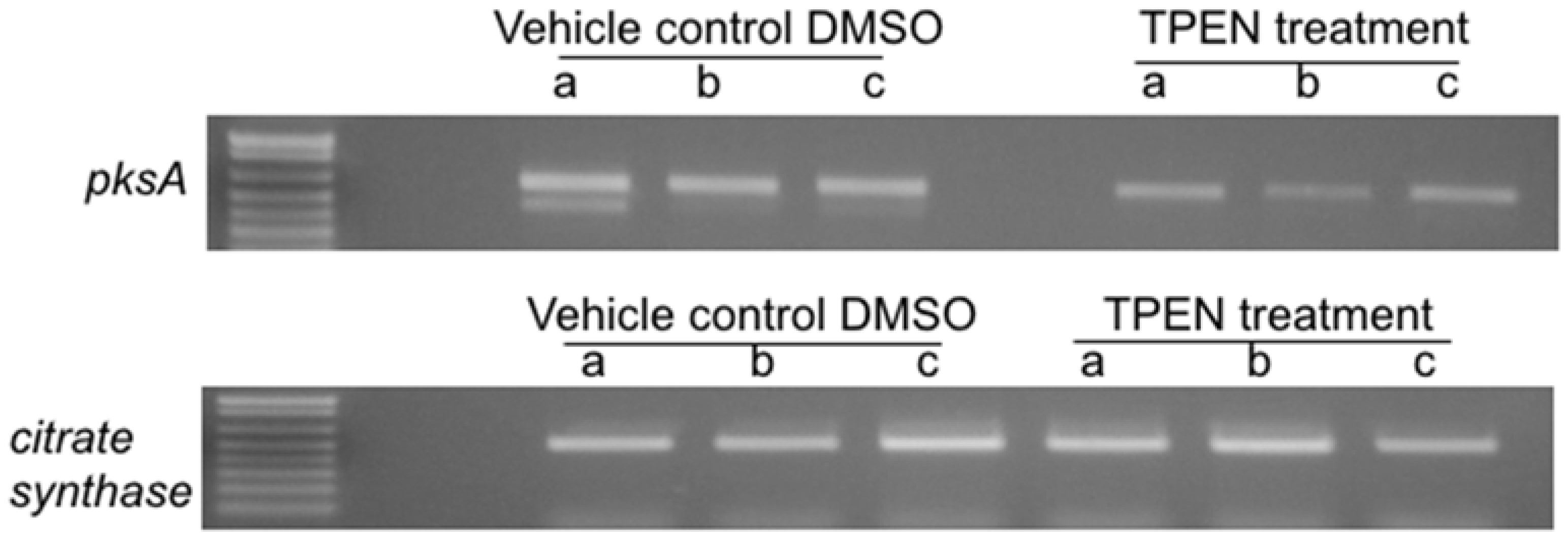
2.2. Effect of TPEN on Aflatoxin Transcript Accumulation in A. parasiticus SU-1
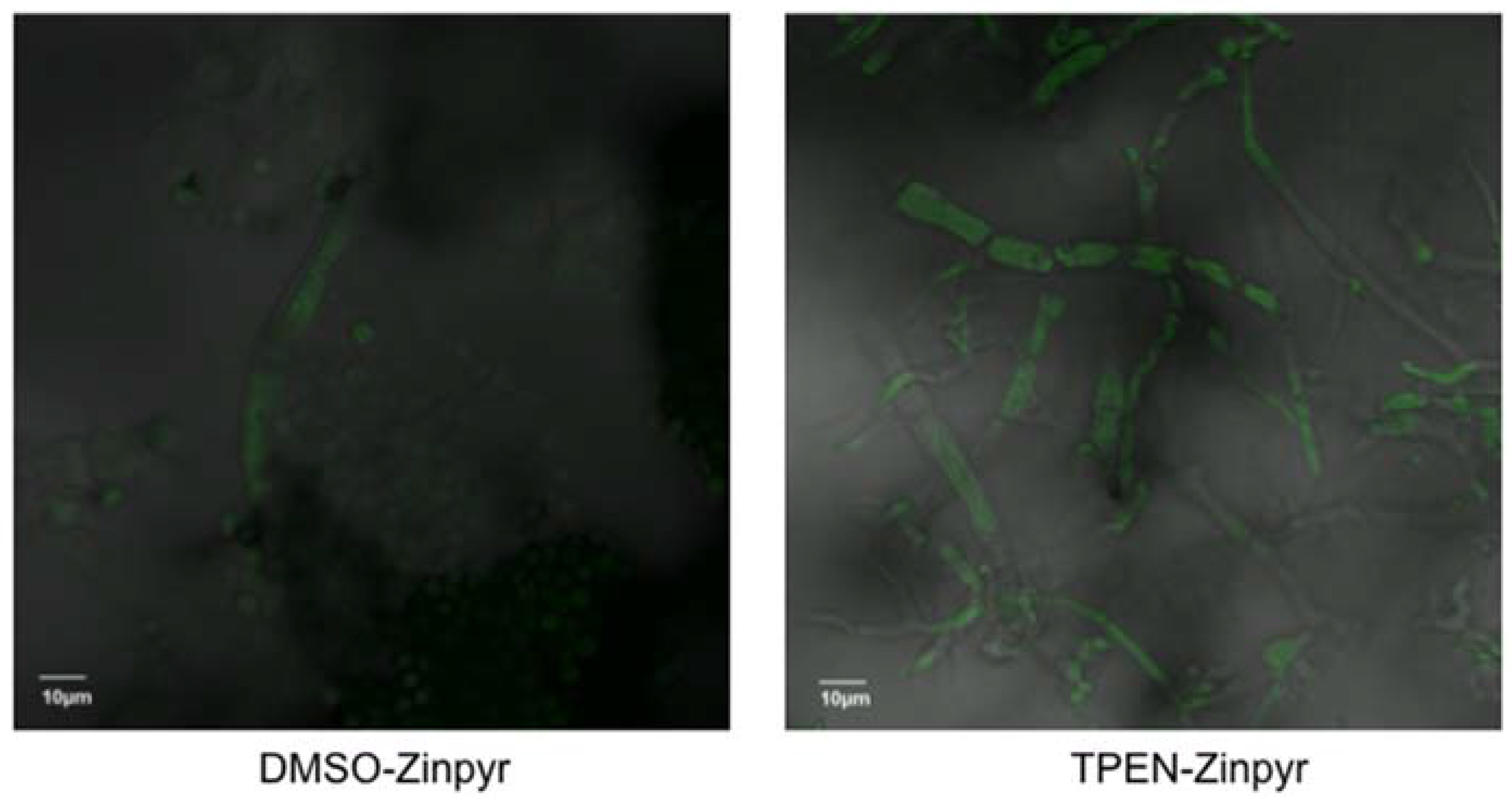
2.3. TPEN Increases Intracellular Zinc Levels
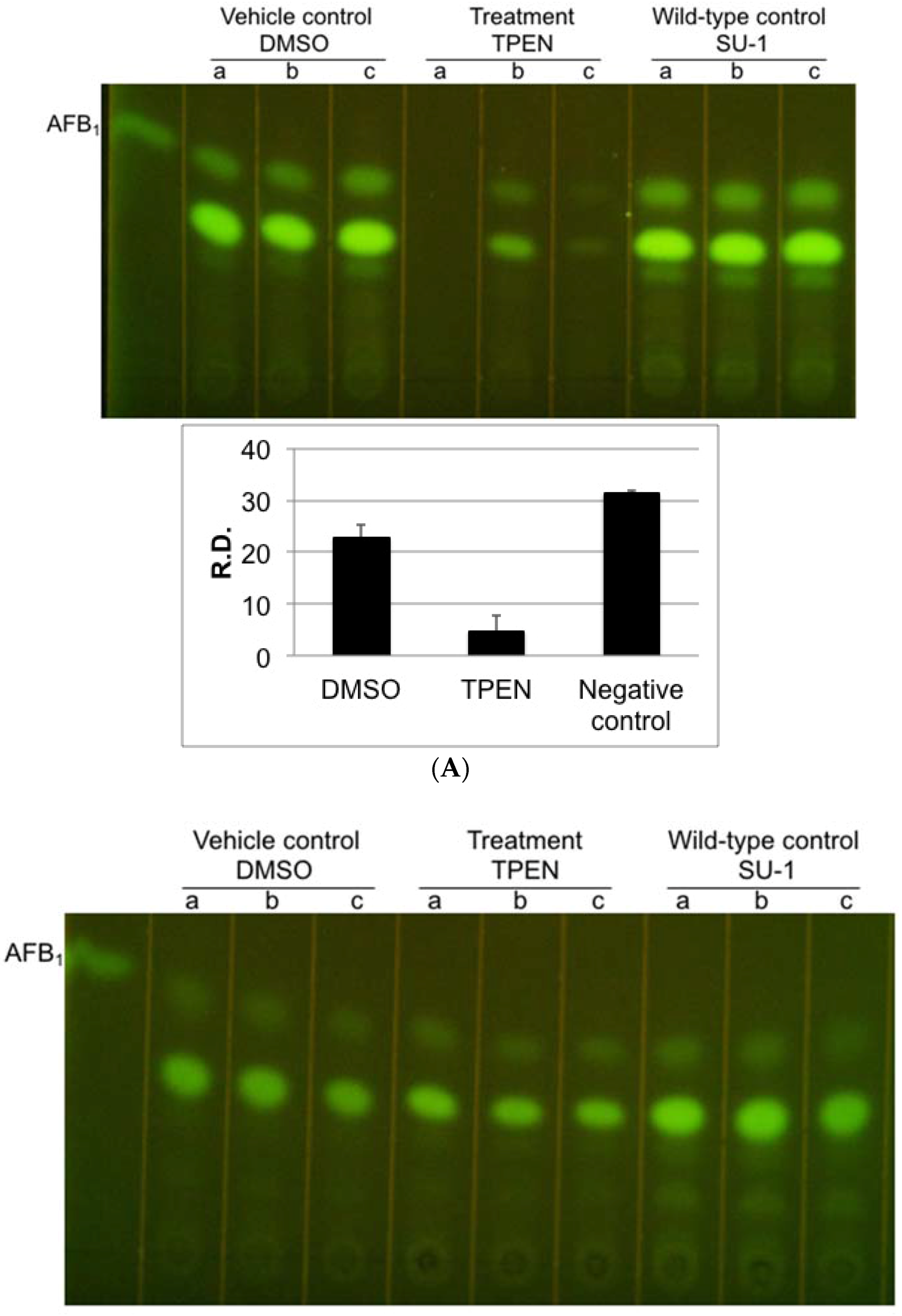
2.4. TPEN Affects Toxin Accumulation in A. parasiticus Grown on Aflatoxin-Inducing Peanut-Derived Medium but Not in Sunflower Seed-Derived Medium
3. Discussion
4. Conclusion and Future Perspectives
5. Material and Methods
5.1. Fungal Strains and Growth Conditions
5.2. Aflatoxins B1, B2, G1, and G2 and Norsolorinic Acid Extraction, Detection, and Analysis
5.3. Total RNA Extraction and Gene Expression Analysis
5.4. Zinpyr-1 and Confocal Scanning Laser Microscopy
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Strosnider, H.; Azziz-Baumgartner, E.; Banziger, M.; Bhat, R.V.; Breiman, R.; Brune, M.-N.; DeCock, K.; Dilley, A.; Groopman, J.; Hell, K.; et al. Workgroup report: Public health strategies for reducing aflatoxin exposure in developing countries. Environ. Health Perspect. 2006, 114, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Wu, F. Global impacts of aflatoxin in maize: Trade and human health. World Mycotoxin J. 2015, 8, 137–142. [Google Scholar] [CrossRef]
- Aziz, N.H.; Moussa, L.A. Influence of white light, near-UV irradiation and other environmental conditions on production of aflatoxin B1 by Aspergillus flavus and ochratoxin A by Aspergillus ochraceus. Nahrung 1997, 41, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Roze, L.V.; Beaudry, R.M.; Arthur, A.E.; Calvo, A.M.; Linz, J.E. Aspergillus volatiles regulate aflatoxin synthesis and asexual sporulation in Aspergillus parasiticus. Appl. Environ. Microbiol. 2007, 73, 7268–7276. [Google Scholar] [CrossRef] [PubMed]
- Roze, L.V.; Laivenieks, M.; Hong, S.-Y.; Wee, J.; Wong, S.-S.; Vanos, B.; Awad, D.; Ehrlich, K.C.; Linz, J.E. Aflatoxin biosynthesis is a novel source of reactive oxygen species—A potential redox signal to initiate resistance to oxidative stress? Toxins 2015, 7, 1411–1430. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Maggon, K.K.; Venkitasubramanian, T.A. Effect of zinc on adenine nucleotide pools in relation to aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 1976, 32, 753–756. [Google Scholar] [PubMed]
