Open Field Study of Some Zea mays Hybrids, Lipid Compounds and Fumonisins Accumulation
Abstract
:1. Introduction
2. Results
2.1. Incidence of Fungal Species, F. verticillioides and Fumonisin Production
| Factors | Fungi | AI | FI | FFB | TFB | 9-HODE | 13-HODE | HLP | PHY-CER | NOH |
|---|---|---|---|---|---|---|---|---|---|---|
| Hybrid | n.s. | n.s. | n.s. | ** | * | ** | * | ** | ** | ** |
| H17 | 47.0 | 4.7 | 24.9 | 4109.2 b | 4682.8 b | 7.4 ab | 10.9 | 2.3 ab | 2.7 ab | 2.3 b |
| H18 | 43.1 | 2.7 | 12.9 | 930.3 a | 531.8 a | 10.9 b | 11.3 | 2.9 b | 3.1 b | 2.8 b |
| H19 | 40.6 | 1.6 | 16.3 | 588.8 a | 411.1 a | 6.4 a | 8.6 | 1.9 a | 1.7 a | 1.3 a |
| H20 | 55.6 | 3.4 | 24.1 | 1943.2 ab | 2709.3 ab | 8.3 ab | 9.0 | 2.8 b | 1.8 a | 1.6 a |
| Sampling Time | ** | n.s. | ** | ** | ** | n.s. | ** | ** | ** | ** |
| 1 | 16.2 a | 7.9 | 7.4 a | 0.0 a | 0.0 a | 7.4 | 12.7 b | 2.8 b | 2.8 b | 2.9 b |
| 2 | 33.9 ab | 0.6 | 12.8 a | 481.4 a | 571.8 b | 9.7 | 9.8 ab | 2.8 b | 3.0 b | 2.4 b |
| 3 | 50.2 b | 3.9 | 16.4 a | 1690.6 b | 1734.7 b | 9.5 | 9.5 a | 2.2 ab | 2.4 b | 1.6 a |
| 4 | 85.9 c | 0.0 | 41.6 b | 5399.4 c | 6028.6 b | 6.4 | 7.8 a | 1.9 a | 1.1 a | 1.1 a |
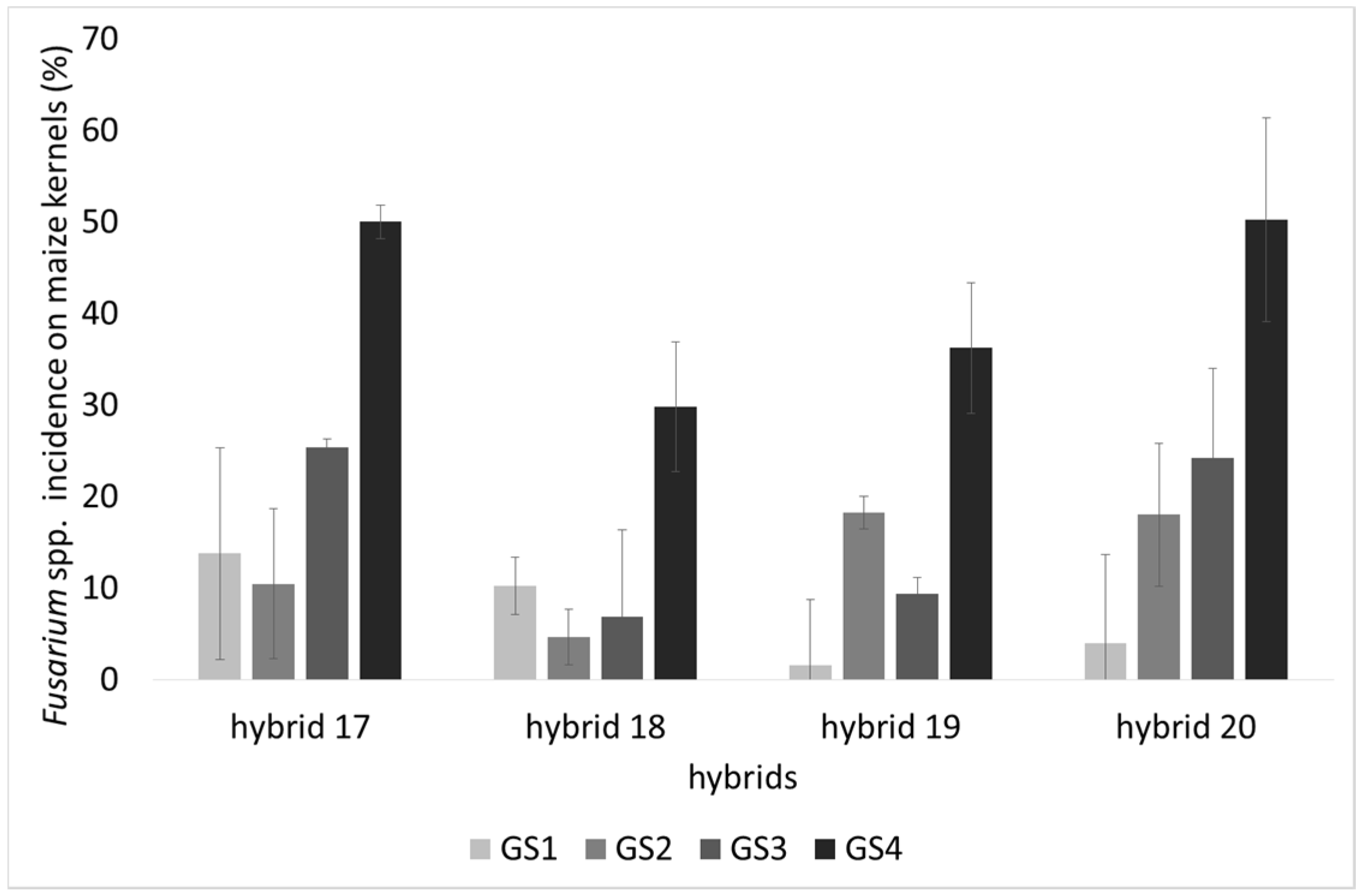
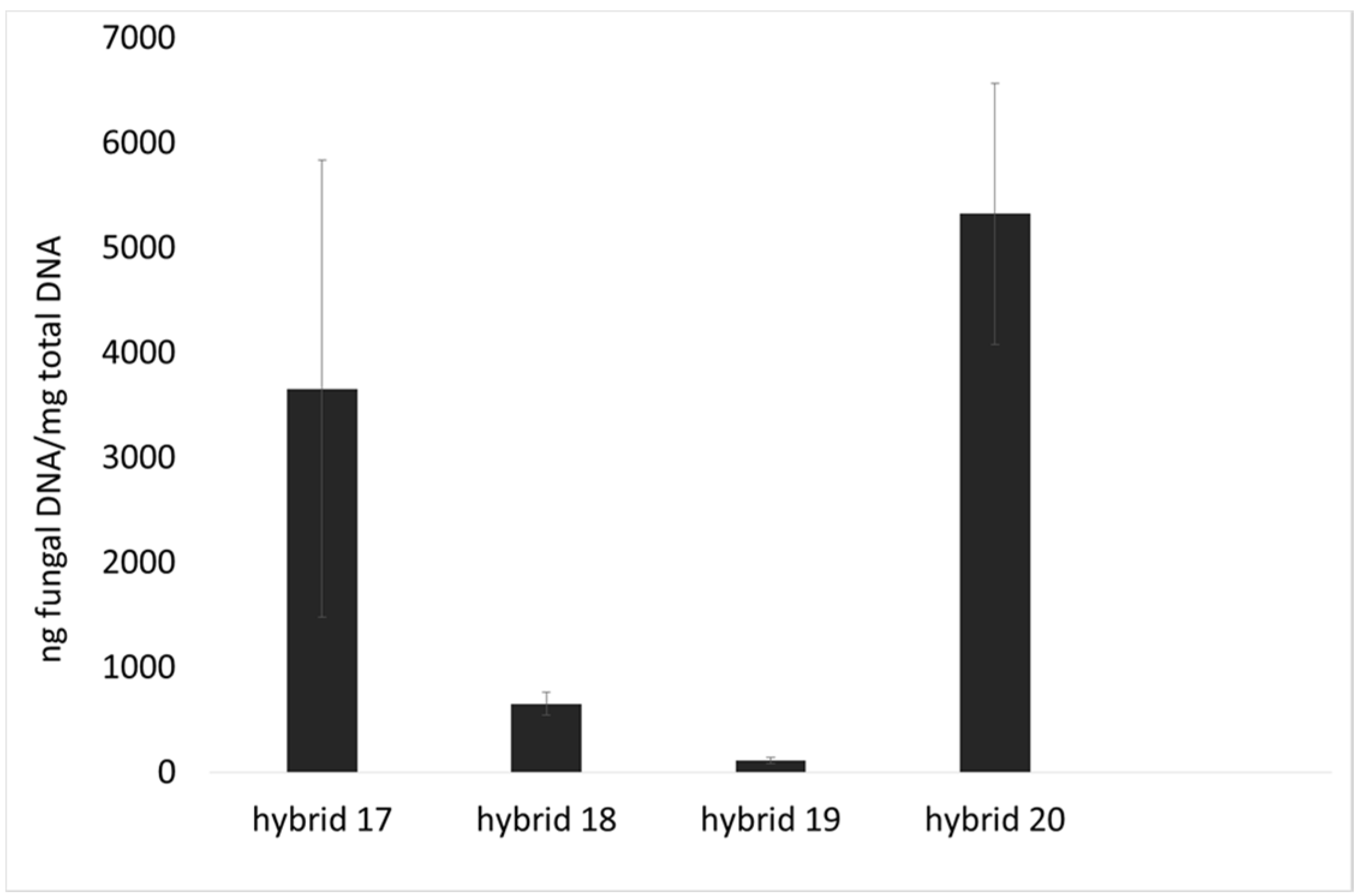
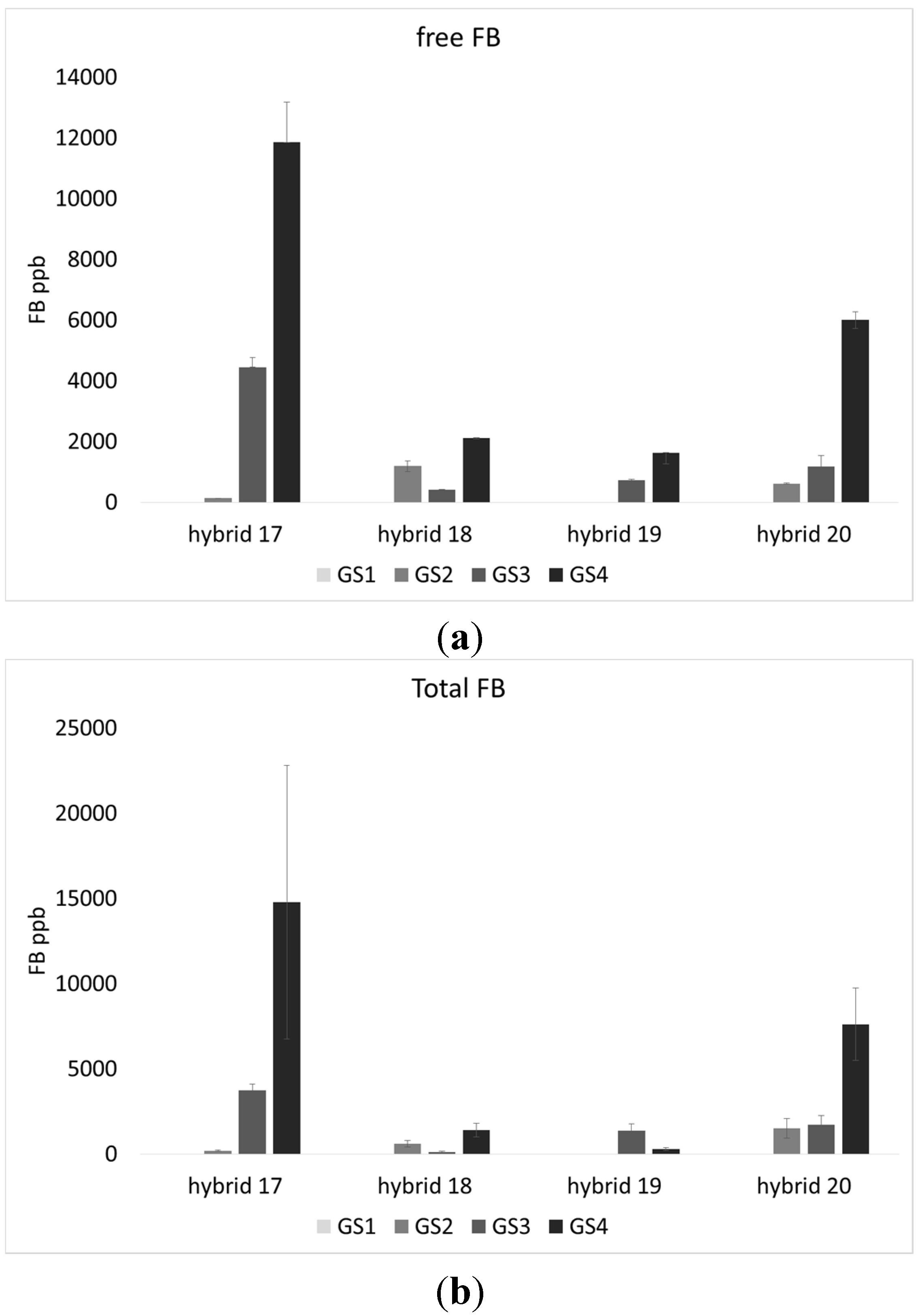
2.2. Lipid Profile of Maize Challenged with F. verticillioides at the Field Level
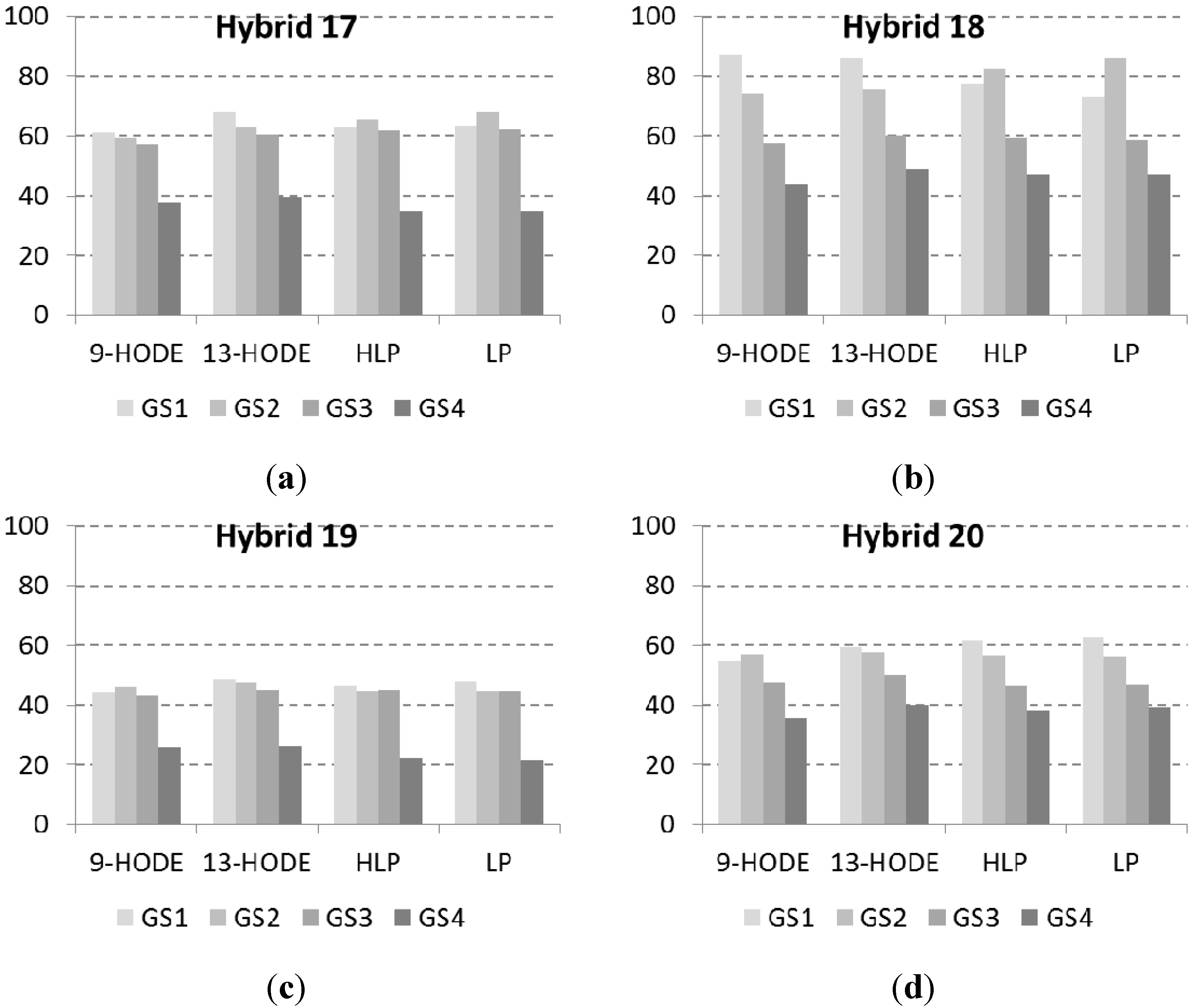
2.3. Correlations
| Variable | 13-HODE | HLP | LP | FFB | TFB | FI |
|---|---|---|---|---|---|---|
| 9-HODE | 0.371 c | 0.482 c | 0.205 a | −0.095 | −0.082 | −0.130 |
| 13-HODE | 0.683 c | 0.715 c | −0.208 a | −0.107 | −0.381 c | |
| HLP | 0.805 c | −0.221 a | −0.185 | −0.413 c | ||
| LP | −0.226 a | −0.179 | −0.416 c | |||
