Analgesic Effects of Bee Venom Derived Phospholipase A2 in a Mouse Model of Oxaliplatin-Induced Neuropathic Pain
Abstract
:1. Introduction
2. Results
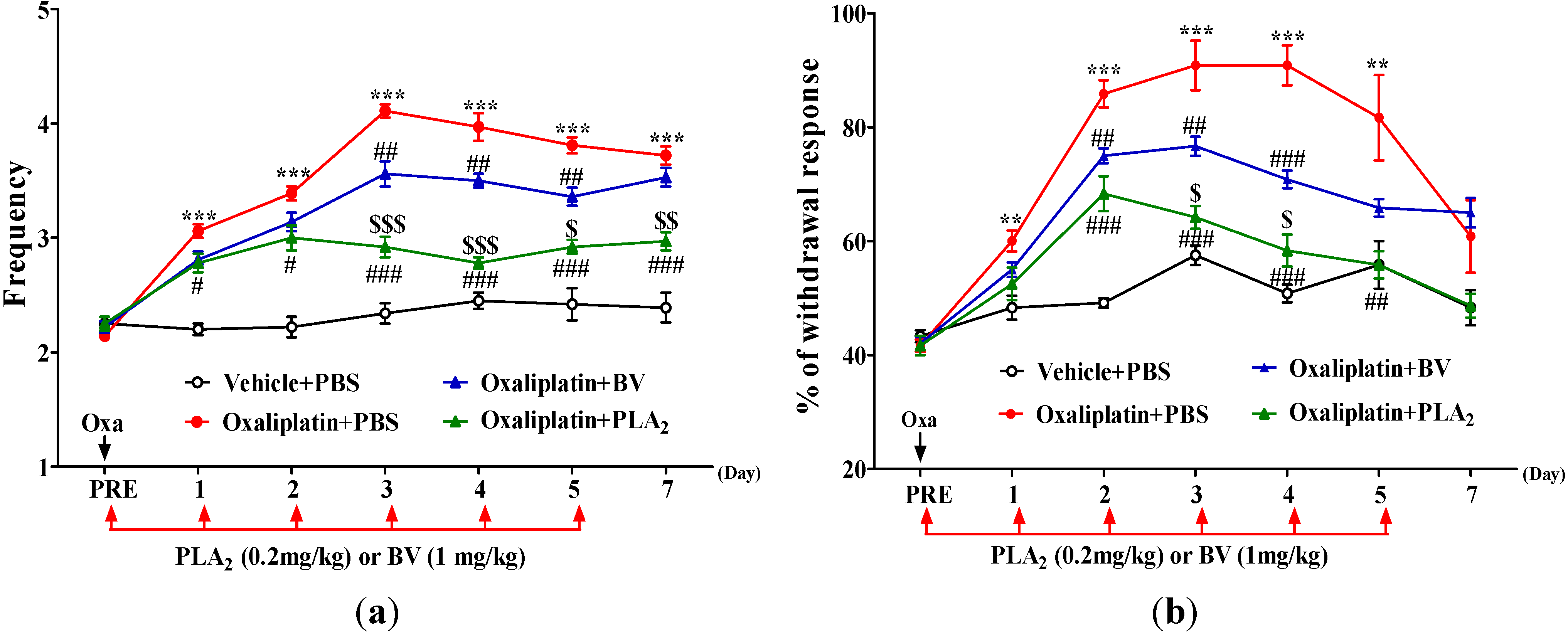
2.1. Effects of BV and bvPLA2 on Oxaliplatin-Induced Cold and Mechanical Hypersensitivity
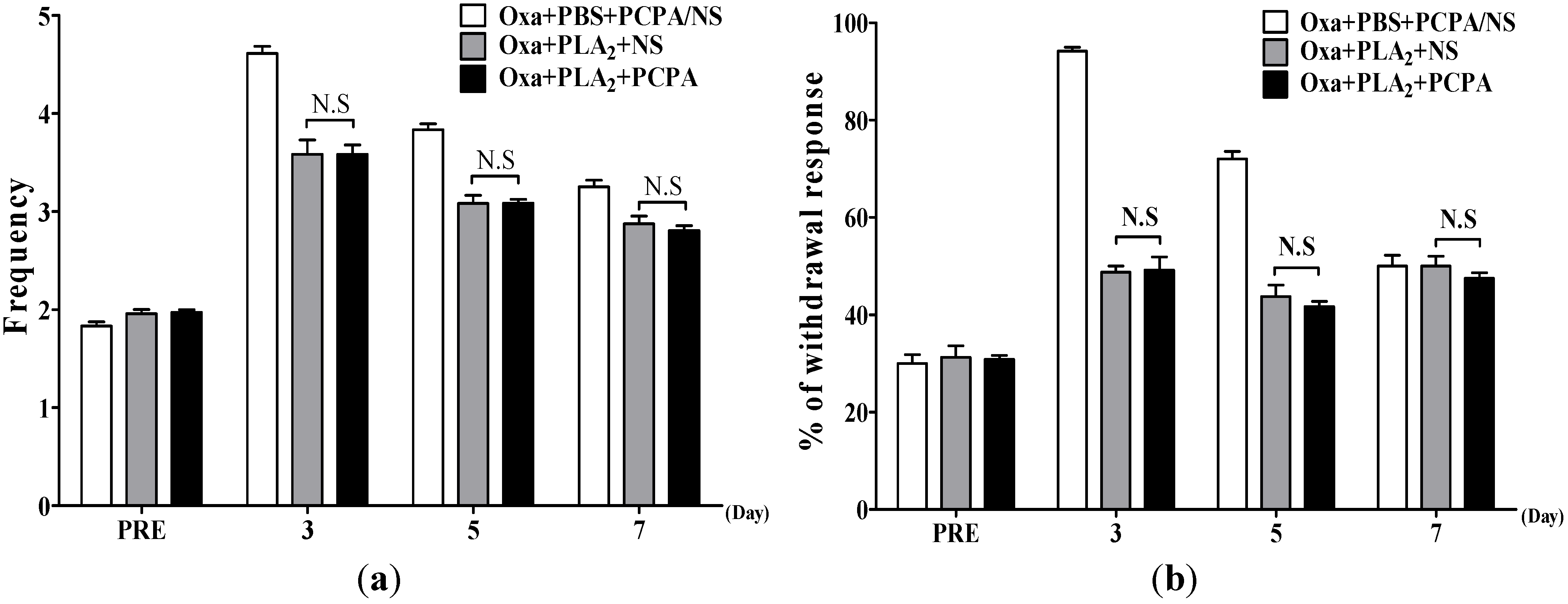
2.2. Effects of BvPLA2 on Oxaliplatin-Induced Cold and Mechanical Allodynia in Serotonin Depleted Mice
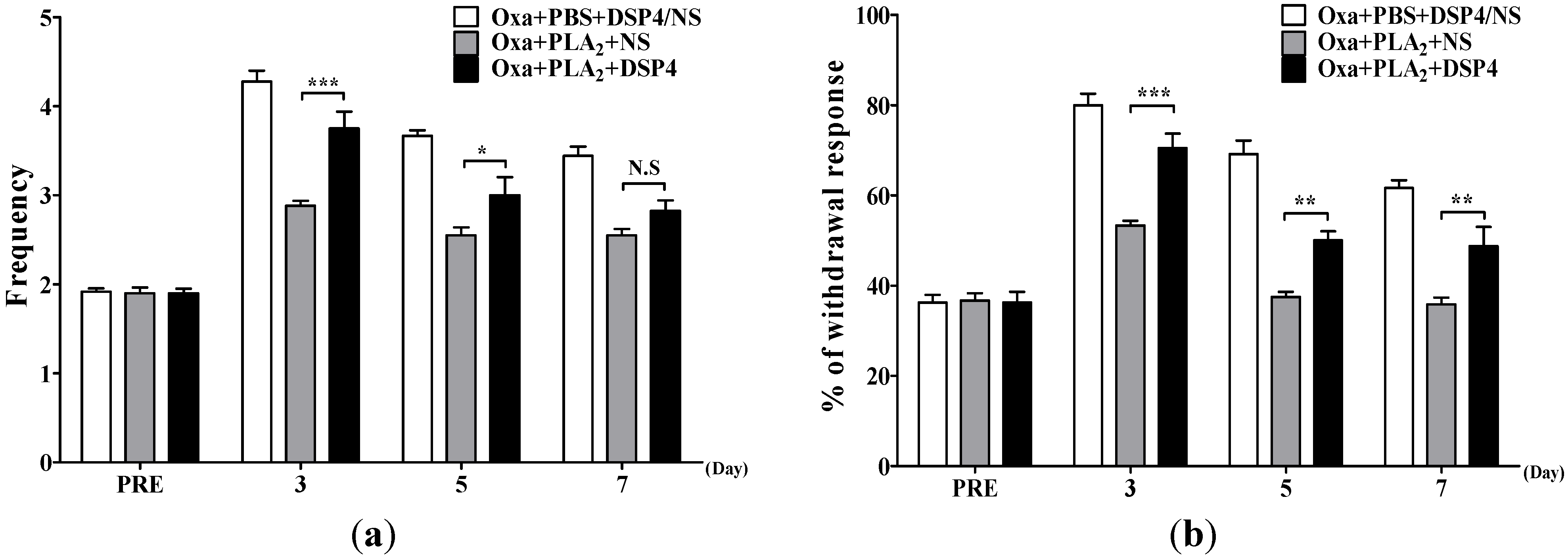
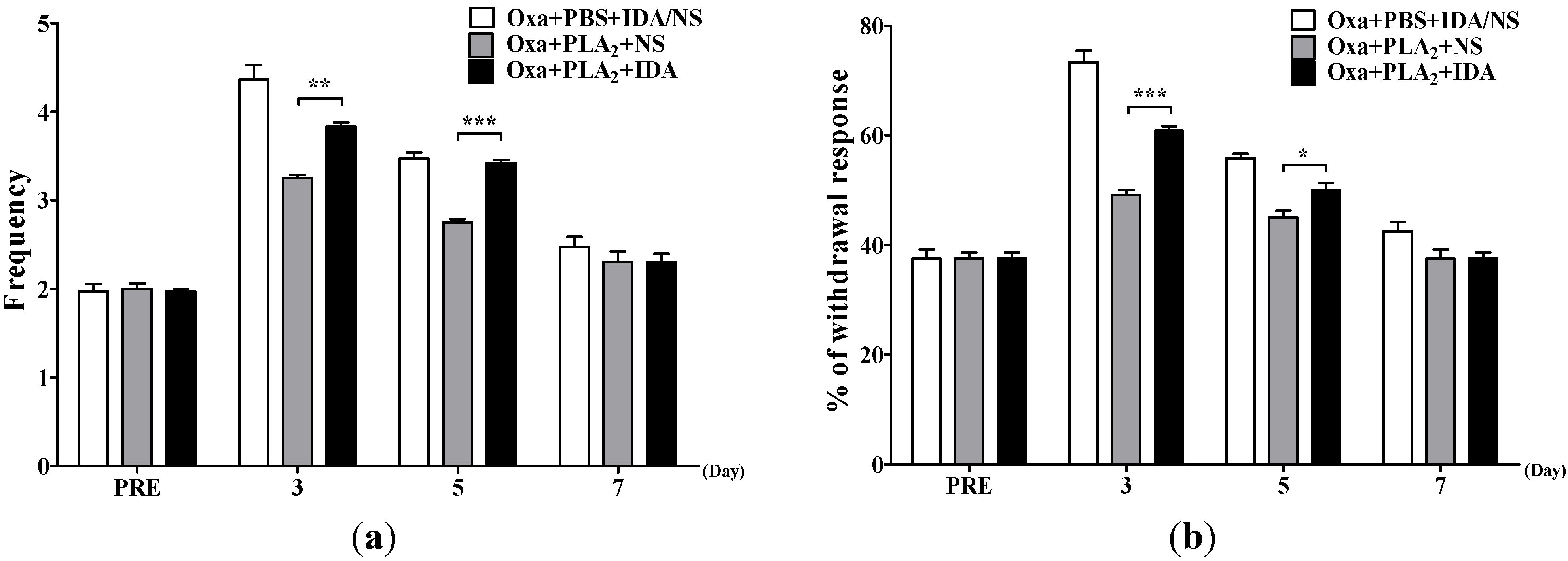
2.3. Noradrenergic Mechanism of the Anti-Allodynic Effects of BvPLA2 in Oxaliplatin-Administered Mice
| Post-Oxaliplatin Day | D0 | D3 | D5 | D7 |
|---|---|---|---|---|
| Acetone test | Frequency | |||
| Oxa + PBS + PRA/NS | 2.1 ± 0.04 | 4.4 ± 0.08 | 3.6 ± 0.15 | 3.3 ± 0.09 |
| Oxa + PLA2 + NS | 2.0 ± 0.06 | 3.5 ± 0.15 *** | 3.0 ± 0.10 ** | 2.6 ± 0.10 *** |
| Oxa + PLA2 + PRA | 2.1 ± 0.06 | 3.1 ± 0.12 *** | 3.1 ± 0.09 * | 2.8 ± 0.07 ** |
