Sequence Variability in Staphylococcal Enterotoxin Genes seb, sec, and sed
Abstract
:1. Introduction
2. Results
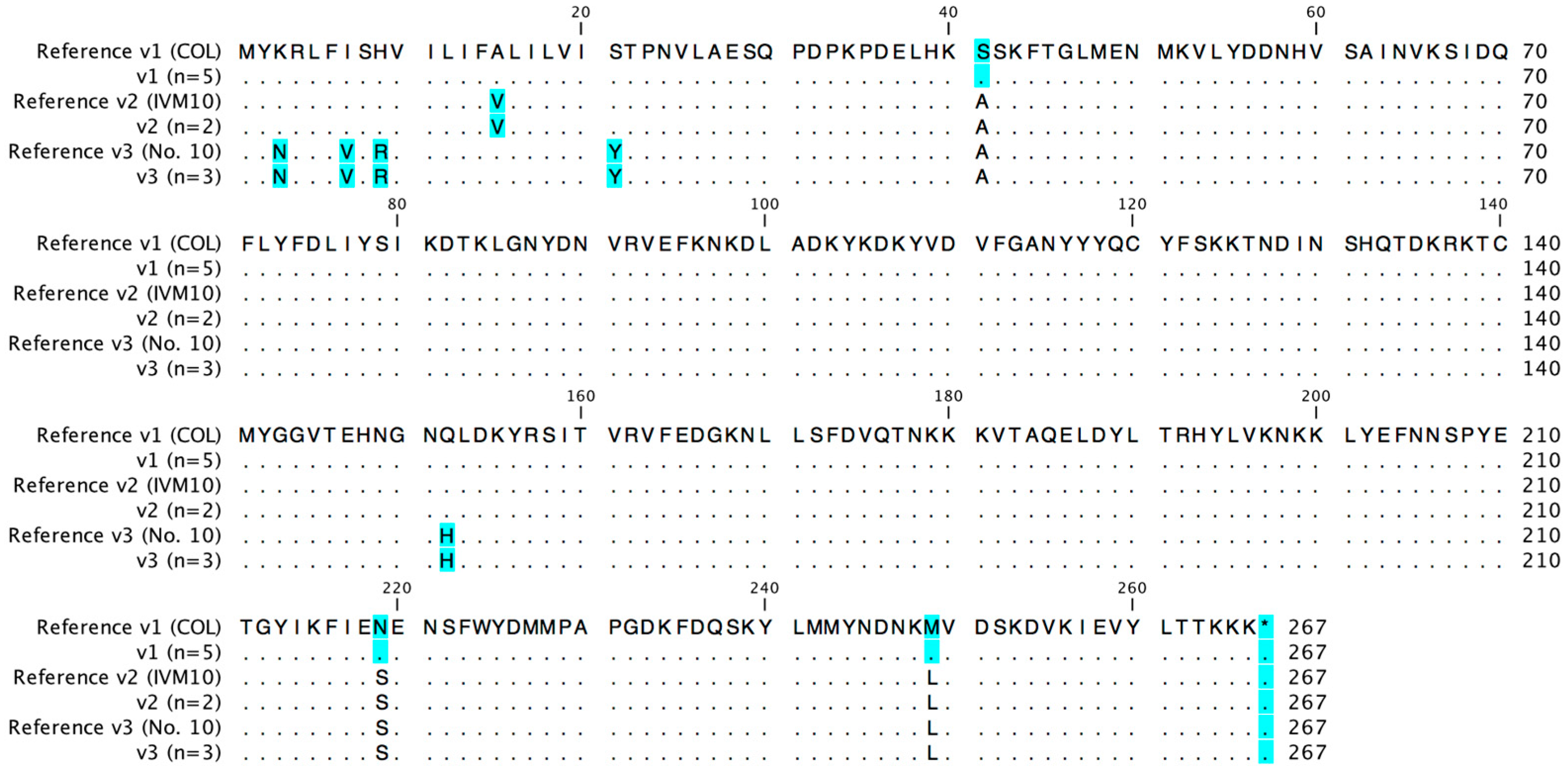
2.1. Seb Promoter and Gene Sequences
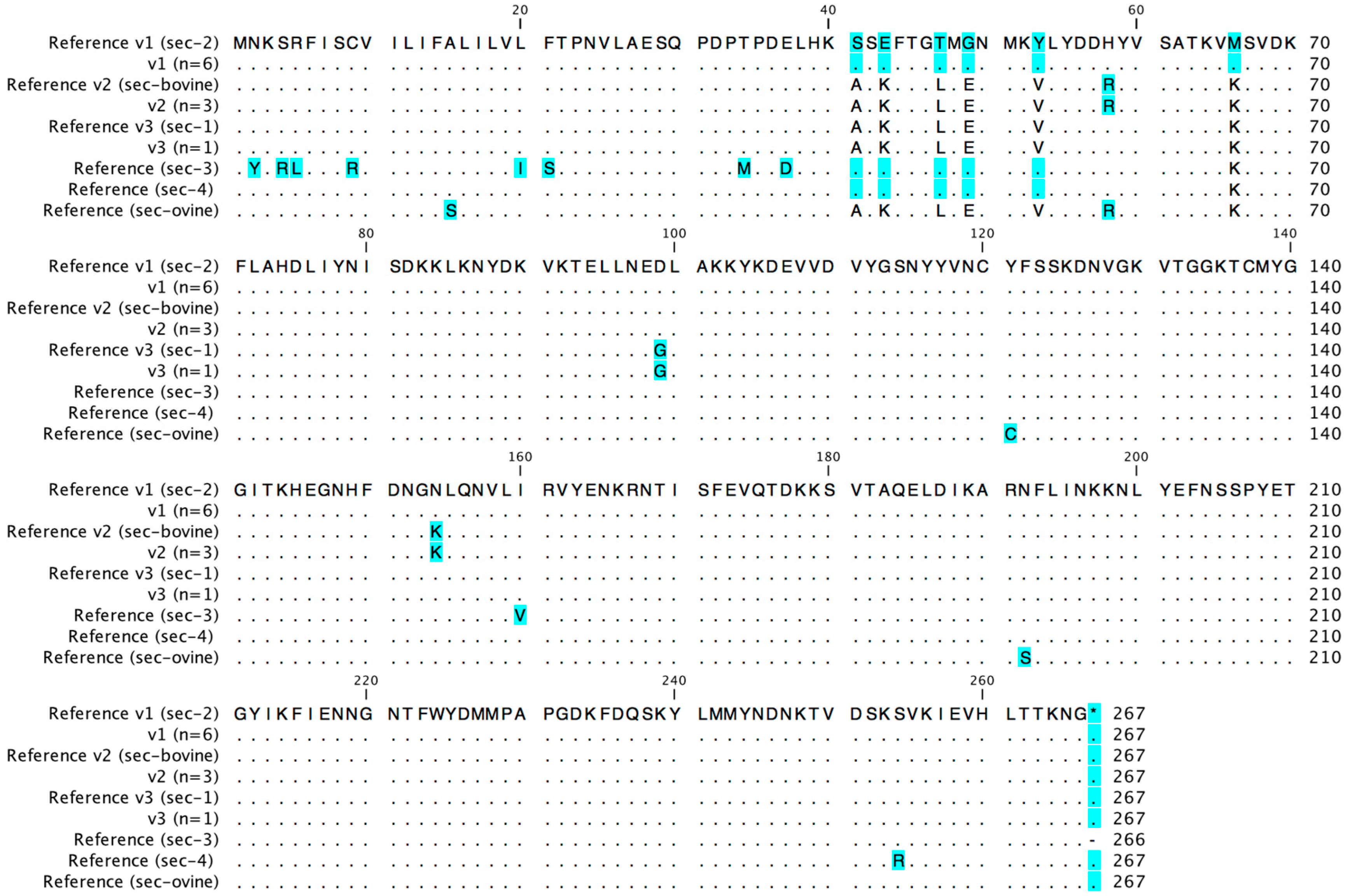
2.2. Sec Promoter and Gene Sequences
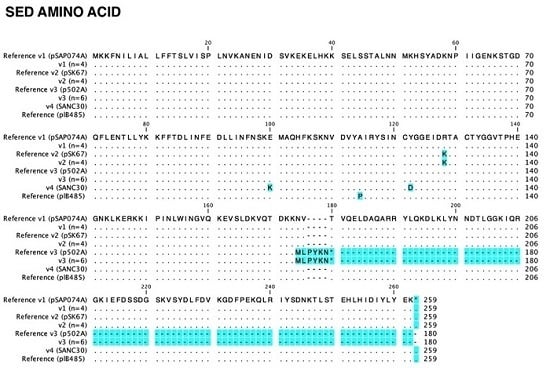
2.3. Sed Promoter and Gene Sequences
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Bacterial Strains
5.2. DNA Extraction and PCR Amplification
5.3. PCR Purification and Sequencing
5.4. Toxin Detection by SET-RPLA
5.5. Amino Acid Identity
5.6. Accession Numbers
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Tranter, H.S. Foodborne staphylococcal illness. Lancet 1990, 336, 1044–1046. [Google Scholar] [CrossRef]
- Sihto, H.M.; Tasara, T.; Stephan, R.; Johler, S. Growth behavior and temporal enterotoxin D expression of Staphylococcus aureus strains under glucose and lactic acid stress. Food Control 2016, 62, 69–73. [Google Scholar] [CrossRef]
- Sihto, H.-M.; Budi Susilo, Y.; Tasara, T.; Rådström, P.; Stephan, R.; Schelin, J.; Johler, S. Effect of sodium nitrite and regulatory mutations Δagr, ΔsarA, and ΔsigB on the mRNA and protein levels of staphylococcal enterotoxin D. Food Control 2016, 65, 37–45. [Google Scholar] [CrossRef]
- Sihto, H.-M.; Tasara, T.; Stephan, R.; Johler, S. Temporal expression of the staphylococcal enterotoxin D gene under NaCl stress conditions encountered during food production and preservation. FEMS Microbiol. Lett. submitted for publication. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wallin-Carlquist, N.; Cao, R.; Márta, D.; da Silva, A.S.; Schelin, J.; Rådström, P. Acetic acid increases the phage-encoded enterotoxin A expression in Staphylococcus aureus. BMC Microbiol. 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Zeaki, N.; Wallin-Carlquist, N.; Skandamis, P.N.; Schelin, J.; Rådström, P. Elevated enterotoxin A expression and formation in Staphylococcus aureus and its association with prophage Induction. Appl. Environ. Microbiol. 2012, 78, 4942–4948. [Google Scholar] [CrossRef] [PubMed]
- Sumby, P.; Waldor, M.K. Transcription of the toxin genes present within the staphylococcal phage φSa3ms is intimately linked with the phage’s life cycle. J. Bacteriol. 2003, 185, 6841–6851. [Google Scholar] [CrossRef] [PubMed]