| TFB | 0.976 c | 0.491 c | ||||
| HFB | 0.309 b |
3. Discussion
4. Materials and Methods
4.1. Maize Sample Collection
4.2. Incidence of Infected Kernels
4.3. Fungal Growth by qPCR
4.4. Chemicals
4.5. Lipid Analysis
| Species | Precursor/Product Ion m/z | CE (eV) | Fragmentor (eV) | RT (min) |
|---|---|---|---|---|
| 9-HODE d4 (ISTD) | 299.2–>172.2 | −20 | −140 | 1.22 |
| 9-HODE | 295.2–>171.2 | −20 | −140 | 1.22 |
| 13-HODE d4 (ISTD) | 299.2–>198.2 | −20 | −140 | 1.23 |
| 13-HODE | 295.2–>195.2 | −20 | −140 | 1.23 |
| C16-D31 Ceramide (ISTD) | 568.6–>264.2 | +30 | +140 | 15.8 |
| Cer (t18:0/24:0(2OH)) | 684.6–>282.2 | +34 | +140 | 17.95 |
| Cer (t18:0/24:0) | 668.6–>282.2 | +34 | +140 | 18.35 |
4.6. Free and Total Fumonisins
4.7. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Cer | Ceramide |
| Free FB | Free fumonisins B |
| Hidden FB | Hidden fumonisins B |
| FB1 | fumonisin B1 |
| HODE | hydroxyoctadecenoic acids |
| MRM | multiple reaction monitoring |
| HLP | N-(2ʹ-hydroxylignoceroyl)-phytosphingosine |
| LP | N-lignoceroyl-phytosphingosine |
| qPCR | quantitative PCR |
| ROS | reactive oxygen species |
| Sph | sphingosine |
| S1P | sphingosine-1-phosphate |
| FBtot | total fumonisins |
| QqQ | triple quadrupole mass spectrometer |
References
- Woloshuk, C.P.; Shim, W.B. Aflatoxins, fumonisins, and trichothecenes: A convergence of knowledge. FEMS Microbiol. Rev. 2013, 37, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.D.; Glenn, A.E.; Zimeri, A.M.; Bacon, C.W.; Smith, M.A.; Riley, R.T. Fumonisin disruption of ceramide biosynthesis in maize roots and the effects on plant development and Fusarium verticillioides-induced seedling disease. J. Agric. Food Chem. 2007, 55, 2937–2946. [Google Scholar] [CrossRef] [PubMed]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans, Beryllium, Cadmium, Mercury, and Exposures in the Glass Manufacturing Industry; IARC: Lyon, France, 1993; Volume 58. [Google Scholar]
