Screening of Cytotoxic B. cereus on Differentiated Caco-2 Cells and in Co-Culture with Mucus-Secreting (HT29-MTX) Cells
Abstract
:1. Introduction
2. Results
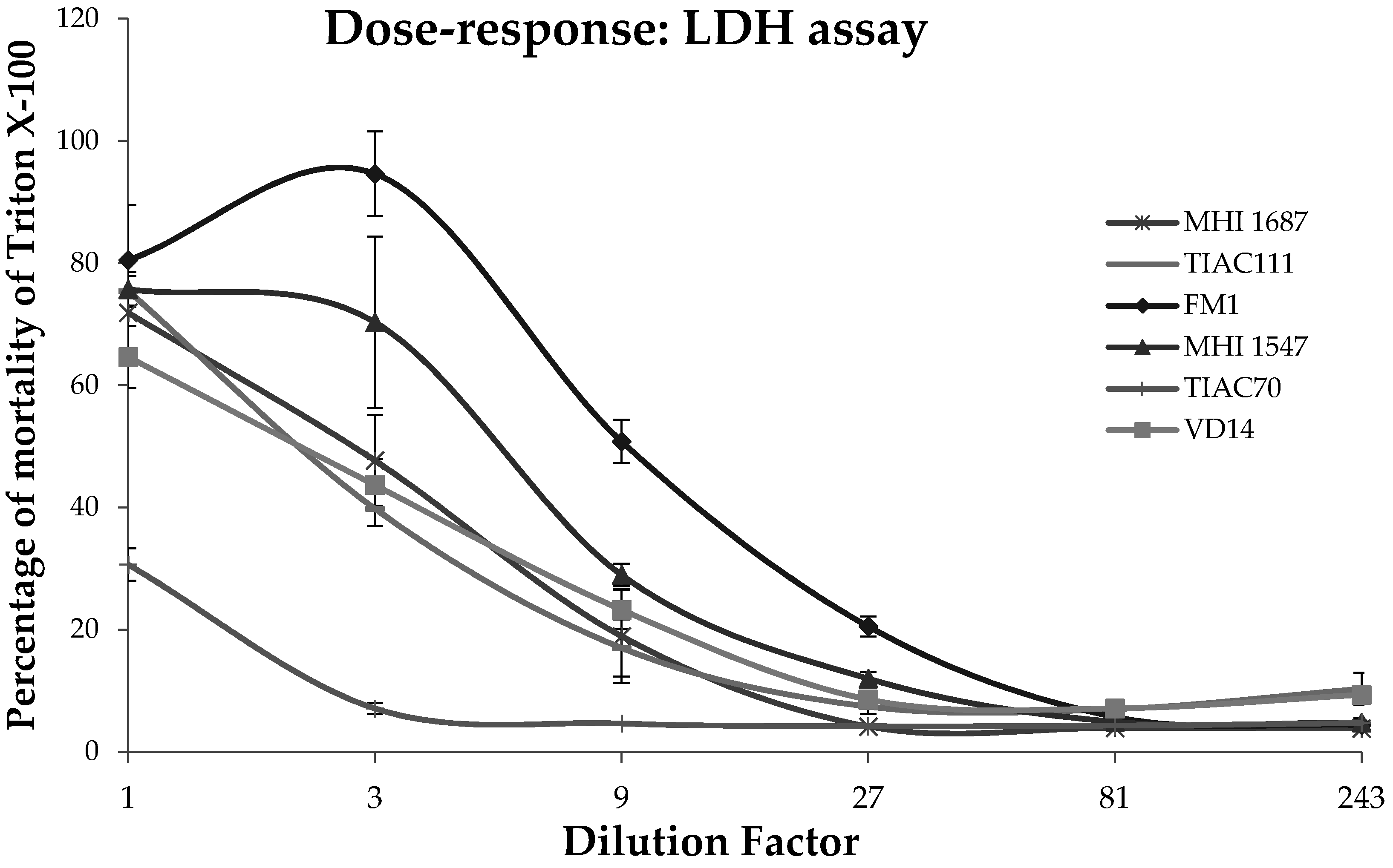
2.1. Cytotoxicity on Caco-2 Cell Monoculture
2.2. Distribution of Potential Enterotoxin and Emetic Toxin Genetic Determinants in the B. cereus Collection
2.2.1. Enterotoxin Genetic Determinant Profiles and Strain Origin
2.2.2. Enterotoxin Genetic Determinant Profiles and Cytotoxicity
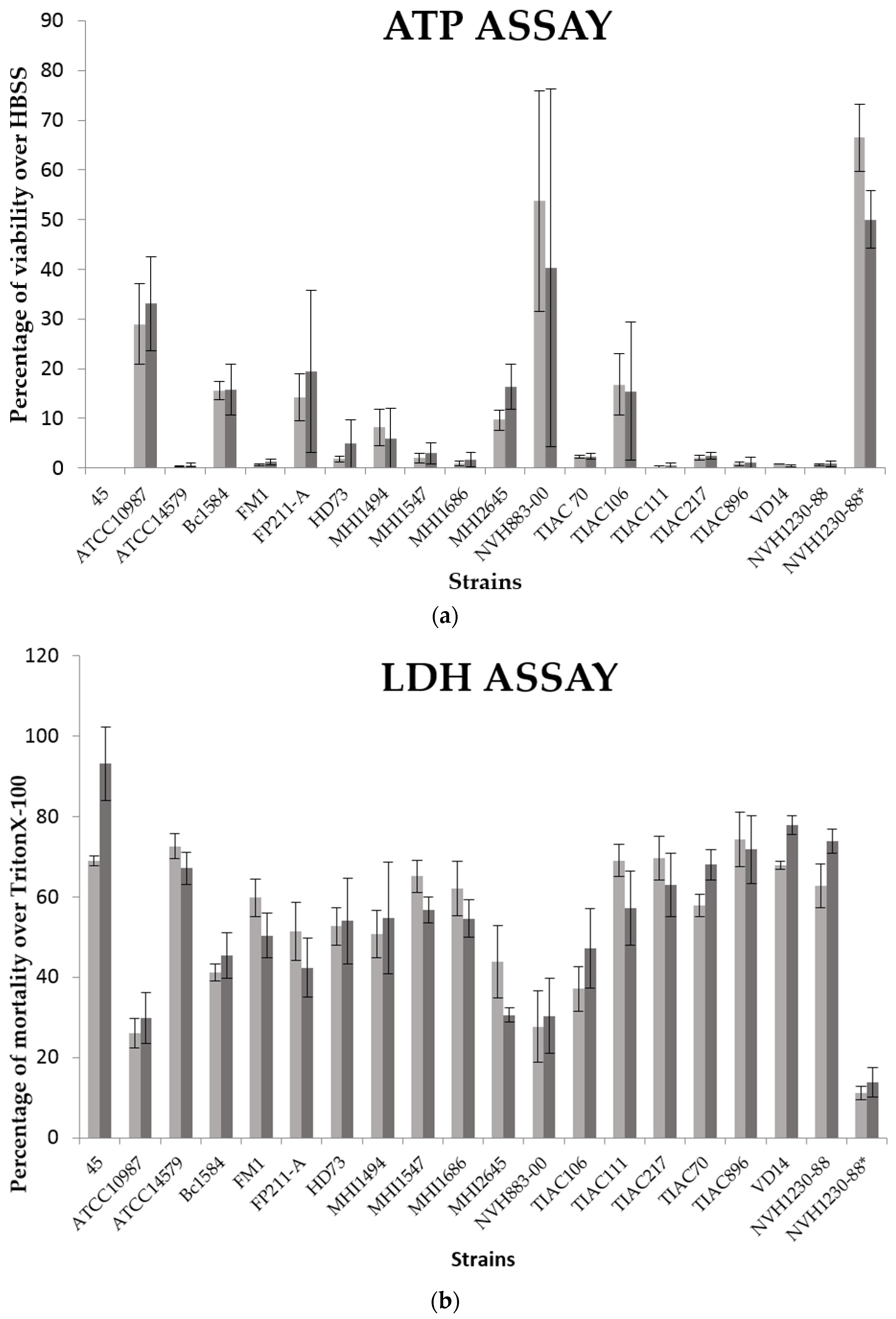
2.3. Comparison of Cytotoxicity between Mono-(Caco-2 Cells) and Co-Cultures (Caco-2/HT29-MTX Cells)
3. Discussion
3.1. Toxicity Prediction
3.2. Choice of Cellular Model to Assess Enteropathogenicity
4. Materials and Methods
4.1. B. cereus Collection and DNA Extraction
4.2. Detection of Virulence Factors and Enterotoxin Genes
4.3. Cell Line Cultures
4.4. Cytotoxicity Assays
4.4.1. Neutral Red Accumulation Assays
4.4.2. LDH Release Assays
4.4.3. ATP Content Assays
4.5. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| C | Clinical isolates |
| Caco-2 | Human epithelial colorectal adenocarcinoma cells |
| ces | Cereulide genetic determinants |
| CerO | Cereolysin O |
| CFU | Colony Forming Unit |
| CHO | Chinese Hamster Ovary |
| CytK | Cytotoxin K |
| DMEM | Dubelcco’s Modified Eagle’s minimal essential medium |
| E | Environmental isolates |
| EntFM | Enterotoxin FM |
| EntS | Enterotoxin S |
| F | Food isolates |
| FP | Food Poisoning isolates |
| HBSS | Hank’s Balanced Salt Solution |
| HBL | Haemolysin BL |
| HlyII | Haemolysin II |
| HT29-MTX | HT29 cells were treated with methotrexate and able to produce mucin |
| kDA | kilo Daltons |
| LB | Lysogenic Broth |
| LDH | Lactate Dehydrogenase |
| MCA | Multiple Correspondence Analysis |
| Nhe | Non-haemolytic enterotoxin |
| NprA | Metalloprotease: bacillolysin (nprA: genetic determinants of NprA) |
| NR | Neutral Red |
| PBS | Phosphate Buffer Saline |
| PCR | Polymerase Chain Reaction |
| PI-PLC | Phosphatidylinositol Phospholipase C (pipl-C: genetic determinants of PI-PLC) |
| SD | Standard Deviation |
| SEM | Standard Error of the Mean |
| Smase | Sphingomyelinase (sph: genetic determinants of Smase) |
| U | isolates with unknown origin |
Appendixes
| Treatments | LDH Assay | NR Assay | ||
|---|---|---|---|---|
| % of Mortality over Triton X-100 | % of Viability over HBSS | |||
| Mean | ±SD | Mean | ±SD | |
| 15 | 58.14 | ±18.21 | 36.69 | ±34.12 |
| 45 | 89.85 | ±0.39 | 2.61 | ±2.43 |
| 27409 | 67.51 | ±30.44 | 46.72 | ±39.54 |
| 390-88 | 46.26 | ±9.98 | 57.10 | ±12.94 |
| A1 | 0.05 | ±1.26 | 84.56 | ±24.82 |
| A12 | 21.09 | ±15.14 | 92.60 | ±20.74 |
| AH621 | 4.38 | ±2.75 | 103.99 | ±18.93 |
| AH676 | 8.43 | ±8.15 | 92.31 | ±17.34 |
| ATCC10876 | 59.06 | ±42.11 | 34.48 | ±49.43 |
| ATCC10987 | 79.17 | ±4.79 | 6.56 | ±11.03 |
| ATCC14579 | 69.16 | ±8.85 | 1.59 | ±2.21 |
| B16 | 64.88 | ±11.98 | 13.55 | ±11.53 |
| B7 | 80.78 | ±13.21 | 1.71 | ±1.22 |
| Bc1558 | 74.26 | ±10.96 | 2.16 | ±1.02 |
| Bc1576 | 66.55 | ±15.58 | 12.02 | ±9.31 |
| Bc1584 | 53.47 | ±12.23 | 35.81 | ±27.40 |
| F4501-83 | 80.28 | ±13.88 | 1.32 | ±1.08 |
| F4810-72 | 2.33 | ±0.48 | 98.14 | ±4.63 |
| F5063/95 | 84.60 | ±9.99 | 11.79 | ±11.87 |
| FM-1 | 90.68 | ±9.43 | 0.84 | ±0.79 |
| FP211-A | 84.04 | ±3.97 | 1.54 | ±1.23 |
| H3081/91 | 5.07 | ±1.66 | 76.34 | ±26.38 |
| HD73 | 61.96 | ±15.59 | 33.74 | ±34.40 |
| I13-2 | 72.21 | ±10.60 | 2.17 | ±1.31 |
| I8-5 | 80.81 | ±12.06 | 4.72 | ±9.17 |
| ISP2954 | 43.98 | ±4.00 | 51.82 | ±11.63 |
| ISP3191 | 76.64 | ±14.21 | 13.00 | ±15.67 |
| MHI13 | 28.34 | ±11.33 | 71.17 | ±18.98 |
| MHI1494 | 66.60 | ±30.73 | 16.67 | ±22.17 |
| MHI1497 | 61.18 | ±13.58 | 10.85 | ±15.85 |
| MHI1547 | 92.74 | ±14.85 | 7.25 | ±10.58 |
| MHI1686 | 84.69 | ±6.17 | 2.29 | ±1.82 |
| MHI1698 | 2.32 | ±1.46 | 92.54 | ±8.19 |
| MHI2645 | 84.03 | ±5.92 | 1.94 | ±1.27 |
| MHI69 | 24.79 | ±6.22 | 89.46 | ±8.93 |
| NVH391-98 | 57.11 | ±13.49 | 5.09 | ±1.89 |
| NVH883-00 | 42.59 | ±37.08 | 51.39 | ±41.88 |
| TIAC106 | 86.59 | ±11.64 | 1.38 | ±1.54 |
| TIAC108 | 71.72 | ±5.13 | 5.86 | ±11.44 |
| TIAC111 | 86.85 | ±15.55 | 2.45 | ±1.48 |
| TIAC132 | 75.37 | ±12.88 | 4.36 | ±4.07 |
| TIAC139 | 2.25 | ±1.23 | 97.76 | ±8.09 |
| TIAC179 | 63.24 | ±28.70 | 49.29 | ±36.63 |
| TIAC180 | 41.55 | ±32.90 | 7.69 | ±12.26 |