- Borst, D.W.; Betley, M.J. Phage-associated differences in staphylococcal enterotoxin A gene (sea) expression correlate with sea allele class. Infect. Immun. 1994, 62, 113–118. [Google Scholar] [PubMed]
- Mahmood, R.; Khan, S.A. Role of upstream sequences in the expression of the staphylococcal enterotoxin B gene. J. Biol. Chem. 1990, 265, 4652–4656. [Google Scholar] [PubMed]
- Zhang, S.; Stewart, G.C. Characterization of the promoter elements for the staphylococcal enterotoxin D gene. J. Bacteriol. 2000, 182, 2321–2325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shafer, W.M.; Iandolo, J.J. Chromosomal locus for staphylococcal enterotoxin B. Infect. Immun. 1978, 20, 273–278. [Google Scholar] [PubMed]
- Sato’o, Y.; Omoe, K.; Ono, H.K.; Nakane, A.; Hu, D.-L. A novel comprehensive analysis method for Staphylococcus aureus pathogenicity islands. Microbiol. Immunol. 2013, 57, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.J.A.; Stephan, R.; Johler, S. Complete and assembled genome sequence of Staphylococcus aureus RKI4, a food-poisoning strain exhibiting a novel S. aureus pathogenicity island carrying seb. Genome Announc. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Kohler, P.L.; Greenwood, S.D.; Nookala, S.; Kotb, M.; Kranz, D.M.; Schlievert, P.M. Staphylococcus aureus isolates encode variant staphylococcal enterotoxin B proteins that are diverse in superantigenicity and lethality. PLoS ONE 2012, 7, e41157. [Google Scholar] [CrossRef] [PubMed]
- Couch, J.L.; Betley, M.J. Nucleotide sequence of the type C3 staphylococcal enterotoxin gene suggests that intergenic recombination causes antigenic variation. J. Bacteriol. 1989, 171, 4507–4510. [Google Scholar] [PubMed]
- Marr, J.C.; Lyon, J.D.; Roberson, J.R.; Lupher, M.; Davis, W.C.; Bohach, G.A. Characterization of novel type C staphylococcal enterotoxins: biological and evolutionary implications. Infect. Immun. 1993, 61, 4254–4262. [Google Scholar] [PubMed]
- Bohach, G.A.; Schlievert, P.M. Nucleotide sequence of the staphylococcal enterotoxin C1 gene and relatedness to other pyrogenic toxins. Mol. Gen. Genet. 1987, 209, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.R.; Monday, S.R.; Foster, T.J.; Bohach, G.A.; Hartigan, P.J.; Meaney, W.J.; Smyth, C.J. Characterization of a putative pathogenicity island from bovine Staphylococcus aureus encoding multiple superantigens. J. Bacteriol. 2001, 183, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Bayles, K.W.; Iandolo, J.J. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J. Bacteriol. 1989, 171, 4799–4806. [Google Scholar] [PubMed]