- Lazzaro, I.; Falavigna, C.; Dall’Asta, C.; Galaverna, G.; Battilani, P. Fumonisins B, A and C profile and masking in Fusarium verticillioides strains on fumonisin-inducing and maize-based media. Int. J. Food Microbiol. 2012, 159, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, I.; Falavigna, C.; Galaverna, G.; Dall’Asta, C.; Battilani, P. Cornmeal and starch influence the dynamic of fumonisin B, A and C production and masking in Fusarium verticillioides and F. proliferatum. Int. J. Food Microbiol. 2013, 166, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Mangia, M.; Berthiller, F.; Molinelli, A.; Sulyok, M.; Schumacher, R.; Krska, R.; Galaverna, G.; Dossena, A.; Marchelli, R. Difficulties in fumonisin determination: The issue of hidden fumonisins. Anal. Bioanal. Chem. 2009, 395, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Falavigna, C.; Galaverna, G.; Dossena, A.; Marchelli, R. In vitro digestion assay for determination of hidden fumonisins in maize. J. Agric. Food Chem. 2010, 58, 12042–12047. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Falavigna, C.; Galaverna, G.; Battilani, P. Role of maize hybrids and their chemical composition in Fusarium infection, fumonisin production and masking. J. Agric. Food Chem. 2012, 60, 3800–3808. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Giorni, P.; Cirlini, M.; Reverberi, M.; Gregori, R.; Ludovici, M.; Camera, E.; Fanelli, C.; Battilani, P.; Scala, V. Maize lipids play a pivotal role in the fumonisin accumulation. World Mycotoxin J. 2014, 8, 87–97. [Google Scholar] [CrossRef]