| TIAC182 | 80.20 | ±21.67 | 18.62 | ±23.40 |
| TIAC217 | 75.18 | ±6.41 | 4.56 | ±4.21 |
| TIAC219 | 77.62 | ±11.79 | 2.17 | ±1.77 |
| TIAC247 | 32.42 | ±14.44 | 94.48 | ±19.98 |
| TIAC297 | 36.44 | ±12.30 | 84.47 | ±16.95 |
| TIAC299 | 37.99 | ±11.58 | 67.08 | ±25.43 |
| TIAC30 | 76.23 | ±16.16 | 6.58 | ±7.96 |
| TIAC371 | 57.63 | ±28.46 | 25.71 | ±28.22 |
| TIAC468 | 87.47 | ±27.66 | 11.64 | ±13.58 |
| TIAC67 | 31.77 | ±39.17 | 94.24 | ±19.45 |
| TIAC70 | 84.83 | ±9.49 | 0.90 | ±0.69 |
| TIAC71 | 65.19 | ±25.54 | 31.21 | ±33.68 |
| TIAC72 | 79.57 | ±13.86 | 1.26 | ±0.76 |
| TIAC73 | 79.73 | ±9.57 | 1.52 | ±0.90 |
| TIAC75 | 52.42 | ±24.35 | 47.59 | ±40.58 |
| TIAC76 | 47.30 | ±8.44 | 38.72 | ±18.19 |
| TIAC78 | 37.24 | ±14.40 | 59.31 | ±35.38 |
| TIAC896 | 67.79 | ±10.61 | 21.80 | ±11.95 |
| VD102 | 2.92 | ±0.90 | 96.36 | ±4.86 |
| VD107 | 4.89 | ±4.11 | 105.17 | ±16.05 |
| VD14 | 83.92 | ±31.05 | 2.91 | ±1.58 |
| VD21 | 13.50 | ±6.60 | 101.68 | ±10.11 |
| VD37 | 32.55 | ±15.36 | 76.41 | ±19.75 |
| VD48 | 48.81 | ±16.71 | 44.53 | ±20.15 |
| VD78 | 11.10 | ±6.46 | 101.28 | ±11.32 |
| 1230-88 1 | 70.53 | ±14.42 | 0.71 | ±0.63 |
| 1230-88 1,* | 1.49 | ±0.87 | 91.22 | ±16.26 |
| LB 1 | 1.75 | ±0.31 | 100.60 | ±14.60 |
| Treatments | LDH Assay | ATP Assay | ||||||
|---|---|---|---|---|---|---|---|---|
| % of Mortality over Triton X-100 | % of Viability over HBSS | |||||||
| Caco-2 | Caco-2 + HT29 | Caco-2 | Caco-2 + HT29 | |||||
| Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | |
| 45 | 69.01 | ±2.98 | 93.16 | ±22.36 | 0.11 | ±0.13 | 0.08 | ±0.08 |
| ATCC10987 | 25.98 | ±9.06 | 29.86 | ±15.51 | 28.94 | ±19.82 | 33.11 | ±9.50 |
| ATCC14579 | 72.63 | ±7.59 | 67.10 | ±9.80 | 0.37 | ±0.24 | 0.58 | ±0.40 |
| Bc1584 | 41.18 | ±4.95 | 45.44 | ±13.73 | 15.62 | ±4.39 | 15.74 | ±5.14 |
| FM1 | 59.79 | ±11.34 | 50.37 | ±13.58 | 0.65 | ±0.52 | 1.24 | ±0.53 |
| FP211 | 51.35 | ±17.81 | 42.39 | ±18.11 | 14.31 | ±11.66 | 19.50 | ±16.26 |
| HD73 | 52.66 | ±11.31 | 54.02 | ±26.04 | 1.81 | ±1.42 | 4.95 | ±4.80 |
| MHI1494 | 50.74 | ±14.60 | 54.69 | ±34.00 | 8.19 | ±8.80 | 5.87 | ±6.26 |
| MHI1547 | 65.12 | ±9.82 | 56.79 | ±7.86 | 2.00 | ±2.48 | 2.98 | ±2.13 |
| MHI1686 | 62.11 | ±16.75 | 54.58 | ±11.38 | 0.98 | ±1.07 | 1.72 | ±1.44 |
| MHI2645 | 43.85 | ±15.50 | 30.57 | ±3.12 | 9.72 | ±3.52 | 16.32 | ±4.51 |
| NVH883-00 | 27.72 | ±21.58 | 30.38 | ±22.86 | 53.76 | ±54.37 | 40.25 | ±35.98 |
| TIAC106 | 37.15 | ±13.63 | 47.23 | ±24.35 | 16.77 | ±15.12 | 15.48 | ±13.93 |
| TIAC111 | 69.08 | ±9.64 | 57.20 | ±22.38 | 0.22 | ±0.36 | 0.49 | ±0.55 |
| TIAC217 | 69.59 | ±13.33 | 62.97 | ±19.07 | 2.12 | ±1.01 | 2.47 | ±0.68 |
| TIAC70 | 57.85 | ±6.88 | 68.00 | ±9.08 | 2.33 | ±0.54 | 2.33 | ±0.55 |
| TIAC896 | 74.28 | ±16.66 | 71.80 | ±20.77 | 0.86 | ±0.77 | 1.21 | ±1.04 |
| VD14 | 67.94 | ±2.43 | 77.85 | ±5.59 | 0.88 | ±0.07 | 0.31 | ±0.12 |
| NVH1230-88 1 | 62.72 | ±13.21 | 73.85 | ±7.55 | 0.71 | ±0.46 | 0.90 | ±0.59 |
| NVH1230-88 1,* | 11.22 | ±3.98 | 13.82 | ±9.24 | 66.45 | ±16.72 | 50.02 | ±5.73 |
| Name | Type of Isolates | Country of Isolation | References |
|---|---|---|---|
| 1230-88 | FP | Norway | [48] |
| 15 | U | Norway | [48] |
| 45 | F | Norway | [48] |
| 27409 | F | Belgium | ILVO 1 |
| 390-88 | F | Norway | [48] |
| A1 | E | Antarctica | MIAE 2 |
| A12 | E | France | [43] |
| AH621 | E | Norway | [66] |
| AH676 | E | Norway | [66] |
| ATCC10876 | U | Unknown | [48] |
| ATCC10987 | Food | Canada | [48] |
| ATCC14579 | E | USA | [48] |
| B16 | FP | Canada | [48] |
| B7 | FP | Canada | [48] |
| Bc1558 | FP | Belgium | MIAE |
| Bc1576 | FP | Belgium | MIAE |
| Bc1584 | FP | Belgium | MIAE |
| F4501-83 | C | Norway | [67] |
| F4810-72 | FP | United Kingdom | [43] |
| F5063/95 | U | United Kingdom | K. Grant 3 |
| FM-1 | FP | Japan | [30] |
| FP211-A | FP | Belgium | [43] |
| H3081/97 | FP | USA | [43] |
| HD73 | E | China | [68] |
| I13-2 | Food | China | [69] |
| I8-5 | Food | China | [69] |
| ISP2954 | FP | Belgium | MIAE |
| ISP3191 | FP | Belgium | MIAE |
| MHI13 | Food | Germany | [35] |
| MHI1494 | Food | Germany | [35] |
| MHI1497 | FP | Germany | [35] |
| MHI1547 | Food | Germany | [35] |
| MHI1686 | Food | Germany | [35] |
| MHI1698 | FP | Germany | [35] |
| MHI2645 | Food | Germany | [35] |
| MHI69 | Food | Germany | [35] |
| NVH391-98 | FP | France | [12] |
| NVH883-00 | F | France | [12] |