- Gupta, S.K.; Maggon, K.K.; Venkitasubramanian, T.A. Effect of Zinc on tricarboxylic acid cycle intermediates and enzymes in relation to aflatoxin biosynthesis. J. Gen. Microbiol. 1977, 99, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, W.G.; Failla, L.J. Effect of zinc on versicolorin production by a mutant strain of Aspergillus parasiticus. Exp. Mycol. 1984, 8, 80–84. [Google Scholar] [CrossRef]
- Marsh, P.B.; Simpson, M.E.; Mary, W.; Trucksess, M.W. Effects of Trace Metals on the Production of Aflatoxins by Aspergillus parasiticus. Appl. Environ. Microbiol. 1975, 30, 52–57. [Google Scholar] [PubMed]
- O’Dell, B.L.; Burpo, C.E.; Savage, J.E. Evaluation of zinc availability in foodstuffs of plant and animal origin. J. Nutr. 1972, 102, 653–660. [Google Scholar] [PubMed]
- Jones, F.T.; Hagler, W.M.; Hamilton, P.B. Correlation of aflatoxin contamination with zinc content of chicken feed. Appl. Environ. Microbiol. 1984, 47, 478–480. [Google Scholar] [PubMed]
- Gupta, S.K.; Venkitasubramanian, T.A. Production of Aflatoxin on Soybeans. Appl. Microbiol. 1975, 29, 834–836. [Google Scholar] [PubMed]
- Eide, D.J. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Lulloff, S.J.; Hahn, B.L.; Sohnle, P.G. Fungal susceptibility to zinc deprivation. J. Lab. Clin. Med. 2004, 144, 208–214. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Schneider, R.; de Souza Süffert Fogaça, N.; Kmetzsch, L.; Schrank, A.; Vainstein, M.H.; Staats, C.C. Zap1 regulates zinc homeostasis and modulates virulence in Cryptococcus gattii. PLoS ONE 2012, 7, e43773. [Google Scholar]