- Scala, V.; Giorni, P.; Cirlini, M.; Ludovici, M.; Visentin, I.; Cardinale, F.; Fabbri, A.A.; Fanelli, C.; Reverberi, M.; Battilani, P.; et al. LDS1-produced oxylipins are negative regulators of growth, conidiation and fumonisin synthesis in the fungal maize pathogen Fusarium verticillioides. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.S.; Nemchenko, A.; Park, Y.S.; Borrego, E.; Huang, P.C.; Schmelz, E.A.; Kunze, S.; Feussner, I.; Yalpani, N.; Meeley, R.; et al. The novel monocot-specific 9-lipoxygenase ZmLOX12 is required to mount an effective jasmonate-mediated defense against Fusarium verticillioides in maize. Mol. Plant Microbe Interact. 2014, 27, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Konig, S.; Feussner, K.; Schwarz, M.; Kaever, A.; Iven, T.; Landesfeind, M.; Ternes, P.; Karlovsky, P.; Lipka, V.; Feussner, I. Arabidopsis mutants of sphingolipid fatty acid a-hydroxylases accumulate ceramides and salicylates. New Phytol. 2012, 196, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphin-golipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Van Brocklyn, J.R.; Williams, J.B. The control of the balance between ceramide and sphingosine-1-phosphate by sphingosine kinase: Oxidative stress and the seesaw of cell survival and death. Comp. Biochem. Physiol. 2012, 163, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Berkey, R.; Bendigeri, D.; Xiao, S. Sphingolipids and plant defense/disease: The “death” connection and beyond. Front. Plant Sci. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Guillas, I.; Puyaubert, J.; Baudouin, E. Nitric oxide sphingolipid interplays in plant signaling: An enigma from the Sphinx? Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Pewzner-Jung, Y.; Ben-Dor, S.; Futerman, A.H. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)? Insights into the regulation of ceramide synthesis. J. Biol. Chem. 2006, 281, 25001–25005. [Google Scholar] [CrossRef] [PubMed]