| TIAC106 | FP | Belgium | IPH 4 |
| TIAC108 | FP | Belgium | IPH |
| TIAC111 | FP | Belgium | IPH |
| TIAC132 | FP | Belgium | IPH |
| TIAC139 | FP | Belgium | IPH |
| TIAC179 | FP | Belgium | [48] |
| TIAC180 | FP | Belgium | [48] |
| TIAC182 | FP | Belgium | IPH |
| TIAC217 | FP | Belgium | IPH |
| TIAC219 | FP | Belgium | IPH |
| TIAC247 | FP | Belgium | IPH |
| TIAC297 | FP | Belgium | [48] |
| TIAC299 | FP | Belgium | IPH |
| TIAC30 | FP | Belgium | IPH |
| TIAC371 | FP | Belgium | IPH |
| TIAC468 | FP | Belgium | [48] |
| TIAC67 | FP | Belgium | IPH |
| TIAC70 | FP | Belgium | [48] |
| TIAC71 | FP | Belgium | [48] |
| TIAC72 | FP | Belgium | IPH |
| TIAC73 | FP | Belgium | [48] |
| TIAC75 | FP | Belgium | [48] |
| TIAC76 | FP | Belgium | IPH |
| TIAC78 | FP | Belgium | [48] |
| TIAC896 | FP | Belgium | IPH |
| VD102 | E | Guadeloupe | MIAE |
| VD107 | E | Guadeloupe | MIAE |
| VD14 | E | Spain | MIAE |
| VD21 | E | Belgium | MIAE |
| VD37 | E | Belgium | MIAE |
| VD48 | E | Greenland | MIAE |
| VD78 | E | Greenland | MIAE |
References
- Bottone, E.J. B. cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef] [PubMed]
- Takabe, F.; Oya, M. An autopsy case of food poisoning associated with B. cereus. Forensic. Sci. 1976, 7, 97–101. [Google Scholar] [CrossRef]
- Mahler, H.; Pasi, A.; Kramer, J.M.; Schulte, P.; Scoging, A.C.; Bär, W.; Krähenbühl, S. Fulminant liver failure in association with the emetic toxin of B. cereus. N. Engl. J. Med. 1997, 336, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Dierick, K.; Van Coillie, E.; Swiecicka, I.; Meyfroidt, G.; Devlieger, H.; Meulemans, A.; Hoedemaekers, G.; Fourie, L.; Heyndrickx, M.; Mahillon, J. Fatal family outbreak of B. cereus-associated food poisoning. J. Clin. Microbiol. 2005, 43, 4277–4229. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Saitou, K.; Mizumoto, H.; Matsusaka, M.; Agata, N.; Nakayama, M.; Kage, M.; Tatsumi, S.; Okamoto, A.; Yamaguchi, S.; et al. Rapid detoxification of cereulide in B. cereus food poisoning. Pediatrics 2010, 125, e951–e955. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, M.; Denayer, S.; Botteldoorn, N.; Delbrassinne, L.; Veys, J.; Waegenaere, J.; Sirtaine, N.; Driesen, R.B.; Sipido, K.R.; Mahillon, J.; et al. Sudden death of a young adult associated with B. cereus food poisoning. J. Clin. Microbiol. 2011, 49, 4379–4381. [Google Scholar] [CrossRef] [PubMed]
- Rajkovic, A.; Uyttendaele, M.; Vermeulen, A.; Andjelkovic, M.; Fitz-James, I.; Denon, Q.; Verhé, R.; Debevere, J. Heat resistance of B. cereus emetic toxin, cereulide. Lett. Appl. Microbiol. 2008, 46, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Agata, N.; Ohta, M.; Mori, M.; Isobe, M. A novel dodecadepsipeptide, cereulide, is an emetic toxin of B. cereus. FEMS Microbiol. Lett. 1995, 129, 17–20. [Google Scholar] [PubMed]
- Granum, P. B. cereus and its food poisoning toxins. FEMS Microbiol. Lett. 1997, 157, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Beecher, D.J.; Macmillan, J.D. Characterization of the components of hemolysin BL from B. cereus. Infect. Immun. 1991, 59, 1778–1784. [Google Scholar] [PubMed]
- Lund, T.; Granum, P.E. Characterisation of a non-haemolytic enterotoxin complex from B. cereus isolated after a foodborne outbreak. FEMS Microbiol. Lett. 1996, 141, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.; De Buyser, M.-L.; Granum, P.E. A new cytotoxin from B. cereus that may cause necrotic enteritis. Mol. Microbiol. 2000, 38, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Beecher, D.; Schoeni, J.; Wong, A. Enterotoxic activity of hemolysin BL from B. cereus. Infect. Immun. 1995, 63, 4423–4428. [Google Scholar] [PubMed]
- Lindbäck, T.; Fagerlund, A.; Rødland, M.S.; Granum, P.E. Characterization of the B. cereus Nhe enterotoxin. Microbiology 2004, 150, 3959–3967. [Google Scholar] [CrossRef] [PubMed]
- Fagerlund, A.; Lindbäck, T.; Storset, A.K.; Granum, P.E.; Hardy, S.P. B. cereus Nhe is a pore-forming toxin with structural and functional properties similar to the ClyA (HlyE, SheA) family of haemolysins, able to induce osmotic lysis in epithelia. Microbiology 2008, 154, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Madegowda, M.; Eswaramoorthy, S.; Burley, S.K.; Swaminathan, S. X-ray crystal structure of the B component of Hemolysin BL from B. cereus. Proteins Struct. Funct. Genet. 2008, 71, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Ganash, M.; Phung, D.; Sedelnikova, S.E.; Lindbäck, T.; Granum, P.E.; Artymiuk, P.J. Structure of the NheA component of the Nhe toxin from B. cereus: Implications for function. PLoS ONE 2013, 8, e74748. [Google Scholar] [CrossRef] [PubMed]