- Kauffman, N.M.; Roberts, R.F. Staphylococcal enterotoxin D production by Staphylococcus aureus FRI 100. J. Food Prot. 2006, 69, 1448–1451. [Google Scholar] [PubMed]
- Suzuki, Y.; Kobayashi, M.; Matsushita, S.; Uehara, S.; Kato, R.; Sato’o, Y.; Ono, H.K.; Sadamasu, K.; Kai, A.; Kamata, Y. Detection of the staphylococcal enterotoxin D-like gene from staphylococcal food poisoning isolates over the last two decades in Tokyo. J. Vet. Med. Sci. 2015, 77, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Narechania, A.; Sebra, R.; Deikus, G.; Larussa, S.; Ryan, C.; Smith, H.; Prince, A.; Mathema, B.; Ratner, A.J.; et al. Genome sequence of bacterial interference strain Staphylococcus aureus 502A. Genome Announc. 2014, 2, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Lis, E.; Podkowik, M.; Schubert, J.; Bystroń, J.; Stefaniak, T.; Bania, J. Production of staphylococcal enterotoxin R by Staphylococcus aureus strains. Foodborne Pathog. Dis. 2012, 9, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.L.; Khan, S.A. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J. Bacteriol. 1986, 166, 29–33. [Google Scholar] [PubMed]
- Johns, M.B., Jr.; Khan, S.A. Staphylococcal enterotoxin B gene is associated with a discrete genetic element. J. Bacteriol. 1988, 170, 4033–4039. [Google Scholar] [PubMed]
- Dinges, M.M.; Orwin, P.M.; Schlievert, P.M. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000, 13, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Hovde, C.; Marr, C.; Hoffmann, M.; Hackett, S.; Chi, Y.; Crum, K.; Stevens, D.; Stauffacher, C.; Bohach, G. Investigation of the role of the disulphide bond in the activity and structure of staphylococcal enterotoxin C1. Mol. Microbiol. 1994, 15, 897–909. [Google Scholar] [CrossRef]
