Interactions between Autophagy and Bacterial Toxins: Targets for Therapy?
Abstract
:1. Introduction

2. Overview of Autophagy Mechanisms
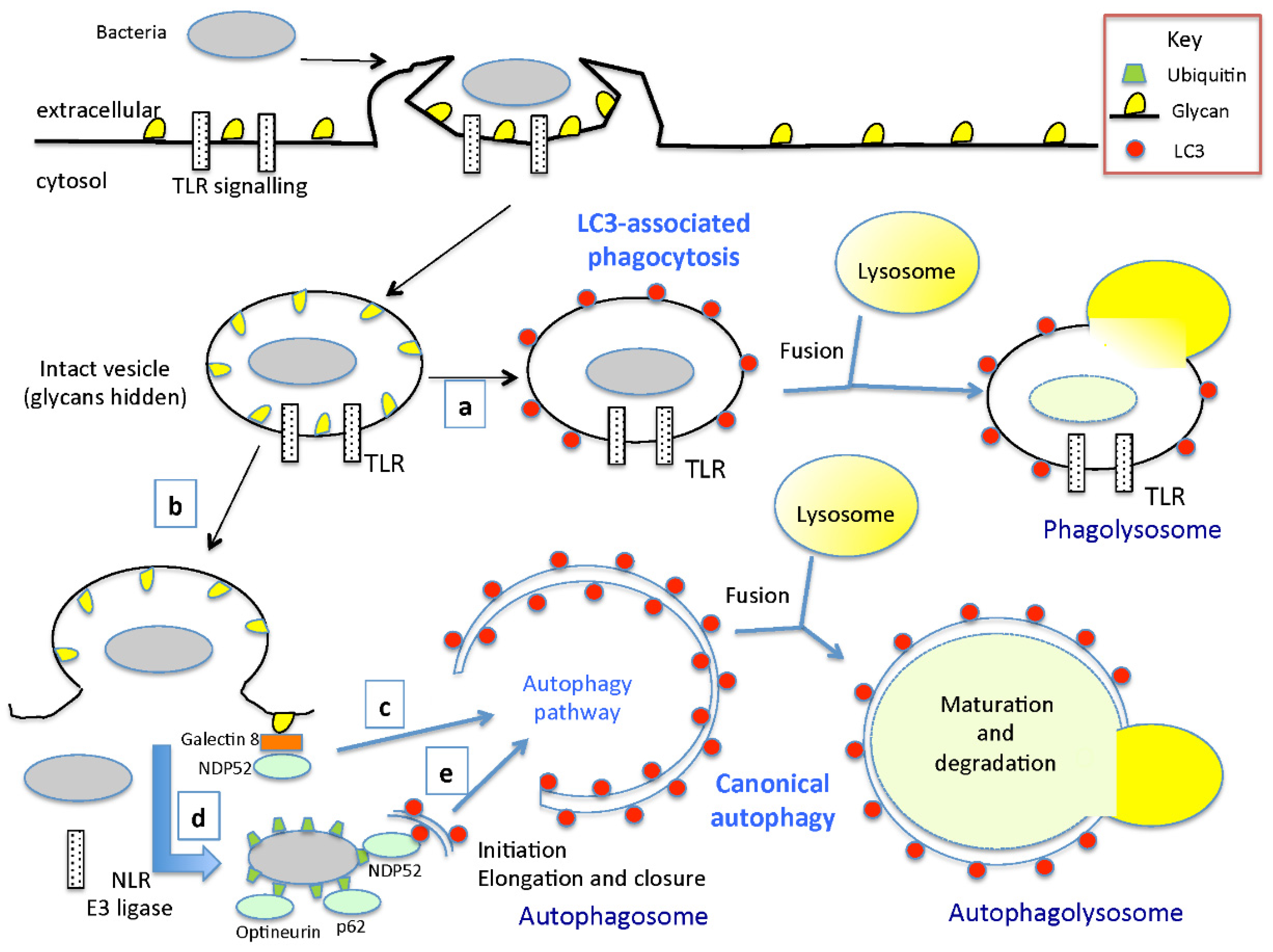
3. Are the Different Interactions of Autophagy and Bacterial Toxins Involved in Bacterial Pathogenesis?
3.1. Lipopolysaccharides
3.2. Bacterial Pore-Forming Toxins (PFTs)
3.3. Bacterial Adenylate Cyclases
| Bacterium | Bacterial factors | Host factors | References |
|---|---|---|---|
| Inhibiting autophagy initiation signaling | |||
| Bacillus anthracis | Edema factor toxin | cAMP | [145] |
| Vibrio cholerae | Cholera toxin | cAMP | [145] |
| Salmonella enterica serovar Typhimurium | Unknown | mTOR, RAG GTPases | [146] |
| Directly interfering with the activity of autophagy components | |||
| Shigella flexneri | VirA | RAB1 | [147] |
| Evading autophagy recognition by masking the bacterial surface | |||
| Shigella flexneri | IcsB | Atg5 and septins | [59] |
| Listeria monocytogenes | ActA and lnlK | MVP and host factors that bind ActA | [157,158] |
| Evading autophagy by escaping from the vacuole | |||
| Listeria monocytogenes | LLO (cholesterol-dependent cytolysin) | Membrane pore formation | [149] |
| Listeria monocytogenes | PI-PLC (phosphatidylinositol-specific phospholipase) | Facilitate vacuole disruption for escape | [31,150] |
| Shigella flexneri | IpaB (membrane pore formation) | Host cell invasion, membrane disruption and escape from the SCV | [31,151] |
| Escaping autophagy by unclear mechanisms | |||
| Burkholderia pseudomallei | T3SS3 effector BopA and translocator BipD, T3SS1 ATPase encodded by bpscN | Unknown | [62,152] |
| Francisella tularensis | DipA | Unknown | [153,154] |
3.4. Other Virulence Factors
4. What Therapeutic Potential Does the Targeting of Molecules Involved in the Autophagy Machinery Have for the Resolution of Bacterial Infections?
| Autophagy inducers | Mechanism of action | References |
|---|---|---|
| FDA-approved-drugs | ||
| Rapamycin | Induces autophagy by inhibiting mTORC1 | [194,195,196] |
| Metformin | Upregulates AMPK, which promotes autophagy by inducing ULK1 phosphorylation | [197,198] |
| Isoniazid | Activates autophagy flux, oxidative stress and upregulates AMPK | [199] |
| Vitamin D3 | Upregulates cathelicidin | [200,201] |
| Vitamin C | Antioxidant | [202] |
| Vitamin E | Antioxidant | [203] |
| Lithium | Lowers inositol and Ins(1,4,5)P" levels | [204] |
| Sodium valproate | Lowers inositol and Ins(1,4,5)P" levels | [196,204] |
| Carbamazepine | Lowers inositol and Ins(1,4,5)P" levels | [196,204] |
| Verapamil | Lowers intracytosolic Ca2+ levels | [196] |
| Clonidine and rilmenidine | Lower cAMP levels | [196] |
| Anti-psychotic drugs | Inhibit autophagy | [205] |
| Statins | Lower membrane cholesterol levels, thereby preventing cholesterol-dependent pore-forming toxins from forming pores | [206] |
| Pharmacological agents | ||
| 17-hydroxy-jolkinolide B | Activates heme oxygenase-1 expression | [207] |
| L-NAME | Decreases nitric oxide formation to induce autophagy | [208] |
| Nutritional compounds | ||
| Resveratrol | Activates sirtuin 1 (histone deacetylase) | [209,210,211] |
| Epicatechins | Inhibit LPS-induced HMGB1 upregulation by stimulating its autophagic degradation | [212,213] |
| Catalase | Antioxidant | [214] |
| Chloroquine | Inhibits autophagosome-lysosome fusion | [215,216] |
| Vinblastine | Inhibits microtubule formation | [217] |
| Nocodazole | Inhibits microtubule formation and inhibits autophagosome-lysosome fusion | [218,219] |
| 3-methyladenine, Wortmannin and LY294002 | Inhibit phosphatidylinositol 3-3-kinase | [220] |
4.1. mTOR Inhibitors
4.2. Polyphenols
4.3. Vitamins
4.4. Adenosine Receptor Agonists
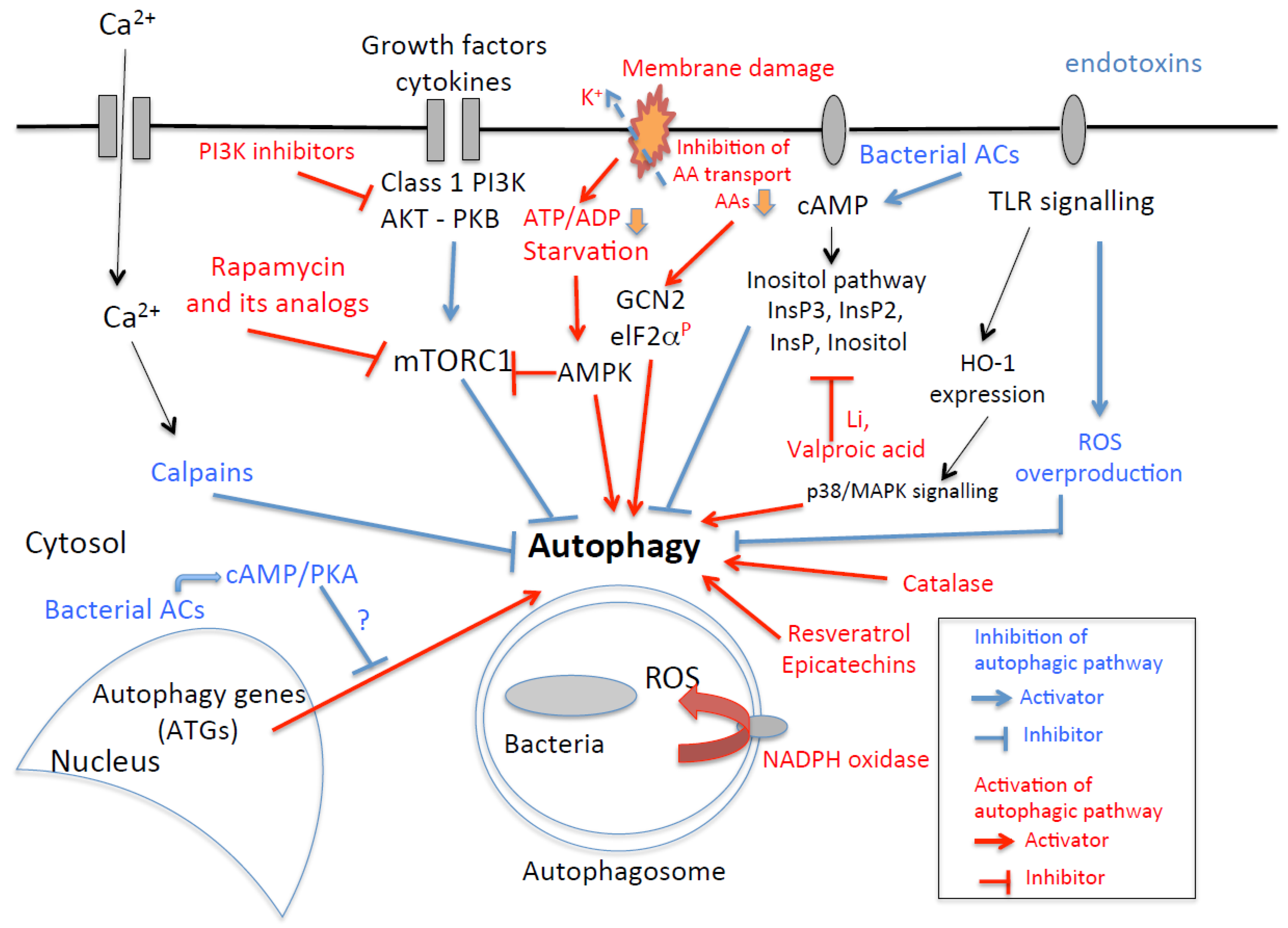
4.5. Antipsychotic Drugs
4.6. Statins
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Shahnazari, S.; Brumell, J. Mechanisms and consequences of bacterial targeting by the autophagy pathway. Curr. Opin. Microbiol. 2011, 14, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.; Codogno, P.; Levine, B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov. 2012, 11, 709–730. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D. Mammalian autophagy: Core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 2010, 22, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Fimia, G.; Piacentini, M. Regulation of autophagy in mammals and its interplay with apoptosis. Cell Mol. Life Sci. 2010, 67, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Marino, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Meijer, A.; Codogno, P. Autophagy: Regulation and role in disease. Crit. Rev. Clin. Lab. Sci. 2009, 46, 210–240. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Yuan, J. Autophagy in cell death: An innocent convict? J. Clin. Invest. 2005, 115, 2679–2688. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Sinha, S.; Kroemer, G. Bcl-2 family members: Dual regulators of apoptosis and autophagy. Autophagy 2008, 4, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.; Abdalla, F.; Abeliovich, H.; Abraham, R.; Acevedo-Arozena, A.; Adeli, K.; Agholme, L.; Agnello, M.; Agostinis, P.; Aguirre-Ghiso, J.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2012, 8, 445–544. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.; Kaushik, S.; Clement, C.C.; Cannizzo, E.S.; Scharf, B.; Follenzi, A.; Potolicchio, I.; Nieves, E.; Cuervo, A.M.; Santambrogio, L. Microautophagy of cytosolic proteins by late endosomes. Dev. Cell 2011, 20, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Santambrogio, L.; Cuervo, A.M. Chasing the elusive mammalian microautophagy. Autophagy 2011, 7, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.; Baehrecke, E.; Brumell, J.; Chu, C.; Codogno, P.; Cuervo, A.; Debnath, J.; Deretic, V.; Elazar, Z.; Eskelinen, E.; et al. A comprehensive glossary of autophagy-related molecules and processes (2nd edition). Autophagy 2011, 7, 1273–1294. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005, 8, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M. Chaperone-mediated autophagy: Selectivity pays off. Trends Endocrinol. Metab. 2010, 21, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Dice, J.F. Chaperone-mediated autophagy. Autophagy 2007, 3, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Bandyopadhyay, U.; Sridhar, S.; Kiffin, R.; Martinez-Vicente, M.; Kon, M.; Orenstein, S.J.; Wong, E.; Cuervo, A.M. Chaperone-mediated autophagy at a glance. J. Cell Sci. 2011, 124, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M.; Dice, J.F. A receptor for the selective uptake and degradation of proteins by lysosomes. Science 1996, 273, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Agarraberes, F.A.; Terlecky, S.R.; Dice, J.F. An intralysosomal hsp70 is required for a selective pathway of lysosomal protein degradation. J. Cell Biol. 1997, 137, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Levine, B. Eating oneself and uninvited guests: Autophagy-related pathways in cellular defense. Cell 2005, 120, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Devenish, R.J.; Lai, S.C. Autophagy and burkholderia. Immunol. Cell Biol. 2015, 93, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Yoshimori, T.; Tooze, S.A. The autophagosome: Origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 2013, 14, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Codogno, P.; Mehrpour, M.; Proikas-Cezanne, T. Canonical and non-canonical autophagy: Variations on a common theme of self-eating? Nat. Rev. Mol. Cell Biol. 2012, 13, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, M.; Milasta, S.; Green, D. Toll-like receptor signaling in the lysosomal pathways. Immunol. Rev. 2009, 227, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Mostowy, S. Autophagy and bacterial clearance: A not so clear picture. Cell Microbiol. 2013, 15, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Haas, A. The phagosome: Compartment with a license to kill. Traffic 2007, 8, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Sansonetti, P.J. Rupture, invasion and inflammatory destruction of the intestinal barrier by Shigella, making sense of prokaryote-eukaryote cross-talks. FEMS Microbiol. Rev. 2001, 25, 3–14. [Google Scholar] [CrossRef]