- Fagerlund, A.; Ween, O.; Lund, T.; Hardy, S.P.; Granum, P.E. Genetic and functional analysis of the cytK family of genes in B. cereus. Microbiology 2004, 150, 2689–2697. [Google Scholar] [CrossRef] [PubMed]
- Fagerlund, A.; Brillard, J.; Fürst, R.; Guinebretière, M.-H.; Granum, P.E. Toxin production in a rare and genetically remote cluster of strains of the B. cereus group. BMC Microbiol. 2007, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Kreft, J.; Berger, H.; Hartlein, M.; Muller, B.; Weidinger, G.; Goebel, W. Cloning and expression in Escherichia coli and Bacillus subtilis of the hemolysin (cereolysin) determinant from B. cereus. J. Bacteriol. 1983, 155, 681–689. [Google Scholar] [PubMed]
- Baida, G.; Budarina, Z.I.; Kuzmin, N.P.; Solonin, A.S. Complete nucleotide sequence and molecular characterization of hemolysin II gene from B. cereus. FEMS Microbiol. Lett. 1999, 180, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Baida, G.E.; Kuzmin, N.P. Cloning and primary structure of a new hemolysin gene from B. cereus. Biochim. Phys. Acta 1995, 1264, 151–154. [Google Scholar] [CrossRef]
- Kuppe, A.; Evans, L.M.; McMillen, D.A.; Griffith, O.H. Phosphatidylinositol-specific phospholipase C of B. cereus: Cloning, sequencing, and relationship to other phospholipases. J. Bacteriol. 1989, 171, 6077–6083. [Google Scholar] [PubMed]
- Lechner, M.; Kupke, T.; Stefanovic, S.; Götz, F. Molecular characterization and sequence of phosphatidylinositol-specific phospholipase C of Bacillus thuringiensis. Mol. Microbiol. 1989, 3, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Schmiel, D.H.; Miller, V.L. Bacterial phospholipases and pathogenesis. Microbes Infect. 1999, 1, 1103–1112. [Google Scholar] [CrossRef]
- Doll, V.M.; Ehling-Schulz, M.; Vogelmann, R. Concerted action of sphingomyelinase and non-hemolytic enterotoxin in pathogenic B. cereus. PLoS ONE 2013, 8, e61404. [Google Scholar] [CrossRef] [PubMed]
- Fedhila, S.; Nel, P.; Lereclus, D. The InhA2 metalloprotease of Bacillus thuringiensis strain 407 is required for pathogenicity in insects infected via the oral route. J. Bacteriol. 2002, 184, 3296–3304. [Google Scholar] [CrossRef] [PubMed]
- Cadot, C.; Tran, S.-L.; Vignaud, M.-L.; De Buyser, M.-L.; Kolstø, A.-B.; Brisabois, A.; Nguyen-Thé, C.; Lereclus, D.; Guinebretière, M.-H.; et al. InhA1, NprA, and HlyII as candidates for markers to differentiate pathogenic from nonpathogenic B. cereus strains. J. Clin. Microbiol. 2010, 48, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Guillemet, E.; Cadot, C.; Tran, S.-L.; Guinebretière, M.-H.; Lereclus, D.; Ramarao, N. The {InhA} metalloproteases of B. cereus contribute concomitantly to virulence. J. Bacteriol. 2010, 192, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Asano, S.I.; Nukumizu, Y.; Bando, H.; Iizuka, T.; Yamamoto, T. Cloning of novel enterotoxin genes from B. cereus and Bacillus thuringiensis. Appl. Environ. Microbiol. 1997, 63, 1054–1057. [Google Scholar] [PubMed]
- Tran, S.-L.; Guillemet, E.; Gohar, M.; Lereclus, D.; Ramarao, N. CwpFM (EntFM) is a B. cereus potential cell wall peptidase implicated in adhesion, biofilm formation, and virulence. J. Bacteriol. 2010, 192, 2638–2642. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Robine-Leon, S.; Appay, M.; Kedlinger, M.; Triadou, N.; Dussaulx, E.; Lacroix, B.; Simon-Assman, P.; Haffen, K.; Fogh, J.; et al. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol. Cell 1983, 47, 323–330. [Google Scholar]