- Tobes, R.; Manrique, M.; Brozynska, M.; Stephan, R.; Pareja, E.; Johler, S. Noncontiguous finished genome sequence of Staphylococcus aureus KLT6, a staphylococcal enterotoxin B-positive strain involved in a food poisoning outbreak in Switzerland. Genome Announc. 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Wattinger, L.; Stephan, R.; Layer, F.; Johler, S. Comparison of Staphylococcus aureus isolates associated with food intoxication with isolates from human nasal carriers and human infections. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Merz, A.; Stephan, R.; Johler, S. Genotyping and DNA microarray based characterization of Staphylococcus aureus isolates from rabbit carcasses. Meat Sci. 2016, 112, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Johler, S.; Layer, F.; Stephan, R. Comparison of virulence and antibiotic resistance genes of food poisoning outbreak isolates of Staphylococcus aureus with isolates obtained from bovine mastitis milk and pig carcasses. J. Food Prot. 2011, 74, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
| Gene | Strain ID | Identical Reference 1 | Promoter Variant 2 (Reference) | Gene Variant 2 (Reference) | Amino Acid Variant (Reference) | Source | Clonal Complex/spa Type | Reference |
|---|---|---|---|---|---|---|---|---|
| seb | KLT6 | COL | sebp v1 | seb v1 | sebaa v1 | SFP 3 | CC12/t160 | [29] |
| SANC31 | COL | sebp v1 | seb v1 | sebaa v1 | Human nasal colonization | CC59/t216 | [30] | |
| SANC49 | COL | sebp v1 | seb v1 | sebaa v1 | Human nasal colonization | CC59/t216 | [30] | |
| SAK9 | COL | sebp v1 | seb v1 | sebaa v1 | Rabbit | CC5/t8456 | [31] | |
| SAK18 | COL | sebp v1 | seb v1 | sebaa v1 | Rabbit | CC5/t8456 | [31] | |
| SAI10 | COL | sebp v1 | seb v1 | sebaa v1 | Human infection | CC59/t216 | [30] | |
| SAI50 | COL | sebp v1 | seb v1 | sebaa v1 | Human infection | CC59/t015 | [30] | |
| SANC14 | IVM10 | sebp v2 | seb v2 | sebaa v2 | Human nasal colonization | CC45/t630 | [30] | |
| RKI4 | novel 4 | sebp v3 (No. 10) | seb v3 (novel) 4 | sebaa v3 (No. 10) | SFP | CC9/t733 | [14] | |
| SAI40 | novel 4 | sebp v4 (novel) 4 | seb v3 (No. 10) | sebaa v3 (No. 10) | Human infection | CC15/t084 | [30] | |
| SAI33 | novel 4 | sebp v4 (novel) 4 | seb v3 (novel) 4 | sebaa v3 (No. 10) | Human infection | CC20/t164 | [30] | |
| SAI45 | novel 4 | sebp v5 (novel) 4 | seb v4 (IVM10) | sebaa v2 (IVM10) | Human infection | CC121/t272 | [30] | |
| sec | BW10 | 79_S10 | secp v1 | sec v1 = SEC-2 | secaa v1 = SEC-2 | SFP | CC45/t383 | Medical Department of the German Federal Armed Forces, Germany |