- Cossart, P.; Sansonetti, P.J. Bacterial invasion: The paradigms of enteroinvasive pathogens. Science 2004, 304, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, G.N.; Hilbi, H. Molecular pathogenesis of Shigella spp.: Controlling host cell signaling, invasion, and death by type III secretion. Clin. Microbiol. Rev. 2008, 21, 134–156. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.; Marteyn, B.; Sansonetti, P.; Tang, C. Life on the inside: The intracellular lifestyle of cytosolic bacteria. Nat. Rev. Microbiol. 2009, 7, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Gilliam, E.A.; Li, L. Innate immune programing by endotoxin and its pathological consequences. Front. Immunol. 2014, 5, 680. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H. Lipopolysaccharide: Basic biochemistry, intracellular signaling, and physiological impacts in the gut. Intest. Res. 2014, 12, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Chen, Y.; Wang, H.Y.; Wang, R.F. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum. Vaccin Immunother. 2014, 10, 3270–3285. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Yang, J.H.; Kim, K.M.; Jang, C.H.; Jung, J.Y.; Cho, I.J.; Shin, S.M.; Ki, S.H. Regulation of Toll-like receptor-mediated Sestrin2 induction by AP-1, Nrf2, and the ubiquitin-proteasome system in macrophages. Toxicol. Sci. 2015, 144, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Tweten, R. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 2005, 73, 6199–6209. [Google Scholar] [CrossRef] [PubMed]
- Cemma, M.; Brumell, J. Interactions of pathogenic bacteria with autophagy systems. Curr. Biol. 2012, 22, R540–R545. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V.; Saitoh, T.; Akira, S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 2013, 13, 722–737. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V.; Levine, B. Autophagy, immunity, and microbial adaptations. Cell. Host Microbe 2009, 5, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Byrne, B.; Swanson, M. Macrophages rapidly transfer pathogens from lipid raft vacuoles to autophagosomes. Autophagy 2005, 1, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.; Cregg, J.; Dunn, W.J.; Emr, S.; Sakai, Y.; Sandoval, I.; Sibirny, A.; Subramani, S.; Thumm, M.; Veenhuis, M.; et al. A unified nomenclature for yeast autophagy-related genes. Dev. Cell 2003, 5, 539–545. [Google Scholar] [CrossRef]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Weidberg, H.; Shvets, E.; Shpilka, T.; Shimron, F.; Shinder, V.; Elazar, Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J. 2010, 29, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Cherra, S.J., 3rd; Kulich, S.M.; Uechi, G.; Balasubramani, M.; Mountzouris, J.; Day, B.W.; Chu, C.T. Regulation of the autophagy protein LC3 by phosphorylation. J. Cell Biol. 2010, 190, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Hanada, T.; Noda, N.; Satomi, Y.; Ichimura, Y.; Fujioka, Y.; Takao, T.; Inagaki, F.; Ohsumi, Y. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J. Biol. Chem. 2007, 282, 37298–37302. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Arakawa, S.; Fujitani, K.; Yamaguchi, H.; Mizuta, T.; Kanaseki, T.; Komatsu, M.; Otsu, K.; Tsujimoto, Y.; Shimizu, S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 2009, 461, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Arakawa, S.; Nishida, Y. Autophagy takes an alternative pathway. Autophagy 2010, 6, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.A.; de Maziere, A.; van Dijk, S.; Carlsson, F.; Klumperman, J.; Brown, E.J. Atg5-independent sequestration of ubiquitinated mycobacteria. PLoS Pathog. 2009, 5, e1000430. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Henault, J.; Kolbeck, R.; Sanjuan, M. Noncanonical autophagy: One small step for LC3, one giant leap for immunity. Curr. Opin. Immunol. 2014, 26, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Randow, F.; Munz, C. Autophagy in the regulation of pathogen replication and adaptive immunity. Trends Immunol. 2012, 33, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Celli, J. LRSAM1, an E3 Ubiquitin ligase with a sense for bacteria. Cell Host Microbe 2012, 12, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Quezada, C.M.; Hicks, S.W.; Galan, J.E.; Stebbins, C.E. A family of Salmonella virulence factors functions as a distinct class of autoregulated E3 ubiquitin ligases. Proc. Natl. Acad. Sci. USA 2009, 106, 4864–4869. [Google Scholar] [CrossRef] [PubMed]
- Huett, A.; Heath, R.J.; Begun, J.; Sassi, S.O.; Baxt, L.A.; Vyas, J.M.; Goldberg, M.B.; Xavier, R.J. The LRR and RING domain protein LRSAM1 is an E3 ligase crucial for ubiquitin-dependent autophagy of intracellular Salmonella Typhimurium. Cell Host Microbe 2012, 12, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.W.; Xu, W.C.; Luo, J.G.; Guo, X.J.; Sun, T.; Zhao, X.L.; Fu, Z.J. WW domain containing E3 ubiquitin protein ligase 1 (WWP1) negatively regulates TLR4-mediated TNF-alpha and IL-6 production by proteasomal degradation of TNF receptor associated factor 6 (TRAF6). PLoS ONE 2013, 8, e67633. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.T.; Shahnazari, S.; Brech, A.; Lamark, T.; Johansen, T.; Brumell, J.H. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J. Immunol. 2009, 183, 5909–5916. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, C.L.; Smith, A.C.; Bakowski, M.A.; Yoshimori, T.; Brumell, J.H. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 2006, 281, 11374–11383. [Google Scholar] [CrossRef]
- Cemma, M.; Kim, P.K.; Brumell, J.H. The ubiquitin-binding adaptor proteins p62/SQSTM1 and NDP52 are recruited independently to bacteria-associated microdomains to target Salmonella to the autophagy pathway. Autophagy 2011, 7, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Thurston, T.L.; Ryzhakov, G.; Bloor, S.; von Muhlinen, N.; Randow, F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat. Immunol. 2009, 10, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Mostowy, S.; Sancho-Shimizu, V.; Hamon, M.; Simeone, R.; Brosch, R.; Johansen, T.; Cossart, P. p62 and NDP52 proteins target intracytosolic Shigella and Listeria to different autophagy pathways. J. Biol. Chem. 2011, 286, 26987–26995. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, M.; Dillon, C.; Tait, S.; Moshiach, S.; Dorsey, F.; Connell, S.; Komatsu, M.; Tanaka, K.; Cleveland, J.; Withoff, S.; et al. Toll-Like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007, 450, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Canadien, V.; Lam, G.; Steinberg, B.; Dinauer, M.; Magalhaes, M.; Glogauer, M.; Grinstein, S.; Brumell, J. Activation of antibacterial autophagy by NADPH oxidases. Proc. Natl. Acad. Sci. USA 2009, 106, 6226–6231. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Cullinane, M.; Treerat, P.; Ramm, G.; Prescott, M.; Adler, B.; Boyce, J.; Devenish, R. The Burkholderia pseudomallei type III secretion system and BopA are required for evasion of LC3-associated phagocytosis. PLoS ONE 2011, 6, e17852. [Google Scholar] [CrossRef] [PubMed]
- Lam, G.; Cemma, M.; Muise, A.; Higgins, D.; Brumell, J. Host and bacterial factors that regulate LC3 recruitment to Listeria monocytogenes during the early stages of macrophage infection. Autophagy 2013, 9, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Almendinger, J.; Oberst, A.; Ness, R.; Dillon, C.P.; Fitzgerald, P.; Hengartner, M.O.; Green, D.R. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl. Acad. Sci. USA 2011, 108, 17396–17401. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.C.; Devenish, R.J. LC3-Associated Phagocytosis (LAP): Connections with Host Autophagy. Cells 2012, 1, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Lippai, M.; Low, P. The role of the selective adaptor p62 and ubiquitin-like proteins in autophagy. Biomed. Res. Int. 2014, 2014, 832704. [Google Scholar] [CrossRef] [PubMed]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Overvatn, A.; Bjorkoy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef] [PubMed]