- Puerto, M.; Pichardo, S.; Jos, A.; Cameón, A.M. Comparison of the toxicity induced by microcystin-RR and microcystin-YR in differentiated and undifferentiated Caco-2 cells. Toxicon 2009, 54, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Nollevaux, G.; Devillé, C.; El Moualij, B.; Zorzi, W.; Deloyer, P.; Schneider, Y.-J.; Peulen, O.; Dandrifosse, G. Development of a serum-free co-culture of human intestinal epithelium cell-lines (Caco-2/HT29–5M21). BMC Cell Biol. 2006, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Moravek, M.; Dietrich, R.; Buerk, C.; Broussolle, V.; Guinebretière, M.-H.; Granum, P.E.; Märtlbauer, E. Determination of the toxic potential of B. cereus isolates by quantitative enterotoxin analyses. FEMS Microbiol. Lett. 2006, 257, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Swiecicka, I.; Van der Auwera, G.A.; Mahillon, J. Hemolytic and nonhemolytic enterotoxin genes are broadly distributed among Bacillus thuringiensis isolated from wild mammals. Microb. Ecol. 2006, 52, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-B.; Kim, J.-B.; Shin, S.-W.; Kim, J.-C.; Cho, S.-H.; Lee, B.-K.; Ahn, J.; Kim, J.-M.; Oh, D.-H. Prevalence, genetic diversity, and antibiotic susceptibility of B. cereus strains isolated from rice and cereals collected in Korea. J. Food Prot. 2009, 72, 612–617. [Google Scholar] [PubMed]
- Hwang, J.-Y.; Park, J.-H. Characteristics of enterotoxin distribution, hemolysis, lecithinase, and starch hydrolysis of B. cereus isolated from infant formulas and ready-to-eat foods. J. Dairy Sci. 2015, 98, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Minnaard, J.; Delfederico, L.; Vasseur, V.; Hollmann, A.; Rolny, I.; Semorile, L.; Pérez, P.F. Virulence of B. cereus: A multivariate analysis. Int. J. Food Microbiol. 2007, 116, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Han, J.-K.; Park, J.-S.; Lee, J.-S.; Lee, S.-H.; Cho, J.-I.; Kim, K.-S. Various enterotoxin and other virulence factor genes widespread among B. cereus and Bacillus thuringiensis strains. J. Microbiol. Biotechnol. 2015, 25, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Celandroni, F.; Salvetti, S.; Gueye, S.A.; Mazzantini, D.; Lupetti, A.; Senesi, S.; Ghelardi, E. Identification and pathogenic potential of clinical Bacillus and Paenibacillus isolates. PLoS ONE 2016, 11, e0152831. [Google Scholar] [CrossRef] [PubMed]
- Hoton, F.M.; Fornelos, N.; N’guessan, E.; Hu, X.; Swiecicka, I.; Dierick, K.; Jääskeläinen, E.; Salkinoja-Salonen, M.; Mahillon, J. Family portrait of B. cereus and Bacillus weihenstephanensis cereulide-producing strains. Environ. Microbiol. Rep. 2009, 1, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Castiaux, V.; N’guessan, E.; Swiecicka, I.; Delbrassinne, L.; Dierick, K.; Mahillon, J. Diversity of pulsed-field gel electrophoresis patterns of cereulide-producing isolates of B. cereus and Bacillus weihenstephanensis. FEMS Microbiol. Lett. 2014, 353, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, P.C.; Kramer, J.M.; Jörgensen, K.; Gilbert, R.J.; Melling, J. Properties and production characteristics of vomiting, diarrheal, and necrotizing toxins of B. cereus. Am. J. Clin. Nutr. 1979, 32, 219–228. [Google Scholar] [PubMed]
- Hoffmaster, A.R.; Novak, R.T.; Marston, C.K.; Gee, J.E.; Helsel, L.; Pruckler, J.M.; Wilkins, P.P. Genetic diversity of clinical isolates of B. cereus using multilocus sequence typing. BMC Microbiol. 2008, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, R.; Moravek, M.; Bürk, C.; Granum, P.E.; Märtlbauer, E. Production and characterization of antibodies against each of the three subunits of the B. cereus nonhemolytic enterotoxin complex. Appl. Environ. Microbiol. 2005, 71, 8214–8220. [Google Scholar] [CrossRef] [PubMed]
- Jeßberger, N.; Dietrich, R.; Bock, S.; Didier, A.; Märtlbauer, E. B. cereus enterotoxins act as major virulence factors and exhibit distinct cytotoxicity to different human cell lines. Toxicon 2014, 77, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Castiaux, V.; Liu, X.; Delbrassinne, L.; Mahillon, J. Is Cytotoxin K from B. cereus a bona fide enterotoxin? Int. J. Food Microbiol. 2015, 211, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Jeßberger, N.; Krey, V.; Rademacher, C.; Böhm, M.; Mohr, A.; Ehling-Schulz, M.; Scherer, S.; Märtlbauer, E. From genome to toxicity: A combinatory approach highlights the complexity of enterotoxin production in B. cereus. Front. Microbiol. 2015, 6, 560. [Google Scholar] [CrossRef] [PubMed]
- Gilois, N.; Ramarao, N.; Bouillaut, L.; Perchat, S.; Aymerich, S.; Nielsen-Leroux, C.; Lereclus, D.; Gohar, M. Growth-related variations in the B. cereus secretome. Proteomics 2007, 7, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, E.L.; Teplova, V.; Andersson, M.A.; Andersson, L.C.; Tammela, P.; Andersson, M.C.; Pirhonen, T.I.; Saris, N.-E.; Vuorela, P.; Salkinoja-Salonen, M.S. In vitro assay for human toxicity of cereulide, the emetic mitochondrial toxin produced by food poisoning B. cereus. Toxicol. Vitro 2003, 17, 737–744. [Google Scholar] [CrossRef]