| LRA1 | 79_S10 | secp v1 | sec v1 = SEC-2 | secaa v1 = SEC-2 | SFP | CC73/t015 | Bavarian State Office of Health and Food Safety, Germany | |
| SANC23 | 79_S10 | secp v1 | sec v1 = SEC-2 | secaa v1 = SEC-2 | Human nasal colonization | CC8/t8016 | [30] | |
| SANC48 | 79_S10 | secp v1 | sec v1 = SEC-2 | secaa v1 = SEC-2 | Human nasal colonization | CC45/t015 | [30] | |
| NB6 | 79_S10 | secp v1 | sec v1 = SEC-2 | secaa v1 = SEC-2 | SFP | CC45/t6969 | Bavarian State Office of Health and Food Safety, Germany | |
| SAR1 | RF122 | secp v2 | sec v2 = SEC-bovine | secaa v2 = SEC-bovine | Bovine mastitis milk | CC151/t529 | [32] | |
| SAR38 | RF122 | secp v2 | sec v2 = SEC-bovine | secaa v2 = SEC-bovine | Bovine mastitis milk | CC151/t529 | [32] | |
| SAR50 | RF122 | secp v2 | sec v2 = SEC-bovine | secaa v2 = SEC-bovine | Bovine mastitis milk | CC151/t529 | [32] | |
| SAI3 | novel 4 | secp v3 (H-EMRSA-15) | sec v3 = SEC-1 (B1085) | secaa v3 = SEC-1 (B1085) | Human infection | CC8/t148 | [30] | |
| SAI48 | novel 4 | secp v1 (79_S10) | sec v4 (novel) 4 | secaa v1 = SEC-2 (79_S10) | Human infection | CC5/t002 | [30] | |
| sed | KLT8 | pSAP074A | sedp v1 | sed v1 | sedaa v1 | SFP | CC5/t8017 | Cantonal Laboratory Thurgau, Switzerland |
| SAI8 | pSAP074A | sedp v1 | sed v1 | sedaa v1 | Human infection | CC5/t954 | [30] | |
| SAI41 | pSAP074A | sedp v1 | sed v1 | sedaa v1 | Human infection | CC5/t8017 | [30] | |
| SAI48 | novel 4 | sedp v3 (novel) 4 | sed v1 (pSAP074A) | sedaa v1 (pSAP074A) | Human infection | CC5/t002 | [30] | |
| BW10 | pSK67 | sedp v2 | sed v2 | sedaa v2 | SFP | CC45/t383 | Medical Department of the German Federal Armed Forces, Germany | |
| RKI1 | pSK67 | sedp v2 | sed v2 | sedaa v2 | SFP | CC8/t648 | Robert Koch Institute, Germany | |
| RKI2 | pSK67 | sedp v2 | sed v2 | sedaa v2 | SFP | CC8/t008 | Robert Koch Institute, Germany | |
| SAR35 | novel 4 | sedp v1 (pSAP074A) | sed v2 (pSK67) | sedaa v2 (pSK67) | Bovine mastitis milk | CC8/t2953 | [32] | |
| SAK8 | novel 4 | sedp v1 (pSAP074A) | sed v3 (p502A) | sedaa v3 (p502A) | Rabbit | CC5/t179 | [31] | |
| SAK9 | novel 4 | ND 5 | sed v3 (p502A) | sedaa v3 (p502A) | Rabbit | CC5/t8456 | [31] | |
| SAK11 | novel 4 | sedp v1 (pSAP074A) | sed v3 (p502A) | sedaa v3 (p502A) | Rabbit | CC5/t179 | [31] | |
| SAK13 | novel 4 | sedp v1 (pSAP074A) | sed v3 (p502A) | sedaa v3 (p502A) | Rabbit | CC5/t179 | [31] | |