- Ciani, B.; Layfield, R.; Cavey, J.R.; Sheppard, P.W.; Searle, M.S. Structure of the ubiquitin-associated domain of p62 (SQSTM1) and implications for mutations that cause Paget’s disease of bone. J. Biol. Chem. 2003, 278, 37409–37412. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Waguri, S.; Koike, M.; Sou, Y.S.; Ueno, T.; Hara, T.; Mizushima, N.; Iwata, J.; Ezaki, J.; Murata, S.; et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 2007, 131, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Nezis, I.P.; Simonsen, A.; Sagona, A.P.; Finley, K.; Gaumer, S.; Contamine, D.; Rusten, T.E.; Stenmark, H.; Brech, A. Ref(2)P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. J. Cell Biol. 2008, 180, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Boyle, K.B.; Randow, F. The role of “eat-me” signals and autophagy cargo receptors in innate immunity. Curr. Opin. Microbiol. 2013, 16, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Duran, A.; Amanchy, R.; Linares, J.F.; Joshi, J.; Abu-Baker, S.; Porollo, A.; Hansen, M.; Moscat, J.; Diaz-Meco, M.T. p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol. Cell 2011, 44, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, R.; Tanaka, K.; Komatsu, M. Dissection of the role of p62/Sqstm1 in activation of Nrf2 during xenophagy. FEBS Lett. 2014, 588, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Girardin, S.E. Emerging themes in bacterial autophagy. Curr. Opin. Microbiol. 2015, 23C, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Philpott, D.J.; Girardin, S.E. Nod-like receptors: Sentinels at host membranes. Curr. Opin. Immunol. 2010, 22, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Travassos, L.H.; Carneiro, L.A.; Ramjeet, M.; Hussey, S.; Kim, Y.G.; Magalhaes, J.G.; Yuan, L.; Soares, F.; Chea, E.; Le Bourhis, L.; et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat. Immunol. 2010, 11, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Thurston, T.L.; Wandel, M.P.; von Muhlinen, N.; Foeglein, A.; Randow, F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 2012, 482, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Shahnazari, S.; Yen, W.L.; Birmingham, C.L.; Shiu, J.; Namolovan, A.; Zheng, Y.T.; Nakayama, K.; Klionsky, D.J.; Brumell, J.H. A diacylglycerol-dependent signaling pathway contributes to regulation of antibacterial autophagy. Cell Host Microbe 2010, 8, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Shahnazari, S.; Namolovan, A.; Klionsky, D.J.; Brumell, J.H. A role for diacylglycerol in antibacterial autophagy. Autophagy 2011, 7, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Moreau, K.; Lacas-Gervais, S.; Fujita, N.; Sebbane, F.; Yoshimori, T.; Simonet, M.; Lafont, F. Autophagosomes can support Yersinia pseudotuberculosis replication in macrophages. Cell Microbiol. 2010, 12, 1108–1123. [Google Scholar] [CrossRef] [PubMed]
- Cooney, R.; Baker, J.; Brain, O.; Danis, B.; Pichulik, T.; Allan, P.; Ferguson, D.; Campbell, B.; Jewell, D.; Simmons, A. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat. Med. 2010, 16, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Shenderov, K.; Huang, N.; Kabat, J.; Abu-Asab, M.; Fitzgerald, K.; Sher, A.; Kehrl, J. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 2012, 13, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Knodler, L.; Celli, J. Eating the strangers within: Host control of intracellular bacteria via xenophagy. Cell Microbiol. 2011, 13, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, S. Chaperones and transport proteins regulate TLR4 trafficking and activation. Immunobiology 2009, 214, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Fujita, N.; Jang, M.; Uematsu, S.; Yang, B.; Satoh, T.; Omori, H.; Noda, T.; Yamamoto, N.; Komatsu, M.; et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008, 456, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Haspel, J.; Rathinam, V.; Lee, S.; Dolinay, T.; Lam, H.; Englert, J.; Rabinovitch, M.; Cernadas, M.; Kim, H.; et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011, 12, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Deretic, V. Toll-like receptors in control of immunological autophagy. Cell Death Differ. 2009, 16, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Kehrl, J. MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J. Biol. Chem. 2008, 283, 33175–33182. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Pattingre, S.; Sinha, S.; Bassik, M.; Levine, B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 2008, 30, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Scherz-Shouval, R.; Elazar, Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem. Sci. 2011, 36, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Cloonan, S.; Mizumura, K.; Choi, A.; Ryter, S. Autophagy: A crucial moderator of redox balance, inflammation, and apoptosis in lung disease. Antioxid Redox Signal. 2014, 20, 474–494. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Nour, M.; Tsalikis, J.; Kleinman, D.; Girardin, S. The emerging role of mTOR signalling in antibacterial immunity. Immunol. Cell. Biol. 2014, 92, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Ellebedy, A.; Ahmed, R. TOR in the immune system. Curr. Opin. Cell Biol. 2011, 23, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Tsalikis, J.; Croitoru, D.; Philpott, D.; Girardin, S. Nutrient sensing and metabolic stress pathways in innate immunity. Cell Microbiol. 2013, 15, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Salh, B.; Wagey, R.; Marotta, A.; Tao, J.; Pelech, S. Activation of phosphatidylinositol 3-kinase, protein kinase B, and p70 S6 kinases in lipopolysaccharide-stimulated Raw 264.7 cells: Differential effects of rapamycin, Ly294002, and wortmannin on nitric oxide production. J. Immunol. 1998, 161, 6947–6954. [Google Scholar] [PubMed]
- Potter, M.; Shah, S.; Elbirt, K.; Callery, M. Endotoxin (LPS) stimulates 4E-BP1/PHAS-I phosphorylation in macrophages. J. Surg. Res. 2001, 97, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Wullschleger, S.; Loewith, R.; Hall, M. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Frost, R. Endotoxin disrupts the leucine-signaling pathway involving phosphorylation of mTOR, 4E-BP1, and S6K1 in skeletal muscle. J. Cell Physiol. 2005, 203, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.; Delattre, A.; de Longueville, F.; Bult, H.; Raes, M. Gene expression profiling of LPS-stimulated murine macrophages and role of the NF-kappaB and PI3K/mTOR signaling pathways. Ann. N. Y. Acad. Sci. 2007, 1096, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Troutman, T.; Bazan, J.; Pasare, C. Toll-like receptors, signaling adapters and regulation of the pro-inflammatory response by PI3K. Cell Cycle 2012, 11, 3559–3567. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kuo, H.; Chen, C.; Hsu, J.; Chou, C.; Wei, Y.; Sun, H.; Li, L.; Ping, B.; Huang, W.; et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell 2007, 130, 440–455. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.; Tee, A.; Logsdon, M.; Blenis, J.; Cantley, L. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell 2002, 10, 151–162. [Google Scholar] [CrossRef]
- Weichhart, T.; Costantino, G.; Poglitsch, M.; Rosner, M.; Zeyda, M.; Stuhlmeier, K.; Kolbe, T.; Stulnig, T.; Horl, W.; Hengstschlager, M.; et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 2008, 29, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Girardin, S.E.; Boneca, I.G.; Carneiro, L.A.; Antignac, A.; Jehanno, M.; Viala, J.; Tedin, K.; Taha, M.K.; Labigne, A.; Zahringer, U.; et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 2003, 300, 1584–1587. [Google Scholar] [CrossRef] [PubMed]