- Zitomer, N.C.; Mitchell, T.; Voss, K.A.; Bondy, G.S.; Pruett, S.T.; Garnier-Amblard, E.C.; Liebeskind, L.S.; Park, H.E.; Wang, M.C.; Sullards, A H., Jr.; et al. Ceramide Synthase Inhibition by Fumonisin B1 Causes Accumulation of 1-Deoxysphinganine. J. Biol. Chem. 2009, 284, 4786–4795. [Google Scholar] [CrossRef] [PubMed]
- Glenn, A.E.; Zitomer, N.C.; Zimeri, A.M.; Williams, L.D.; Riley, R.T.; Proctor, R.H. Transformation-mediated complementation of a FUM gene cluster deletion in Fusarium verticillioides restores both fumonisin production and pathogenicity on maize seedlings. Mol. Plant Microbe Interact. 2008, 21, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.L.; Wenk, M.R. Mass spectrometry-based profiling of phospholipids and sphingolipids in extracts from Saccharomyces cerevisiae. Yeast 2006, 23, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Fester, T. Plant metabolite profiles and the buffering capacities of ecosystems. Phytochemistry 2015, 110, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. Regime shifts, resilience, and biodiversity in ecosystem management. Ann. Rev. Ecol. Evol. Syst. 2004, 35, 557–581. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Pons, S.; Pinson-Gadais, L.; Picot, A.; Marchegay, G.; Bonnin-Verdal, M.N.; Ducos, C.; Barreau, C.; Roucolle, J.; Sehabiague, P.; et al. Chlorogenic acid and maize ear rot resistance: A dynamic study investigating Fusarium graminearum development, deoxynivalenol production, and phenolic acid accumulation. Mol. Plant Microbe Interact. 