- Georgianna, D.R.; Fedorova, N.D.; Burroughs, J.L.; Dolezal, A.L.; Bok, J.W.; Horowitz-Brown, S.; Woloshuk, C.P.; Yu, J.; Keller, N.P.; Payne, G.A. Beyond aflatoxin: Four distinct expression patterns and functional roles associated with Aspergillus flavus secondary metabolism gene clusters. Mol. Plant Pathol. 2010, 11, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Georgianna, D.R.; Payne, G.A. Genetic regulation of aflatoxin biosynthesis: From gene to genome. Fungal Genet. Biol. 2009, 46, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Cary, J.W.; Ehrlich, K.C.; Kale, S.P.; Calvo, A.M.; Bhatnagar, D.; Cleveland, T.E. Regulatory elements in aflatoxin biosynthesis. Mycotoxin Res. 2006, 22, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Roze, L.V.; Chanda, A.; Laivenieks, M.; Beaudry, R.M.; Artymovich, K.A.; Koptina, A.V; Awad, D.W.; Valeeva, D.; Jones, A.D.; Linz, J.E. Volatile profiling reveals intracellular metabolic changes in Aspergillus parasiticus: veA regulates branched chain amino acid and ethanol metabolism. BMC Biochem. 2010, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Gunterus, A.; Roze, L.V.; Beaudry, R.; Linz, J.E. Ethylene inhibits aflatoxin biosynthesis in Aspergillus parasiticus grown on peanuts. Food Microbiol. 2007, 24, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Roze, L.V.; Linz, J.E. Evidence that a wortmannin-sensitive signal transduction pathway regulates aflatoxin biosynthesis. Mycologia 2007, 99, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Roze, L.V.; Calvo, A.M.; Gunterus, A.; Beaudry, R.; Kall, M.; Linz, J.E. Ethylene modulates development and toxin biosynthesis in aspergillus possibly via an ethylene sensor-mediated signaling pathway. J. Food Prot. 2004, 67, 438–447. [Google Scholar] [PubMed]
- Roze, L.; Koptina, A.; Laivenieks, M.; Beaudry, R.; Jones, D.; Kanarsky, A.; Linz, J. Willow volatiles influence growth, development, and secondary metabolism in Aspergillus parasiticus. Appl. Microbiol. Biotechnol. 2011, 92, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Baidya, S.; Duran, R.; Lohmar, J.M.; Harris-Coward, P.Y.; Cary, J.W.; Hong, S.