- Kufer, T.A.; Banks, D.J.; Philpott, D.J. Innate immune sensing of microbes by Nod proteins. Ann. N. Y. Acad. Sci. 2006, 1072, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Warner, N.; Viani, K.; Nunez, G. Function of Nod-like receptors in microbial recognition and host defense. Immunol. Rev. 2009, 227, 106–128. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.R.; Bischofberger, M.; Pernot, L.; van der Goot, F.G.; Freche, B. Bacterial pore-forming toxins: The (w)hole story? Cell Mol. Life Sci. 2008, 65, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Lakey, J.H.; Slatin, S.L. Pore-forming colicins and their relatives. Curr. Top. Microbiol. Immunol. 2001, 257, 131–161. [Google Scholar] [PubMed]
- Pezard, C.; Berche, P.; Mock, M. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect. Immun. 1991, 59, 3472–3477. [Google Scholar] [PubMed]
- Parker, M.W.; Feil, S.C. Pore-forming protein toxins: From structure to function. Prog. Biophys. Mol. Biol. 2005, 88, 91–142. [Google Scholar] [CrossRef] [PubMed]
- Pellizzari, R.; Guidi-Rontani, C.; Vitale, G.; Mock, M.; Montecucco, C. Lethal factor of Bacillus anthracis cleaves the N-terminus of MAPKKs: Analysis of the intracellular consequences in macrophages. Int. J. Med. Microbiol. 2000, 290, 421–427. [Google Scholar] [CrossRef]
- Agarwal, N.; Bishai, W. cAMP signaling in Mycobacterium tuberculosis. Indian J. Exp. Biol. 2009, 47, 393–400. [Google Scholar] [PubMed]
- Gutierrez, M.; Saka, H.; Chinen, I.; Zoppino, F.; Yoshimori, T.; Bocco, J.; Colombo, M. Protective role of autophagy against Vibrio cholerae cytolysin, a pore-forming toxin from V. cholerae. Proc. Natl. Acad. Sci. USA 2007, 104, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Thelestam, M.; Mollby, R. Survival of cultured cells after functional and structural disorganization of plasma membrane by bacterial haemolysins and phospholipases. Toxicon 1983, 21, 805–815. [Google Scholar] [CrossRef]
- Walev, I.; Palmer, M.; Martin, E.; Jonas, D.; Weller, U.; Hohn-Bentz, H.; Husmann, M.; Bhakdi, S. Recovery of human fibroblasts from attack by the pore-forming alpha-toxin of Staphylococcus aureus. Microb Pathog. 1994, 17, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Dragneva, Y.; Anuradha, C.D.; Valeva, A.; Hoffmann, A.; Bhakdi, S.; Husmann, M. Subcytocidal attack by staphylococcal alpha-toxin activates NF-kappaB and induces interleukin-8 production. Infect. Immun. 2001, 69, 2630–2635. [Google Scholar] [CrossRef] [PubMed]
- Walev, I.; Hombach, M.; Bobkiewicz, W.; Fenske, D.; Bhakdi, S.; Husmann, M. Resealing of large transmembrane pores produced by streptolysin O in nucleated cells is accompanied by NF-kappaB activation and downstream events. FASEB J. 2002, 16, 237–239. [Google Scholar] [PubMed]
- Haugwitz, U.; Bobkiewicz, W.; Han, S.R.; Beckmann, E.; Veerachato, G.; Shaid, S.; Biehl, S.; Dersch, K.; Bhakdi, S.; Husmann, M. Pore-forming Staphylococcus aureus alpha-toxin triggers epidermal growth factor receptor-dependent proliferation. Cell Microbiol. 2006, 8, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Kloft, N.; Neukirch, C.; Bobkiewicz, W.; Veerachato, G.; Busch, T.; von Hoven, G.; Boller, K.; Husmann, M. Pro-autophagic signal induction by bacterial pore-forming toxins. Med. Microbiol. Immunol. 2010, 199, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Von Hoven, G.; Kloft, N.; Neukirch, C.; Ebinger, S.; Bobkiewicz, W.; Weis, S.; Boller, K.; Janda, K.; Husmann, M. Modulation of translation and induction of autophagy by bacterial exoproducts. Med. Microbiol. Immunol. 2012, 201, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, I.; Amano, A.; Mizushima, N.; Yamamoto, A.; Yamaguchi, H.; Kamimoto, T.; Nara, A.; Funao, J.; Nakata, M.; Tsuda, K.; et al. Autophagy defends cells against invading group A Streptococcus. Science 2004, 306, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- von Muhlinen, N.; Thurston, T.; Ryzhakov, G.; Bloor, S.; Randow, F. NDP52, a novel autophagy receptor for ubiquitin-decorated cytosolic bacteria. Autophagy 2010, 6, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Mestre, M.B.; Fader, C.M.; Sola, C.; Colombo, M.I. Alpha-hemolysin is required for the activation of the autophagic pathway in Staphylococcus aureus-infected cells. Autophagy 2010, 6, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Mostowy, S.; Cossart, P. Bacterial autophagy: Restriction or promotion of bacterial replication? Trends Cell Biol. 2012, 22, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, Y.; Ogawa, M.; Hain, T.; Yoshida, M.; Fukumatsu, M.; Kim, M.; Mimuro, H.; Nakagawa, I.; Yanagawa, T.; Ishii, T.; et al. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nat. Cell Biol. 2009, 11, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Talloczy, Z.; Jiang, W.; Virgin, H.W., 4th; Leib, D.A.; Scheuner, D.; Kaufman, R.J.; Eskelinen, E.L.; Levine, B. Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proc. Natl. Acad. Sci. USA 2002, 99, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.E.; Bubeck Wardenburg, J. Host autophagy combating S. aureus: Alpha-toxin will be tolerated. Cell Host Microbe 2015, 17, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Inoshima, I.; Inoshima, N.; Wilke, G.A.; Powers, M.E.; Frank, K.M.; Wang, Y.; Bubeck Wardenburg, J. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat. Med. 2011, 17, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.E.; Kim, H.K.; Wang, Y.; Bubeck Wardenburg, J. ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J. Infect. Dis. 2012, 206, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Maurer, K.; Torres, V.J.; Cadwell, K. Autophagy is a key tolerance mechanism during Staphylococcus aureus infection. Autophagy 2015, 11, 1184–1186. [Google Scholar] [CrossRef] [PubMed]
- Maurer, K.; Reyes-Robles, T.; Alonzo, F., 3rd; Durbin, J.; Torres, V.J.; Cadwell, K. Autophagy mediates tolerance to Staphylococcus aureus alpha-toxin. Cell Host Microbe 2015, 17, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Genisset, C.; Puhar, A.; Calore, F.; de Bernard, M.; Dell'Antone, P.; Montecucco, C. The concerted action of the Helicobacter pylori cytotoxin VacA and of the v-ATPase proton pump induces swelling of isolated endosomes. Cell Microbiol. 2007, 9, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.; Shaughnessy, L.; Loessner, M.J.; Alberti-Segui, C.; Higgins, D.E.; Swanson, J.A. Cytolysin-dependent delay of vacuole maturation in macrophages infected with Listeria monocytogenes. Cell Microbiol. 2006, 8, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, C.L.; Canadien, V.; Kaniuk, N.A.; Steinberg, B.E.; Higgins, D.E.; Brumell, J.H. Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature 2008, 451, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Ligeon, L.; Moreau, K.; Barois, N.; Bongiovanni, A.; Lacorre, D.; Werkmeister, E.; Proux-Gillardeaux, V.; Galli, T.; Lafont, F. Role of VAMP3 and VAMP7 in the commitment of Yersinia pseudotuberculosis to LC3-associated pathways involving single- or double-membrane vacuoles. Autophagy 2014, 10, 1588–1602. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Shin, D.M.; Ramakrishna, L.; Goussetis, D.J.; Platanias, L.C.; Xiong, H.; Morse, H.C., 3rd; Ozato, K. IRF8 directs stress-induced autophagy in macrophages and promotes clearance of Listeria monocytogenes. Nat. Commun. 2015, 6, 6379. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, L.K.; Jones, N.L. Modulation of autophagy by Helicobacter pylori and its role in gastric carcinogenesis. Trends Microbiol. 