2012, 25, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Zitomer, N.C.; Riley, R.T. Extraction and analysis of fumonisins and compounds indicative of fumonisin exposure in plant and mammalian tissues and cultured cells. Methods Mol. Biol. 2011, 739, 171–185. [Google Scholar] [PubMed]
- Wang, H.; Jones, C.; Ciacci-Zanella, J.; Holt, T.; Gilchrist, D.G. Fumonisins and Alternaria alternata lycopersici toxins: Sphinganine analog mycotoxins induce apoptosis in monkey kidney cells. Proc. Natl. Acad. Sci. USA 1996, 93, 3461–3466. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.B.; Kaloyanova, D.V.; Strating, J.R.P.; van Hellemond, J.J.; van der Schaar, H.M.; Tielens, A.G.M.; van Kuppeveld, F.J.M.; Brouwers, J.F. Targeting of the hydrophobic metabolome by pathogens. Traffic 2015, 16, 439–460. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.E.; Plattner, R.D.; Nelsen, T.C.; Leslie, J.F. Genetic analysis of fumonisin production and virulence of Gibberella fujikuroi mating population A (Fusarium moniliforme) on maize (Zea mays) seedlings. Appl. Environ. Microbiol. 1995, 61, 79–86. [Google Scholar] [PubMed]
- Sanchez-Rangel, D.; Sanchez-Nieto, S.; Plasencia, J. Fumonisin B1, a toxin produced by Fusarium verticillioides, modulates maize beta-1,3-glucanase activities involved in defense response. Planta 2011, 235, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Bleiholder, H. Explanations of the BBCH decimal codes for the growth stages of maize, rape, faba beans, sunflowers and peas—With illustrations. Gesunde Pflanzen 1990, 42, 308–321. [Google Scholar]
- Lancashire, P.D.; Bleiholder, H.; Boom, T.; Langeluddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Summerell, B.A.; Salleh, B.; Leslie, J.F. A utilitarian approach to Fusarium identification. Plant Dis. 2003, 87, 117–128. [Google Scholar] [CrossRef]