| SAK18 | novel 4 | ND 5 | sed v3 (p502A) | sedaa v3 (p502A) | Rabbit | CC5/t8456 | [31] | |
| SAK64 | novel 4 | ND 5 | sed v3 (p502A) | sedaa v3 (p502A) | Rabbit | CC5/t160 | [31] | |
| SANC30 | novel 4 | sedp v1 (pSAP074A) | sed v4 (novel)4 | sedaa v4 (novel) 4 | Human nasal colonization | CC5/t002 | [30] |
| Variant | SEB v1 | SEB v2 | SEB v3 | SEC-1 | SEC-2 | SEC-3 | SEC-4 | SEC-bovine | SEC-ovine | SED v1 | SED v2 | SED v3 | SED v4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEB v1 | 100 | 99 | 97 | 68 | 67 | 69 | 67 | 69 | 67 | 36 | 35 | 25 | 36 |
| SEB v2 | - | 100 | 98 | 67 | 66 | 67 | 66 | 68 | 67 | 36 | 36 | 25 | 36 |
| SEB v3 | - | - | 100 | 67 | 66 | 67 | 66 | 68 | 67 | 36 | 35 | 24 | 35 |
| SEC-1 | - | - | - | 100 | 97 | 94 | 97 | 99 | 98 | 32 | 32 | 22 | 32 |
| SEC-2 | - | - | - | - | 100 | 96 | 99 | 97 | 96 | 33 | 32 | 22 | 32 |
| SEC-3 | - | - | - | - | - | 100 | 96 | 94 | 93 | 33 | 33 | 23 | 33 |
| SEC-4 | - | - | - | - | - | - | 100 | 97 | 96 | 33 | 33 | 22 | 33 |
| SEC-bovine | - | - | - | - | - | - | - | 100 | 99 | 32 | 32 | 22 | 32 |
| SEC-ovine | - | - | - | - | - | - | - | - | 100 | 32 | 32 | 21 | 32 |
| SED v1 | - | - | - | - | - | - | - | - | - | 100 | 99 | 66 | 99 |
| SED v2 | - | - | - | - | - | - | - | - | - | - | 100 | 66 | 99 |
| SED v3 | - | - | - | - | - | - | - | - | - | - | - | 100 | 65 |
| SED v4 | - | - | - | - | - | - | - | - | - | - | - | - | 100 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johler, S.; Sihto, H.-M.; Macori, G.; Stephan, R. Sequence Variability in Staphylococcal Enterotoxin Genes seb, sec, and sed. Toxins 2016, 8, 169. https://doi.org/10.3390/toxins8060169
Johler S, Sihto H-M, Macori G, Stephan R. Sequence Variability in Staphylococcal Enterotoxin Genes seb, sec, and sed. Toxins. 2016; 8(6):169. https://doi.org/10.3390/toxins8060169
Chicago/Turabian StyleJohler, Sophia, Henna-Maria Sihto, Guerrino Macori, and Roger Stephan. 2016. "Sequence Variability in Staphylococcal Enterotoxin Genes seb, sec, and sed" Toxins 8, no. 6: 169. https://doi.org/10.3390/toxins8060169
APA StyleJohler, S., Sihto, H.-M., Macori, G., & Stephan, R. (2016). Sequence Variability in Staphylococcal Enterotoxin Genes seb, sec, and sed. Toxins, 8(6), 169. https://doi.org/10.3390/toxins8060169