2013, 21, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Terebiznik, M.; Raju, D.; Vazquez, C.; Torbricki, K.; Kulkarni, R.; Blanke, S.; Yoshimori, T.; Colombo, M.; Jones, N. Effect of Helicobacter pylori’s vacuolating cytotoxin on the autophagy pathway in gastric epithelial cells. Autophagy 2009, 5, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Di Venanzio, G.; Stepanenko, T.; Garcia Vescovi, E. Serratia marcescens ShlA pore-forming toxin is responsible for early induction of autophagy in host cells and is transcriptionally regulated by RcsB. Infect. Immun. 2014, 82, 3542–3554. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.B.; Croxen, M.A.; Marchiando, A.M.; Ferreira, R.B.; Cadwell, K.; Foster, L.J.; Finlay, B.B. Autophagy facilitates Salmonella replication in HeLa cells. MBio 2014, 5, e00865-14. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Birmingham, C.L.; Shahnazari, S.; Shiu, J.; Zheng, Y.T.; Smith, A.C.; Campellone, K.G.; Heo, W.D.; Gruenheid, S.; Meyer, T.; et al. Antibacterial autophagy occurs at PI(3)P-enriched domains of the endoplasmic reticulum and requires Rab1 GTPase. Autophagy 2011, 7, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, N.; Kumar, P.; Bhatnagar, R. The adenylate cyclase toxins. Crit. Rev. Microbiol. 2004, 30, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Thammavongsa, V.; Kern, J.; Missiakas, D.; Schneewind, O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J. Exp. Med. 2009, 206, 2417–2427. [Google Scholar] [CrossRef] [PubMed]
- Tournier, J.; Quesnel-Hellmann, A.; Mathieu, J.; Montecucco, C.; Tang, W.; Mock, M.; Vidal, D.; Goossens, P. Anthrax edema toxin cooperates with lethal toxin to impair cytokine secretion during infection of dendritic cells. J. Immunol. 2005, 174, 4934–4941. [Google Scholar] [CrossRef] [PubMed]
- Shahnazari, S.; Namolovan, A.; Mogridge, J.; Kim, P.; Brumell, J. Bacterial toxins can inhibit host cell autophagy through cAMP generation. Autophagy 2011, 7, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Tattoli, I.; Sorbara, M.; Vuckovic, D.; Ling, A.; Soares, F.; Carneiro, L.; Yang, C.; Emili, A.; Philpott, D.; Girardin, S. Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe 2012, 11, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Zhu, Y.; Lu, Q.; Hu, L.; Zheng, Y.; Shao, F. Structurally distinct bacterial TBC-like GAPs link Arf GTPase to Rab1 inactivation to counteract host defenses. Cell 2012, 150, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Yoshimori, T.; Suzuki, T.; Sagara, H.; Mizushima, N.; Sasakawa, C. Escape of intracellular Shigella from autophagy. Science 2005, 307, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.A.; Ribet, D.; Stavru, F.; Cossart, P. Listeriolysin O: The Swiss army knife of Listeria. Trends Microbiol. 2012, 20, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.A.; Marquis, H.; Jones, S.; Johnston, N.C.; Portnoy, D.A.; Goldfine, H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 1995, 63, 4231–4237. [Google Scholar] [PubMed]
- Picking, W.L.; Nishioka, H.; Hearn, P.D.; Baxter, M.A.; Harrington, A.T.; Blocker, A.; Picking, W.D. IpaD of Shigella flexneri is independently required for regulation of Ipa protein secretion and efficient insertion of IpaB and IpaC into host membranes. Infect. Immun. 2005, 73, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- D’Cruze, T.; Gong, L.; Treerat, P.; Ramm, G.; Boyce, J.; Prescott, M.; Adler, B.; Devenish, R. Role for the Burkholderia pseudomallei type three secretion system cluster 1 bpscN gene in virulence. Infect. Immun. 2011, 79, 3659–3664. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.; Wehrly, T.; Child, R.; Hansen, B.; Hwang, S.; Virgin, H.; Celli, J. Cytosolic clearance of replication-deficient mutants reveals Francisella tularensis interactions with the autophagic pathway. Autophagy 2012, 8, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Checroun, C.; Wehrly, T.; Fischer, E.; Hayes, S.; Celli, J. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc. Natl. Acad. Sci. USA 2006, 103, 14578–14583. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Brumell, J. Bacteria-autophagy interplay: A battle for survival. Nat. Rev. Microbiol. 2014, 12, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Asrat, S.; de Jesus, D.; Hempstead, A.; Ramabhadran, V.; Isberg, R. Bacterial pathogen manipulation of host membrane trafficking. Annu. Rev. Cell Dev. Biol. 2014, 30, 79–109. [Google Scholar] [CrossRef] [PubMed]
- Py, B.; Lipinski, M.; Yuan, J. Autophagy limits Listeria monocytogenes intracellular growth in the early phase of primary infection. Autophagy 2007, 3, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Dortet, L.; Mostowy, S.; Cossart, P. Listeria and autophagy escape: Involvement of InlK, an internalin-like protein. Autophagy 2012, 8, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Feoktistov, I.; Biaggioni, I. Adenosine A2B receptors. Pharmacol. Rev. 1997, 49, 381–402. [Google Scholar] [PubMed]
- Budovskaya, Y.; Stephan, J.; Deminoff, S.; Herman, P. An evolutionary proteomics approach identifies substrates of the cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 2005, 102, 13933–13938. [Google Scholar] [CrossRef] [PubMed]
- Stephan, J.; Yeh, Y.; Ramachandran, V.; Deminoff, S.; Herman, P. The Tor and PKA signaling pathways independently target the Atg1/Atg13 protein kinase complex to control autophagy. Proc. Natl. Acad. Sci. USA 2009, 106, 17049–17054. [Google Scholar] [CrossRef] [PubMed]
- Yorimitsu, T.; Zaman, S.; Broach, J.; Klionsky, D. Protein kinase A and Sch9 cooperatively regulate induction of autophagy in Saccharomyces cerevisiae. Mol. Biol. Cell 2007, 18, 4180–4189. [Google Scholar] [CrossRef] [PubMed]
- Dal Molin, F.; Zornetta, I.; Puhar, A.; Tonello, F.; Zaccolo, M.; Montecucco, C. cAMP imaging of cells treated with pertussis toxin, cholera toxin, and anthrax edema toxin. Biochem. Biophys. Res. Commun. 2008, 376, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.; Pethe, K.; Russell, D.; Purdy, G. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc. Natl. Acad. Sci. USA 2007, 104, 6031–6036. [Google Scholar] [CrossRef] [PubMed]
- Ponpuak, M.; Davis, A.; Roberts, E.; Delgado, M.; Dinkins, C.; Zhao, Z.; Virgin, H.W.; Kyei, G.; Johansen, T.; Vergne, I.; et al. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity 2010, 32, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Butchar, J.; Cremer, T.; Clay, C.; Gavrilin, M.; Wewers, M.; Marsh, C.; Schlesinger, L.; Tridandapani, S. Microarray analysis of human monocytes infected with Francisella tularensis identifies new targets of host response subversion. PLoS ONE 2008, 3, e2924. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fux, B.; Goodwin, M.; Dunay, I.; Strong, D.; Miller, B.; Cadwell, K.; Delgado, M.; Ponpuak, M.; Green, K.; et al. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe 2008, 4, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Barnett, T.C.; Liebl, D.; Seymour, L.M.; Gillen, C.M.; Lim, J.Y.; Larock, C.N.; Davies, M.R.; Schulz, B.L.; Nizet, V.; Teasdale, R.D.; et al. The globally disseminated M1T1 clone of group A Streptococcus evades autophagy for intracellular replication. Cell Host Microbe 2013, 14, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Grumati, P.; Coletto, L.; Schiavinato, A.; Castagnaro, S.; Bertaggia, E.; Sandri, M.; Bonaldo, P. Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles. Autophagy 2011, 7, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Niu, Y.; Yuan, H.; Huang, J.; Fu, L. AMPK binds to Sestrins and mediates the effect of exercise to increase insulin-sensitivity through autophagy. Metabolism 2015, 64, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Thullier, P.; Avril, A.; Mathieu, J.; Behrens, C.; Pellequer, J.; Pelat, T. Mapping the epitopes of a neutralizing antibody fragment directed against the lethal factor of Bacillus anthracis and cross-reacting with the homologous edema factor. PLoS ONE 2013, 8, e65855. [Google Scholar] [CrossRef] [PubMed]