-Y.; Roze, L.V.; Linz, J.E.; Calvo, A.M. VeA is associated with the response to oxidative stress in the aflatoxin-producer Aspergillus flavus. Eukaryot. Cell 2014, 13, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.Y.; Linz, J.E. Functional expression and sub-cellular localization of the early aflatoxin pathway enzyme Nor-1 in Aspergillus parasiticus. Mycol. Res. 2009, 113, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Woloshuk, C.P.; Foutz, K.R.; Brewer, J.F.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A. Molecular characterization of aflR, a regulatory locus for aflatoxin biosynthesis. Appl. Environ. Microbiol. 1994, 60, 2408–2414. [Google Scholar] [PubMed]
- Liang, S.H.; Skory, C.D.; Linz, J.E. Characterization of the function of the ver-1A and ver-1B genes, involved in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 1996, 62, 4568–4575. [Google Scholar] [PubMed]
- Sinclair, S.A.; Sherson, S.M.; Jarvis, R.; Camakaris, J.; Cobbett, C.S. The use of the zinc-fluorophore, Zinpyr-1, in the study of zinc homeostasis in Arabidopsis roots. New Phytol. 2007, 174, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Chulze, S.; Fusero, S.; Dalcero, A.; Etcheverry, M.; Varsavsky, E. Aflatoxins in sunflower seeds: Effect of zinc in aflatoxin production by two strains of Aspergillus parasiticus. Mycopathologia 1987, 99, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Dorner, J.W.; Cole, R.J.; Sanders, T.H.; Blankenship, P.D. Interrelationship of kernel water activity, soil temperature, maturity, and phytoalexin production in preharvest aflatoxin contamination of drought-stressed peanuts. Mycopathologia 1989, 105, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Kana, J.R.; Gnonlonfin, B.G.J.; Harvey, J.; Wainaina, J.; Wanjuki, I.; Skilton, R.A.; Teguia, A. Assessment of Aflatoxin Contamination of Maize, Peanut Meal and Poultry Feed Mixtures from Different Agroecological Zones in Cameroon. Toxins 2013, 5, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Staats, C.C.; Kmetzsch, L.; Schrank, A.; Vainstein, M.H. Fungal zinc metabolism and its connections to virulence. Front. Cell. Infect. Microbiol. 2013, 3, 65. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Sakata-Sogawa, K.; Hasegawa, A.; Suzuki, T.; Kabu, K.; Sato, E.; Kurosaki, T.; Yamashita, S.; Tokunaga, M.; Nishida, K.; et al. Zinc is a novel intracellular second messenger. J. Cell Biol. 2007, 177, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Blaurock-Busch, E.; Busch, Y.M. Comparison of Chelating Agents DMPS, DMSA and EDTA for the Diagnosis and Treatment of Chronic Metal Exposure. Br. J. Med. Med. Res. 2014, 4, 1821–1835. [Google Scholar] [CrossRef]
- Richter, M.; Cantin, A.M.; Beaulieu, C.; Cloutier, A.; Larivée, P. Zinc chelators inhibit eotaxin, RANTES, and MCP-1 production in stimulated human airway epithelium and fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L719–L729. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, R.L.; Lewis, D.F. Regulation of aflatoxin biosynthesis: Effect of glucose on activities of various glycolytic enzymes. Appl. Environ. Microbiol. 1984, 48, 306–310. [Google Scholar] [PubMed]
- Abdollahi, A.; Buchanan, R.L. Regulation of Aflatoxin Biosynthesis: Induction of Aflatoxin Production by Various Carbohydrates. J. Food Sci. 1981, 46, 633–635. [Google Scholar] [CrossRef]
- Roze, L.V.; Miller, M.J.; Rarick, M.; Mahanti, N.; Linz, J.E. A Novel cAMP-response Element, CRE1, Modulates Expression of nor-1 in Aspergillus parasiticus. J. Biol. Chem. 2004, 279, 27428–27439. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, T.; Zhang, L.; Hinz, A.J.; Parr, C.J.; Mah, T.-F. The Biofilm-Specific Antibiotic Resistance Gene ndvB Is Important for Expression of Ethanol Oxidation Genes in Pseudomonas aeruginosa Biofilms. J. Bacteriol. 2012, 194, 3128–3136. [Google Scholar] [CrossRef] [PubMed]
- Carraway, R.E.; Dobner, P.R. Zinc pyrithione induces ERK- and PKC-dependent necrosis distinct from TPEN-induced apoptosis in prostate cancer cells. Biochim. Biophys. Acta 2012, 1823, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Di Angelantonio, S.; Murana, E.; Cocco, S.; Scala, F.; Bertollini, C.; Molinari, M.G.; Lauro, C.; Bregestovski, P.; Limatola, C.; Ragozzino, D. A role for intracellular zinc in glioma alteration of neuronal chloride equilibrium. Cell Death Dis. 2014, 5, e1501. [Google Scholar] [CrossRef] [PubMed]