- Rajkovic, A.; Grootaert, C.; Butorac, A.; Cucu, T.; De Meulenaer, B.; van Camp, J.; Bracke, M.; Uyttendaele, M.; Bačun-Družina, V.; Cindrić, M. Sub-emetic toxicity of B. cereus toxin cereulide on cultured human enterocyte-like Caco-2 cells. Toxins (Basel) 2014, 6, 2270–2290. [Google Scholar] [CrossRef] [PubMed]
- Guinebretière, M.-H.; Velge, P.; Couvert, O.; Carlin, F.; Debuyser, M.-L.; Nguyen-The, C. Ability of B. cereus group strains to cause food poisoning varies according to phylogenetic affiliation (groups I to VII) rather than species affiliation. J. Clin. Microbiol. 2010, 48, 3388–3391. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Tsilia, V.; Kerckhof, F.-M.; Rajkovic, A.; Heyndrickx, M.; van de Wiele, T. B. cereus NVH 0500/00 can adhere to mucin, but cannot produce enterotoxins during gastrointestinal simulation. Appl. Environ. Microbiol. 2015, 82, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Fricker, M.; Scherer, S. Identification of emetic toxin producing B. cereus strains by a novel molecular assay. FEMS Microbiol. Lett. 2004, 232, 189–195. [Google Scholar] [CrossRef]
- Guinebretière, M.-H.; Fagerlund, A.; Granum, P.E.; Nguyen-The, C. Rapid discrimination of cytK-1 and cytK-2 genes in B. cereus strains by a novel duplex PCR system. FEMS Microbiol. Lett. 2006, 259, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Guinebretière, M.-H.; Broussolle, V.; Nguyen-The, C. Enterotoxigenic profiles of food-poisoning and food-borne B. cereus strains. J. Clin. Microbiol. 2002, 40, 3053–3056. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, P.H.; Jacobsen, C.S.; Sørensen, J. Development and application of a primer set for specific detection of Bacillus thuringiensis and B. cereus in soil using magnetic capture hybridization and PCR amplification. Syst. Appl. Microbiol. 1996, 19, 436–441. [Google Scholar] [CrossRef]
- Hsieh, Y.M.; Sheu, S.J.; Chen, Y.L.; Tsen, H.Y. Enterotoxigenic profiles and polymerase chain reaction detection of B. cereus group cells and B. cereus strains from foods and food-borne outbreaks. J. Appl. Microbiol. 1999, 87, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Lasarow, R.M.; Isseroff, R.R.; Gomez, E.C. Quantitative in vitro assessment of phototoxicity by a fibroblast-neutral red assay. J. Invest. Dermatol. 1992, 98, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, C.; Coiffard, C.; Coiffard, L.J.M.; Rivalland, P.; De Roeck-Holtzhauer, Y. In vitro correlation between two colorimetric assays and the pyruvic acid consumption by fibroblasts cultured to determine the sodium laurylsulfate cytotoxicity. J. Pharmacol. Toxicol. Methods 1998, 39, 143–146. [Google Scholar] [CrossRef]
- Dufrane, D.; Delloye, C.; Mckay, I.J.; De Aza, P.N.; De Aza, S.; Schneider, Y.-J.; Anseau, M. Indirect cytotoxicity evaluation of pseudowollastonite. J. Mater. Sci. Mater. Med. 2003, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Stenfors, L.P.; Mayr, R.; Scherer, S.; Granum, P.E. Pathogenic potential of fifty Bacillus weihenstephanensis strains. FEMS Microbiol. Lett. 2002, 215, 47–51. [Google Scholar] [CrossRef] [PubMed]
- From, C.; Pukall, R.; Schumann, P.; Hormazábal, V.; Granum, P.E. Toxin—Producing ability among Bacillus spp. outside the B. cereus group. Appl. Environ. Microbiol. 2005, 71, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Zwick, M.E.; Joseph, S.J.; Didelot, X.; Chen, P.E.; Bishop-Lilly, K.A.; Stewart, A.C.; Willner, K.; Nolan, N.; Lentz, S.; Thomason, M.K.; et al. Genomic characterization of the B. cereus sensu lato species: Backdrop to the evolution of Bacillus anthracis. Genome Res. 2012, 22, 1512–1524. [Google Scholar] [CrossRef] [PubMed]
- Stenfors, L.P.; Granum, P.E. Psychrotolerant species from the B. cereus group are not necessarily Bacillus weihenstephanensis. FEMS Microbiol. Lett. 2001, 197, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Song, L.; Shu, C.; Wang, P.; Deng, C.; Peng, Q.; Lereclus, D.; Wang, X.; Huang, D.; Zhang, J.; et al. Complete genome sequence of Bacillus thuringiensis subsp. kurstaki strain HD73. Genome Announc. 2013, 1, 2–3. [Google Scholar]
- Zhou, G.; Zheng, D.; Dou, L.; Cai, Q.; Yuan, Z. Occurrence of psychrotolerant B. cereus group strains in ice creams. Int. J. Food Microbiol. 2010, 137, 143–146. [Google Scholar] [CrossRef] [PubMed]