- Avril, A.; Froude, J.; Mathieu, J.; Pelat, T.; Thullier, P. Isolation of antibodies from non-human primates for clinical use. Curr. Drug Discov. Technol. 2014, 11, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Tonello, F.; Seveso, M.; Marin, O.; Mock, M.; Montecucco, C. Screening inhibitors of anthrax lethal factor. Nature 2002, 418, 386. [Google Scholar] [CrossRef] [PubMed]
- Karanasios, E.; Ktistakis, N.T. Live-cell imaging for the assessment of the dynamics of autophagosome formation: Focus on early steps. Methods 2015, 75, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Perlstein, E.O.; Imarisio, S.; Pineau, S.; Cordenier, A.; Maglathlin, R.L.; Webster, J.A.; Lewis, T.A.; O’Kane, C.J.; Schreiber, S.L.; et al. Small molecules enhance autophagy and reduce toxicity in Huntington’s disease models. Nat. Chem. Biol. 2007, 3, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, J.; Pan, H.; Hu, P.; Hao, Y.; Cai, W.; Zhu, H.; Yu, A.D.; Xie, X.; Ma, D.; et al. Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc. Natl. Acad. Sci. USA 2007, 104, 19023–19028. [Google Scholar] [CrossRef] [PubMed]
- Hundeshagen, P.; Hamacher-Brady, A.; Eils, R.; Brady, N.R. Concurrent detection of autolysosome formation and lysosomal degradation by flow cytometry in a high-content screen for inducers of autophagy. BMC Biol. 2011, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Joachim, J.; Jiang, M.; McKnight, N.C.; Howell, M.; Tooze, S.A. High-throughput screening approaches to identify regulators of mammalian autophagy. Methods 2015, 75, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Mizushima, N. LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 2015, 75, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.W.; Zou, P.; Udeshi, N.D.; Martell, J.D.; Mootha, V.K.; Carr, S.A.; Ting, A.Y. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science 2013, 339, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Packer, M.; Codogno, P. Development of autophagy inducers in clinical medicine. J. Clin. Invest. 2015, 125, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Wild, P.; Farhan, H.; McEwan, D.G.; Wagner, S.; Rogov, V.V.; Brady, N.R.; Richter, B.; Korac, J.; Waidmann, O.; Choudhary, C.; et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 2011, 333, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.; Popova, T.; Kashanchi, F.; Bailey, C. Targeting the inflammasome and adenosine type-3 receptors improves outcome of antibiotic therapy in murine anthrax. World J. Biol. Chem. 2011, 2, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Choi, A. Autophagy: A potential therapeutic target in lung diseases. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L93–L107. [Google Scholar] [CrossRef] [PubMed]
- Vakifahmetoglu-Norberg, H.; Xia, H.G.; Yuan, J. Pharmacologic agents targeting autophagy. J. Clin. Invest. 2015, 125, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S. Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: Autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem. Soc. Trans. 2013, 41, 1103–1130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Wang, Y.; Paueksakon, P.; Harris, R.C. Epidermal growth factor receptor inhibition slows progression of diabetic nephropathy in association with a decrease in endoplasmic reticulum stress and an increase in autophagy. Diabetes 2014, 63, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S. Chemical screening platforms for autophagy drug discovery to identify therapeutic candidates for Huntington’s disease and other neurodegenerative disorders. Drug Discov. Today Technol. 2013, 10, e137–e144. [Google Scholar] [CrossRef] [PubMed]
- Lonskaya, I.; Hebron, M.L.; Desforges, N.M.; Schachter, J.B.; Moussa, C.E. Nilotinib-induced autophagic changes increase endogenous parkin level and ubiquitination, leading to amyloid clearance. J. Mol. Med. (Berl) 2014, 92, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chiang, H.C.; Wu, W.; Liang, B.; Xie, Z.; Yao, X.; Ma, W.; Du, S.; Zhong, Y. Epidermal growth factor receptor is a preferred target for treating amyloid-beta-induced memory loss. Proc. Natl. Acad. Sci. USA 2012, 109, 16743–16748. [Google Scholar] [CrossRef] [PubMed]
- Hidvegi, T.; Ewing, M.; Hale, P.; Dippold, C.; Beckett, C.; Kemp, C.; Maurice, N.; Mukherjee, A.; Goldbach, C.; Watkins, S.; et al. An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science 2010, 329, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Conway, K.L.; Kuballa, P.; Song, J.H.; Patel, K.K.; Castoreno, A.B.; Yilmaz, O.H.; Jijon, H.B.; Zhang, M.; Aldrich, L.N.; Villablanca, E.J.; et al. Atg16l1 is required for autophagy in intestinal epithelial cells and protection of mice from Salmonella infection. Gastroenterology 2013, 145, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Parihar, S.P.; Guler, R.; Khutlang, R.; Lang, D.M.; Hurdayal, R.; Mhlanga, M.M.; Suzuki, H.; Marais, A.D.; Brombacher, F. Statin therapy reduces the mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J. Infect. Dis. 2014, 209, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, B.; Duden, R.; Rubinsztein, D.C. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum. Mol. Genet. 2002, 11, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, B.; Vacher, C.; Berger, Z.; Davies, J.E.; Luo, S.; Oroz, L.G.; Scaravilli, F.; Easton, D.F.; Duden, R.; O’Kane, C.J.; et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet. 2004, 36, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Sarkar, S.; Cuddon, P.; Ttofi, E.K.; Saiki, S.; Siddiqi, F.H.; Jahreiss, L.; Fleming, A.; Pask, D.; Goldsmith, P.; et al. Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol. 2008, 4, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Meley, D.; Bauvy, C.; Houben-Weerts, J.H.; Dubbelhuis, P.F.; Helmond, M.T.; Codogno, P.; Meijer, A.J. AMP-activated protein kinase and the regulation of autophagic proteolysis. J. Biol. Chem. 2006, 281, 34870–34879. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Shin, D.; Kim, W.; Yuk, J.; Jin, H.; Lee, S.; Cha, G.; Kim, J.; Lee, Z.; et al. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe 2012, 11, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Yuk, J.; Shin, D.; Lee, H.; Yang, C.; Jin, H.; Kim, K.; Lee, Z.; Lee, S.; Kim, J.; Jo, E. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 2009, 6, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Fabri, M.; Modlin, R. A vitamin for autophagy. Cell Host Microbe 2009, 6, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.; Fisher, B.; Kraskauskas, D.; Farkas, D.; Brophy, D.; Fowler, A.A.; Natarajan, R. Vitamin C: A novel regulator of neutrophil extracellular trap formation. Nutrients 2013, 5, 3131–3151. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Birmingham, N.; Jackson-Humbles, D.; Jiang, Q.; Harkema, J.; Peden, D. Supplementation with gamma-tocopherol attenuates endotoxin-induced airway neutrophil and mucous cell responses in rats. Free Radic Biol. Med. 2014, 68, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Floto, R.A.; Berger, Z.; Imarisio, S.; Cordenier, A.; Pasco, M.; Cook, L.J.; Rubinsztein, D.C. Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 2005, 170, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.; Tran, K.; Castoreno, A.; Peloquin, J.; Lassen, K.; Khor, B.; Aldrich, L.; Tan, P.; Graham, D.; Kuballa, P.; et al. Selective modulation of autophagy, innate immunity, and adaptive immunity by small molecules. ACS Chem. Biol. 2013, 8, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Parihar, S.; Guler, R.; Lang, D.; Suzuki, H.; Marais, A.; Brombacher, F. Simvastatin Enhances Protection against Listeria monocytogenes Infection in Mice by Counteracting Listeria-Induced Phagosomal Escape. PLoS ONE 2013, 8, e75490. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Qin, G.W.; Morinaga, O.; Shoyama, Y. 17-Hydroxy-jolkinolide B, a diterpenoid from Euphorbia fischeriana, inhibits inflammatory mediators but activates heme oxygenase-1 expression in lipopolysaccharide-stimulated murine macrophages. Int. Immunopharmacol. 2012, 12, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Korolchuk, V.I.; Renna, M.; Imarisio, S.; Fleming, A.; Williams, A.; Garcia-Arencibia, M.; Rose, C.; Luo, S.; Underwood, B.R.; et al. Complex inhibitory effects of nitric oxide on autophagy. Mol. Cell 2011, 43, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Opipari, A.W., Jr.; Tan, L.; Boitano, A.E.; Sorenson, D.R.; Aurora, A.; Liu, J.R. Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res. 2004, 64, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Armour, S.M.; Baur, J.A.; Hsieh, S.N.; Land-Bracha, A.; Thomas, S.M.; Sinclair, D.A. Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy. Aging (Albany NY) 2009, 1, 515–528. [Google Scholar] [PubMed]