- Raper, K.B.; Fennell, D.I. The Genus Aspergillus; Robert, E., Ed.; Krieger Publishing Company Inc.: Malabar, FL, USA, 1965. [Google Scholar]
- Pitt, J. The Genus Penicillium; Academic Press: London, UK, 1979. [Google Scholar]
- Ludovici, M.; Ialongo, C.; Reverberi, M.; Beccaccioli, M.; Scarpari, M.; Scala, V. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis of Fusarium verticillioides and maize kernels. Food Addit. Contam. 2014, 31, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giorni, P.; Dall'Asta, C.; Reverberi, M.; Scala, V.; Ludovici, M.; Cirlini, M.; Galaverna, G.; Fanelli, C.; Battilani, P. Open Field Study of Some Zea mays Hybrids, Lipid Compounds and Fumonisins Accumulation. Toxins 2015, 7, 3657-3670. https://doi.org/10.3390/toxins7093657
Giorni P, Dall'Asta C, Reverberi M, Scala V, Ludovici M, Cirlini M, Galaverna G, Fanelli C, Battilani P. Open Field Study of Some Zea mays Hybrids, Lipid Compounds and Fumonisins Accumulation. Toxins. 2015; 7(9):3657-3670. https://doi.org/10.3390/toxins7093657
Chicago/Turabian StyleGiorni, Paola, Chiara Dall'Asta, Massimo Reverberi, Valeria Scala, Matteo Ludovici, Martina Cirlini, Gianni Galaverna, Corrado Fanelli, and Paola Battilani. 2015. "Open Field Study of Some Zea mays Hybrids, Lipid Compounds and Fumonisins Accumulation" Toxins 7, no. 9: 3657-3670. https://doi.org/10.3390/toxins7093657
APA StyleGiorni, P., Dall'Asta, C., Reverberi, M., Scala, V., Ludovici, M., Cirlini, M., Galaverna, G., Fanelli, C., & Battilani, P. (2015). Open Field Study of Some Zea mays Hybrids, Lipid Compounds and Fumonisins Accumulation. Toxins, 7(9), 3657-3670. https://doi.org/10.3390/toxins7093657