| Origin 1 | Strain | Enterotoxin Genes or Genetic Determinants of Virulence Factors Tested 2 | Toxicity Values 3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ces | cytK1 | cytK2 | entS | entFM | hblC | hblD | hblA | hlyII | nheA | nheB | nheC | nprA | piplC | sph | LDH | NR | ||
| C | F4501-83 | - | - | + | + | + | + | + | + | + | + | + | + | + | + | + | 3 | 3 |
| E | A1 | - | - | + | + | + | + | + | + | + | + | + | + | + | + | - | 0 | 0 |
| A12 | - | - | - | + | + | - | - | - | + | + | + | + | + | + | + | 0 | 1 | |
| AH621 | - | - | + | + | + | + | + | + | - | + | + | + | + | + | + | 0 | 0 | |
| AH676 | - | - | - | + | + | - | - | - | - | + | + | + | + | - | + | 0 | 0 | |
| ATCC14579 | - | - | + | + | + | + | + | - | + | + | + | + | + | + | + | 3 | 2 | |
| HD73 | - | - | + | + | + | + | + | + | + | + | + | + | + | + | + | 2 | 2 | |
| VD102 | - | - | + | + | + | - | - | - | - | + | + | + | + | - | + | 0 | 0 | |
| VD107 | - | - | - | + | + | - | + | - | - | + | + | + | + | + | - | 0 | 0 | |
| VD14 | - | - | - | + | + | + | - | + | - | + | + | + | + | + | + | 3 | 3 | |
| VD21 | - | - | - | + | - | - | - | - | - | + | + | - | + | - | + | 0 | 0 | |
| VD37 | - | - | + | + | + | + | + | + | - | + | + | + | + | + | + | 0 | 1 | |
| VD48 | - | - | + | + | + | + | + | + | + | + | + | + | + | + | + | 2 | 1 | |
| VD78 | - | - | - | + | + | + | + | + | - | + | + | - | + | - | - | 0 | 0 | |
| F | 45 | - | - | + | + | - | + | - | + | - | + | + | + | + | + | + | 3 | 3 |
| 27409 | - | - | + | + | + | - | - | - | - | + | + | + | + | + | + | 2 | 2 | |
| 390-88 | - | - | - | + | + | - | + | - | + | + | + | + | + | + | + | 1 | 1 | |
| ATCC10987 | - | - | + | + | + | - | - | - | - | + | + | + | + | + | + | 3 | 3 | |
| I13-2 | - | - | + | + | + | + | + | - | + | + | + | + | + | + | + | 3 | 3 | |
| I8-5 | - | - | + | + | + | + | + | + | - | + | + | + | + | + | + | 3 | 3 | |
| ISP2954 | - | - | + | + | + | + | + | + | - | + | + | + | + | + | + | 1 | 1 | |
| ISP3191 | - | - | - | + | + | - | - | - | + | + | + | + | + | + | + | 3 | 3 | |
| MHI13 | - | - | - | + | + | - | - | - | + | + | + | + | + | + | + | 0 | 1 | |
| MHI1494 | - | - | - | + | + | - | - | - | + | + | + | + | + | + | + | 3 | 2 | |
| MHI1547 | - | - | + | + | + | + | + | + | - | + | + | + | + | + | + | 3 | 3 | |
| MHI1686 | - | - | + | + | + | - | - | - | - | + | + | + | + | + | + | 3 | 3 | |
| MHI2645 | - | - | + | + | + | + | + | + | - | + | + | + | + | + | + | 3 | 3 | |
| MHI69 | - | - | - | + | + | - | - | - | - | + | + | + | + | + | + | 0 | 1 | |
| NVH883-00 | - | + | - | + | + | + | + | + | + | + | + | + | + | + | + | 1 | 1 | |
| FP | NVH1230-88 | - | - | + | + | + | + | + | + | + | + | + | + | + | + | + | 3 | 3 |
| B16 | - | - | + | + | + | + | + | + | - | + | + | + | + | + | + | 3 | 2 | |
| B7 | - | - | - | + | + | + | + | - | - | + | + | + | + | + | + | 3 | 3 | |
| Bc1558 | - | - | + | + | + | - | - | - | - | + | + | + | + | + | + | 3 | 3 | |
| Bc1576 | + | - | - | + | + | - | - | - | - | + | + | + | + | + | + | 3 | 2 | |
| Bc1584 | + | - | - | + | + | - | - | - | - | + | + | + | + | + | + | 2 | 2 | |
| F4810-72 | + | - | - | + | + | - | - | - | - | + | + | + | + | + | + | 0 | 0 | |
| FM-1 | - | - | + | + | + | + | + | + | + | + | + | + | + | + | + | 3 | 3 | |
| FP211-A | + | - | - | + | + | - | - | - | - | + | + | + | + | + | + | 3 | 3 | |
| H3081/97 | + | - | - | + | + | - | - | - | - | + | + | + | + | + | + | 0 | 0 | |
| MHI1497 | - | - | + | + | + | + | + | + | - | + | + | + | + | + | + | 3 | 2 | |
| MHI1698 | - | - | - | + | + | - | - | - | - | + | + | + | + | - | + | 0 | 0 | |
| NVH391-98 | - | + | - | + | - | - | - | - | - | + | - | - | + | + | + | 3 | 2 | |
| TIAC106 | - | - | + | + | + | + | + | + | - | + | + | + | + | + | + | 3 | 3 | |
| TIAC108 | - | - | + | + | + | - | - | - | - | + | + | + | + | + | + | 3 | 3 | |
| TIAC111 | - | - | + | + | + | + | + | + | + | + | + | + | + | + | + | 3 | 3 | |
| TIAC132 | - | - | + | + | + | + | + | + | - | + | + | + | + | + | + | 3 | 3 | |
| TIAC139 | - | - | - | + | + | - | - | - | - | + | + | - | + | + | + | 0 | 0 | |
| TIAC179 | - | - | + | + | + | + | + | + | + | + | + | + | + | + | + | 2 | 2 | |
| TIAC180 | - | - | + | + | + | - | - | - | - | + | + | + | + | + | + | 3 | 1 | |