- Jeong, J.K.; Moon, M.H.; Bae, B.C.; Lee, Y.J.; Seol, J.W.; Kang, H.S.; Kim, J.S.; Kang, S.J.; Park, S.Y. Autophagy induced by resveratrol prevents human prion protein-mediated neurotoxicity. Neurosci. Res. 2012, 73, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, S.; Li, J.; Assa, A.; Jundoria, A.; Xu, J.; Fan, S.; Eissa, N.T.; Tracey, K.J.; Sama, A.E.; et al. EGCG stimulates autophagy and reduces cytoplasmic HMGB1 levels in endotoxin-stimulated macrophages. Biochem. Pharmacol. 2011, 81, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Kandadi, M.; Yu, X.; Frankel, A.; Ren, J. Cardiac-specific catalase overexpression rescues anthrax lethal toxin-induced cardiac contractile dysfunction: Role of oxidative stress and autophagy. BMC Med. 2012, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Boya, P.; Gonzalez-Polo, R.A.; Casares, N.; Perfettini, J.L.; Dessen, P.; Larochette, N.; Metivier, D.; Meley, D.; Souquere, S.; Yoshimori, T.; et al. Inhibition of macroautophagy triggers apoptosis. Mol. Cell Biol. 2005, 25, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Amaravadi, R.K.; Yu, D.; Lum, J.J.; Bui, T.; Christophorou, M.A.; Evan, G.I.; Thomas-Tikhonenko, A.; Thompson, C.B. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J. Clin. Invest. 2007, 117, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Punnonen, E.L.; Reunanen, H. Effects of vinblastine, leucine, and histidine, and 3-methyladenine on autophagy in Ehrlich ascites cells. Exp. Mol. Pathol. 1990, 52, 87–97. [Google Scholar] [CrossRef]
- Reunanen, H.; Marttinen, M.; Hirsimaki, P. Effects of griseofulvin and nocodazole on the accumulation of autophagic vacuoles in Ehrlich ascites tumor cells. Exp. Mol. Pathol. 1988, 48, 97–102. [Google Scholar] [CrossRef]
- Webb, J.L.; Ravikumar, B.; Rubinsztein, D.C. Microtubule disruption inhibits autophagosome-lysosome fusion: Implications for studying the roles of aggresomes in polyglutamine diseases. Int. J. Biochem. Cell Biol. 2004, 36, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Blommaart, E.F.; Krause, U.; Schellens, J.P.; Vreeling-Sindelarova, H.; Meijer, A.J. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur. J. Biochem. 1997, 243, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Roe, N.; Xu, X.; Kandadi, M.; Hu, N.; Pang, J.; Weiser-Evans, M.; Ren, J. Targeted deletion of PTEN in cardiomyocytes renders cardiac contractile dysfunction through interruption of Pink1-AMPK signaling and autophagy. Biochim. Biophys. Acta 2014, 1852, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Mostowy, S.; Boucontet, L.; Mazon Moya, M.; Sirianni, A.; Boudinot, P.; Hollinshead, M.; Cossart, P.; Herbomel, P.; Levraud, J.; Colucci-Guyon, E. The zebrafish as a new model for the in vivo study of Shigella flexneri interaction with phagocytes and bacterial autophagy. PLoS Pathog. 2013, 9, e1003588. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Mizumura, K.; Cloonan, S.; Haspel, J.; Choi, A. The emerging importance of autophagy in pulmonary diseases. Chest 2012, 142, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.; Zheng, X.; Forestieri, R.; Balgi, A.; Nodwell, M.; Vollett, S.; Anderson, H.; Andersen, R.; Av-Gay, Y.; Roberge, M. Nitazoxanide stimulates autophagy and inhibits mTORC1 signaling and intracellular proliferation of Mycobacterium tuberculosis. PLoS Pathog. 2012, 8, e1002691. [Google Scholar] [CrossRef] [PubMed]
- Junkins, R.; McCormick, C.; Lin, T. The emerging potential of autophagy-based therapies in the treatment of cystic fibrosis lung infections. Autophagy 2014, 10, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.; Lopez-Alonso, I.; Gonzalez-Lopez, A.; Amado-Rodriguez, L.; Batalla-Solis, E.; Astudillo, A.; Blazquez-Prieto, J.; Fernandez, A.; Galvan, J.; Dos Santos, C.; et al. Defective autophagy impairs ATF3 activity and worsens lung injury during endotoxemia. J. Mol. Med. (Berl) 2014, 92, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Higami, Y.; Komatsu, T.; Tanaka, K.; Honda, S.; Yamaza, H.; Chiba, T.; Ayabe, H.; Shimokawa, I. Acute stress response in calorie-restricted rats to lipopolysaccharide-induced inflammation. Mech. Ageing Dev. 2005, 126, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Carchman, E.; Rao, J.; Loughran, P.; Rosengart, M.; Zuckerbraun, B. Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology 2011, 53, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Choi, A.M. Autophagy: An Integral Component of the Mammalian Stress Response. J. Biochem. Pharmacol. Res. 2013, 1, 176–188. [Google Scholar] [PubMed]
- Kandadi, M.; Frankel, A.; Ren, J. Toll-like receptor 4 knockout protects against anthrax lethal toxin-induced cardiac contractile dysfunction: Role of autophagy. Br. J. Pharmacol. 2012, 167, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Chang, H.; Choi, H.; Shin, J.; Park, S.; Jo, Y.; Choi, E.; Baek, S.; Kim, B.; Chang, J.; et al. Autophagy induced by resveratrol suppresses alpha-MSH-induced melanogenesis. Exp. Dermatol. 2014, 23, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Flemmig, J.; Remmler, J.; Rohring, F.; Arnhold, J. (-)-Epicatechin regenerates the chlorinating activity of myeloperoxidase in vitro and in neutrophil granulocytes. J. Inorg. Biochem. 2014, 130, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Munz, C. Innate and adaptive immunity through autophagy. Immunity 2007, 27, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Kandadi, M.; Hua, Y.; Ma, H.; Li, Q.; Kuo, S.; Frankel, A.; Ren, J. Anthrax lethal toxin suppresses murine cardiomyocyte contractile function and intracellular Ca2+ handling via a NADPH oxidase-dependent mechanism. PLoS ONE 2010, 5, e13335. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aica, I.; Dona, M.; Tonello, F.; Piris, A.; Mock, M.; Montecucco, C.; Garbisa, S. Potent inhibitors of anthrax lethal factor from green tea. EMBO Rep. 2004, 5, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Sakagami, H. Induction of apoptosis by epigallocatechin gallate and autophagy inhibitors in a mouse macrophage-like cell line. Anticancer Res. 2008, 28, 1713–1718. [Google Scholar] [PubMed]
- Kim, H.S.; Montana, V.; Jang, H.J.; Parpura, V.; Kim, J.A. Epigallocatechin gallate (EGCG) stimulates autophagy in vascular endothelial cells: A potential role for reducing lipid accumulation. J. Biol. Chem. 2013, 288, 22693–22705. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Kim, Y.; Jung, J.; Yang, S.; Lee, T.; Shin, D. Resveratrol induces autophagy through death-associated protein kinase 1 (DAPK1) in human dermal fibroblasts under normal culture conditions. Exp. Dermatol. 2013, 22, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; Lang, F.; Chen, Y.; Zhang, H.; Cong, X.; Shen, X.; Su, G. Resveratrol alleviates endotoxin-induced myocardial toxicity via the Nrf2 transcription factor. PLoS ONE 2013, 8, e69452. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A. Resveratrol, sirtuins, and the promise of a DR mimetic. Mech. Ageing Dev. 2010, 131, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Marino, G.; Bennetzen, M.V.; Eisenberg, T.; Megalou, E.; Schroeder, S.; Cabrera, S.; Benit, P.; Rustin, P.; Criollo, A.; et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J. Cell Biol. 2011, 192, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Marino, G.; Lissa, D.; Vacchelli, E.; Malik, S.A.; Niso-Santano, M.; Zamzami, N.; Galluzzi, L.; Maiuri, M.C.; Kroemer, G. Pro-autophagic polyphenols reduce the acetylation of cytoplasmic proteins. Cell Cycle 2012, 11, 3851–3860. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Battistoni, A.; Gambari, R.; Pompella, A.; Bragonzi, A.; Pilolli, F.; Iuliano, L.; Piroddi, M.; Dechecchi, M.; Cabrini, G. Oxidative stress and antioxidant therapy in cystic fibrosis. Biochim. Biophys. Acta 2012, 1822, 690–713. [Google Scholar] [CrossRef] [PubMed]