- Roze, L.V.; Beaudry, R.M.; Keller, N.P.; Linz, J.E. Regulation of aflatoxin synthesis by FadA/cAMP/protein kinase A signaling in Aspergillus parasiticus. Mycopathologia 2004, 158, 219–232. [Google Scholar] [CrossRef] [PubMed]
- McCloy, R.A.; Rogers, S.; Caldon, C.E.; Lorca, T.; Castro, A.; Burgess, A. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle 2014, 13, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.; Vigneron, S.; Brioudes, E.; Labbé, J.-C.; Lorca, T.; Castro, A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc. Natl. Acad. Sci. 2010, 107, 12564–12569. [Google Scholar] [CrossRef] [PubMed]
- Roze, L.V.; Arthur, A.E.; Hong, S.-Y.; Chanda, A.; Linz, J.E. The initiation and pattern of spread of histone H4 acetylation parallel the order of transcriptional activation of genes in the aflatoxin cluster. Mol. Microbiol. 2007, 66, 713–726. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequence | Reference |
|---|---|---|
| aflR | F-5′ TGAGAACGATAAGGACGAC 3′ | [4] |
| R-5′ CATCCTCAATCGAATCAAC 3′ | ||
| ver-1 | F-5′ TTGTATCGTTCGGTCACC 3′ | [45] |
| R-3′ GGTTCAAAGGAGAGAGCC 3′ | ||
| citrate synthase | F-5′ TGCAGTCCGTTGCCTTCAATG 3′ | [5] |
| R-5′ TAGCGTAGGCCTTGGCGAAAG 3′ | ||
| pksA | F-5′ GTTCCTTGGCCGCTGTG 3′ | [45] |
| R-5′ AAAGGCGTGGCAGTCC 3′ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wee, J.; Day, D.M.; Linz, J.E. Effects of Zinc Chelators on Aflatoxin Production in Aspergillus parasiticus. Toxins 2016, 8, 171. https://doi.org/10.3390/toxins8060171
Wee J, Day DM, Linz JE. Effects of Zinc Chelators on Aflatoxin Production in Aspergillus parasiticus. Toxins. 2016; 8(6):171. https://doi.org/10.3390/toxins8060171
Chicago/Turabian StyleWee, Josephine, Devin M. Day, and John E. Linz. 2016. "Effects of Zinc Chelators on Aflatoxin Production in Aspergillus parasiticus" Toxins 8, no. 6: 171. https://doi.org/10.3390/toxins8060171
APA StyleWee, J., Day, D. M., & Linz, J. E. (2016). Effects of Zinc Chelators on Aflatoxin Production in Aspergillus parasiticus. Toxins, 8(6), 171. https://doi.org/10.3390/toxins8060171