| TIAC182 | - | - | + | + | + | + | + | + | - | + | + | + | + | + | + | 3 | 3 | |
| TIAC217 | - | - | + | + | + | + | + | + | - | + | + | + | + | + | + | 3 | 3 | |
| TIAC219 | + | - | + | + | + | + | + | + | + | + | + | + | + | + | + | 3 | 3 | |
| TIAC247 | - | - | - | + | + | - | - | - | - | + | + | + | + | + | + | 0 | 1 | |
| TIAC297 | - | - | + | + | + | + | - | - | - | + | + | + | + | + | + | 0 | 1 | |
| TIAC299 | - | - | + | + | + | + | + | + | - | + | + | + | + | + | + | 1 | 1 | |
| TIAC30 | + | - | + | + | + | - | - | - | - | + | + | + | + | + | + | 3 | 3 | |
| TIAC371 | - | - | - | + | + | + | - | - | + | + | + | + | + | + | + | 2 | 2 | |
| TIAC468 | - | - | - | + | + | - | - | - | - | + | + | + | + | + | + | 3 | 3 | |
| TIAC67 | - | - | - | + | + | + | + | - | - | + | + | - | + | + | + | 0 | 1 | |
| TIAC70 | - | - | + | + | + | + | + | + | + | + | + | + | + | + | + | 3 | 3 | |
| TIAC71 | - | - | + | + | + | + | + | + | + | + | + | + | + | + | + | 2 | 2 | |
| TIAC72 | - | - | + | + | + | - | - | - | - | + | + | + | + | + | + | 3 | 3 | |
| TIAC73 | - | - | + | + | + | - | - | - | - | + | + | + | + | + | + | 3 | 3 | |
| TIAC75 | - | - | + | + | + | + | + | + | - | + | + | + | + | + | + | 2 | 2 | |
| TIAC76 | - | - | + | + | + | + | + | + | + | + | + | - | + | + | + | 2 | 1 | |
| TIAC78 | - | - | + | + | + | + | + | + | + | + | + | + | + | + | + | 1 | 1 | |
| TIAC896 | - | - | + | + | - | + | + | + | + | + | + | + | + | + | + | 2 | 2 | |
| U | 15 | - | - | + | + | + | + | + | + | - | + | + | + | + | + | + | 2 | 2 |
| ATCC10876 | - | - | + | + | + | + | + | + | - | + | + | + | + | + | + | 2 | 2 | |
| F5063/95 | + | - | + | + | + | - | - | - | - | + | + | + | + | + | + | 3 | 3 | |
| Gene | Name | Tm (°C) | Size (bp) | Sequences | References |
|---|---|---|---|---|---|
| Ces | EM1F | 60 | 635 | GACAAGAGAAATTTCTACGAGCAAGTACAAT | [56] |
| EM1R | GCAGCCTTCCAATTACTCCTTCTGCCACAGT | ||||
| cytK-1 | CK1F | 57 | 426 | CAATTCCAGGGGCAAGTGTC | [57] |
| CK1R | CCTCGTGCATCTGTTTCATGAG | ||||
| cytK-2 | CK2F | 57 | 585 | CAATCCCTGGCGCTAGTGCA | [57] |
| CK2R | GTGIAGCCTGGACGAAGTTGG | ||||
| entFM | ENT-A | 52 | 1269 | ATGAAAAAAGTAATTTGCAGG | [30] |
| ENT-B | TTAGTATGCTTTTGTGTAACC | ||||
| entS | TY123 F | 60 | 581 | GGTTTAGCAGCAGCTTCTGTAGCTGGCG | [30] |
| TY125 R | GTTTCGTTAGATACAGCAGAACCACC | ||||
| hlyII | Fhly-II | 48 | 868 | GATTCTAAAGGAACTGTAG | [28] |
| Rhly-II | GGTTATCAAGAGTAACTTG | ||||
| hblC | HC F | 58 | 740 | GATACYAATGTGGCAACTGC | [58] |
| HC R | TTGAGACTGCTCGYTAGTTG | ||||
| hblD | HD F | 58 | 829 | ACCGGTAACACTATTCATGC | [58] |
| HD R | GAGTCCATATGCTTAGATGC | ||||
| hblA | HA F | 56 | 1154 | AAGCAATGGAATACAATGGG | [58] |
| HA R | AGAATCTAAATCATGCCACTGC | ||||
| nheA | NA F | 56 | 755 | GTTAGGATCACAATCACCGC | [58] |
| NA R | ACGAATGTAATTTGAGTCGC | ||||
| nheB | NB F | 54 | 743 | TTTAGTAGTGGATCTGTACGC | [58] |
| NB R | TTAATGTTCGTTAATCCTGC | ||||
| nheC | NC F | 54 | 683 | TGGATTCCAAGATGTAACG | [58] |
| NC R | ATTACGACTTCTGCTTGTGC | ||||
| nprA | F-nprA-d | 55 | 263 | GTATACGGAGATGGTGATGG | [28] |
| R-nprA-d | GGATCACTCATAGAGCGAAG | ||||
| piplC | PC105F | 57 | 569 | CGCTATCAATGGACCATGG | [59] |
| PC106 R | GGACTATTCCATGCTGTACC | ||||
| Sph | Ph1 | 58 | 558 | CGTGCCGATTTAATTGGGGC | [60] |
| Ph2 | CAATGTTTTAAACATGGATGCG |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castiaux, V.; Laloux, L.; Schneider, Y.-J.; Mahillon, J. Screening of Cytotoxic B. cereus on Differentiated Caco-2 Cells and in Co-Culture with Mucus-Secreting (HT29-MTX) Cells. Toxins 2016, 8, 320. https://doi.org/10.3390/toxins8110320
Castiaux V, Laloux L, Schneider Y-J, Mahillon J. Screening of Cytotoxic B. cereus on Differentiated Caco-2 Cells and in Co-Culture with Mucus-Secreting (HT29-MTX) Cells. Toxins. 2016; 8(11):320. https://doi.org/10.3390/toxins8110320
Chicago/Turabian StyleCastiaux, Virginie, Laurie Laloux, Yves-Jacques Schneider, and Jacques Mahillon. 2016. "Screening of Cytotoxic B. cereus on Differentiated Caco-2 Cells and in Co-Culture with Mucus-Secreting (HT29-MTX) Cells" Toxins 8, no. 11: 320. https://doi.org/10.3390/toxins8110320
APA StyleCastiaux, V., Laloux, L., Schneider, Y.-J., & Mahillon, J. (2016). Screening of Cytotoxic B. cereus on Differentiated Caco-2 Cells and in Co-Culture with Mucus-Secreting (HT29-MTX) Cells. Toxins, 8(11), 320. https://doi.org/10.3390/toxins8110320