- Fabri, M.; Realegeno, S.; Jo, E.; Modlin, R. Role of autophagy in the host response to microbial infection and potential for therapy. Curr. Opin. Immunol. 2011, 23, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Hase, K.; Segawa, A.; Takita, T. H89 (N-[2-p-bromocinnamylamino-ethyl]-5-isoquinolinesulphonamide) induces autophagy independently of protein kinase A inhibition. Eur. J. Pharmacol. 2013, 714, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kuniyasu, H. Significance of AKT in gastric cancer (Review). Int. J. Oncol. 2014, 45, 2187–2192. [Google Scholar] [PubMed]
- Hasko, G.; Nemeth, Z.; Vizi, E.; Salzman, A.; Szabo, C. An agonist of adenosine A3 receptors decreases interleukin-12 and interferon-gamma production and prevents lethality in endotoxemic mice. Eur. J. Pharmacol. 1998, 358, 261–268. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.; Joo, J.; Gallos, G.; Chen, J.; Emala, C. A3 adenosine receptor activation decreases mortality and renal and hepatic injury in murine septic peritonitis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R959–R969. [Google Scholar] [CrossRef] [PubMed]
- Csoka, B.; Nemeth, Z.; Rosenberger, P.; Eltzschig, H.; Spolarics, Z.; Pacher, P.; Selmeczy, Z.; Koscso, B.; Himer, L.; Vizi, E.; et al. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J. Immunol. 2010, 185, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Yitzhaki, S.; Huang, C.; Liu, W.; Lee, Y.; Gustafsson, A.; Mentzer, R.J.; Gottlieb, R. Autophagy is required for preconditioning by the adenosine A1 receptor-selective agonist CCPA. Basic Res. Cardiol. 2009, 104, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Factor, P.; Mutlu, G.; Chen, L.; Mohameed, J.; Akhmedov, A.; Meng, F.; Jilling, T.; Lewis, E.; Johnson, M.; Xu, A.; et al. Adenosine regulation of alveolar fluid clearance. Proc. Natl. Acad. Sci. USA 2007, 104, 4083–4088. [Google Scholar] [CrossRef] [PubMed]
- Hasko, G.; Linden, J.; Cronstein, B.; Pacher, P. Adenosine receptors: Therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008, 7, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Karapetyan, Y.; Sferrazza, G.; Zhou, M.; Ottenberg, G.; Spicer, T.; Chase, P.; Fallahi, M.; Hodder, P.; Weissmann, C.; Lasmezas, C. Unique drug screening approach for prion diseases identifies tacrolimus and astemizole as antiprion agents. Proc. Natl. Acad. Sci. USA 2013, 110, 7044–7049. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.; Gelli, A. Astemizole and an analogue promote fungicidal activity of fluconazole against Cryptococcus neoformans var. grubii and Cryptococcus gattii. Med. Mycol. 2010, 48, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.; Chen, X.; Shi, L.; Liu, J.; Sullivan, D.J. A clinical drug library screen identifies astemizole as an antimalarial agent. Nat. Chem. Biol. 2006, 2, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, L.; Higgins, D. A small-molecule screen identifies the antipsychotic drug pimozide as an inhibitor of Listeria monocytogenes infection. Antimicrob. Agents Chemother. 2009, 53, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, L.; Higgins, D. Inhibition of Listeria monocytogenes infection by neurological drugs. Int. J. Antimicrob. Agents 2010, 35, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. The discovery and development of HMG-CoA reductase inhibitors. J. Lipid Res. 1992, 33, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Kwak, B.; Mulhaupt, F.; Myit, S.; Mach, F. Statins as a newly recognized type of immunomodulator. Nat. Med. 2000, 6, 1399–1402. [Google Scholar] [PubMed]
- Liao, J.; Laufs, U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Tleyjeh, I.; Kashour, T.; Hakim, F.; Zimmerman, V.; Erwin, P.; Sutton, A.; Ibrahim, T. Statins for the prevention and treatment of infections: A systematic review and meta-analysis. Arch. Intern. Med. 2009, 169, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Hennen, R.; Keller, A.; Russ, M.; Muller-Werdan, U.; Werdan, K.; Buerke, M. Association of statin therapy and increased survival in patients with multiple organ dysfunction syndrome. Intensive Care Med. 2006, 32, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Souraud, J.B.; Briolant, S.; Dormoi, J.; Mosnier, J.; Savini, H.; Baret, E.; Amalvict, R.; Soulard, R.; Rogier, C.; Pradines, B. Atorvastatin treatment is effective when used in combination with mefloquine in an experimental cerebral malaria murine model. Malar J. 2012, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.A.; Estato, V.; da Silva, T.I.; d’Avila, J.C.; Siqueira, L.D.; Assis, E.F.; Bozza, P.T.; Bozza, F.A.; Tibirica, E.V.; Zimmerman, G.A.; et al. Statins decrease neuroinflammation and prevent cognitive impairment after cerebral malaria. PLoS Pathog. 2012, 8, e1003099. [Google Scholar] [CrossRef] [PubMed]
- Hotze, E.; Tweten, R. Membrane assembly of the cholesterol-dependent cytolysin pore complex. Biochim. Biophys. Acta 2012, 1818, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.R.; Bischofberger, M.; Freche, B.; Ho, S.; Parton, R.G.; van der Goot, F.G. Pore-forming toxins induce multiple cellular responses promoting survival. Cell Microbiol. 2011, 13, 1026–1043. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, C.; Shu, Y.; Ju, X.; Zou, Z.; Wang, H.; Rao, S.; Guo, F.; Liu, H.; Nan, W.; et al. Inhibition of autophagy ameliorates acute lung injury caused by avian influenza A H5N1 infection. Sci. Signal 2012, 5, ra16. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.; Yuan, S.; Hsu, C.; Cheng, Y.; Chang, Y.; Hsueh, H.; Lee, P.; Hsieh, Y. Lc3 over-expression improves survival and attenuates lung injury through increasing autophagosomal clearance in septic mice. Ann. Surg. 2013, 257, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Schein, C.; Chen, D.; Ma, L.; Kanalas, J.; Gao, J.; Jimenez, M.; Sower, L.; Walter, M.; Gilbertson, S.; Peterson, J. Pharmacophore selection and redesign of non-nucleotide inhibitors of anthrax edema factor. Toxins (Basel) 2012, 4, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Shin, J.; Kim, H.; Oh, S.; Lee, P. Neuroprotective effects of mesenchymal stem cells through autophagy modulation in a parkinsonian model. Neurobiol. Aging 2014, 35, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Brandau, S.; Jakob, M.; Bruderek, K.; Bootz, F.; Giebel, B.; Radtke, S.; Mauel, K.; Jager, M.; Flohe, S.; Lang, S. Mesenchymal stem cells augment the anti-bacterial activity of neutrophil granulocytes. PLoS ONE 2014, 9, e106903. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathieu, J. Interactions between Autophagy and Bacterial Toxins: Targets for Therapy? Toxins 2015, 7, 2918-2958. https://doi.org/10.3390/toxins7082918
Mathieu J. Interactions between Autophagy and Bacterial Toxins: Targets for Therapy? Toxins. 2015; 7(8):2918-2958. https://doi.org/10.3390/toxins7082918
Chicago/Turabian StyleMathieu, Jacques. 2015. "Interactions between Autophagy and Bacterial Toxins: Targets for Therapy?" Toxins 7, no. 8: 2918-2958. https://doi.org/10.3390/toxins7082918
APA StyleMathieu, J. (2015). Interactions between Autophagy and Bacterial Toxins: Targets for Therapy? Toxins, 7(8), 2918-2958. https://doi.org/10.3390/toxins7082918