| von Frey test <0.4 g> | % withdrawal | response | ||
| Oxa + PBS + PRA/NS | 40.8 ± 0.83 | 76.7 ± 1.67 | 55.8 ± 1.54 | 44.2 ± 1.54 |
| Oxa + PLA2 + NS | 40.0 ± 1.83 | 59.2 ± 2.39 *** | 48.1 ± 1.29 ** | 41.5 ± 1.12 |
| Oxa + PLA2 + PRA | 40.8 ± 2.01 | 57.5 ± 1.71 *** | 47.5 ± 1.71 ** | 40.33 ± 1.67 |
3. Discussion
4. Experimental Section
4.1. Animals
4.2. Behavioral Tests
4.3. Oxaliplatin Administration and BV/bvPLA2 Treatment
4.4. Depletion of Serotonin or Noradrenaline
4.5. α1- or α2-Adrenergic Receptor Antagonist
4.6. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Andre, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- De Gramont, A.; Figer, A.; Seymour, M.; Homerin, M.; Hmissi, A.; Cassidy, J.; Boni, C.; Cortes-Funes, H.; Cervantes, A.; Freyer, G.; et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J. Clin. Oncol. 2000, 18, 2938–2947. [Google Scholar] [PubMed]
- Cassidy, J.; Misset, J.L. Oxaliplatin-related side effects: Characteristics and management. Semin. Oncol. 2002, 29, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Extra, J.M.; Marty, M.; Brienza, S.; Misset, J.L. Pharmacokinetics and safety profile of oxaliplatin. Semin. Oncol. 1998, 25, 13–22. [Google Scholar] [PubMed]
- Pasetto, L.M.; D’Andrea, M.R.; Rossi, E.; Monfardini, S. Oxaliplatin-related neurotoxicity: How and why? Crit. Rev. Oncol. Hematol. 2006, 59, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A. Oxaliplatin-safety profile: Neurotoxicity. Semin. Oncol. 2003, 30, 5–13. [Google Scholar] [CrossRef]
- Billingham, M.E.; Morley, J.; Hanson, J.M.; Shipolini, R.A.; Vernon, C.A. Letter: An anti-inflammatory peptide from bee venom. Nature 1973, 245, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Park, S.; Choi, T.; Lee, G.; Haam, K.K.; Hong, M.C.; Min, B.I.; Bae, H. Bee venom ameliorates ovalbumin induced allergic asthma via modulating CD4+CD25+ regulatory T cells in mice. Cytokine 2013, 61, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.S.; Moon, H.J.; Li, D.X.; Gil, M.; Min, J.K.; Lee, G.; Bae, H.; Kim, S.K.; Min, B.I. Effect of bee venom acupuncture on oxaliplatin-induced cold allodynia in rats. Evid. Based Complement. Alternat. Med. 2013, 2013, 369324. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Yeo, J.H.; Han, S.D.; Bong, D.J.; Oh, B.; Roh, D.H. Diluted bee venom injection reduces ipsilateral mechanical allodynia in oxaliplatin-induced neuropathic mice. Biol. Pharm. Bull. 2013, 36, 1787–1793. [Google Scholar] [CrossRef] [PubMed]
- Somerfield, S.D.; Brandwein, S. Bee venom and adjuvant arthritis. J. Rheumatol. 1988, 15, 1878. [Google Scholar] [PubMed]
- Roh, D.H.; Kwon, Y.B.; Kim, H.W.; Ham, T.W.; Yoon, S.Y.; Kang, S.Y.; Han, H.J.; Lee, H.J.; Beitz, A.J.; Lee, J.H. Acupoint stimulation with diluted bee venom (apipuncture) alleviates thermal hyperalgesia in a rodent neuropathic pain model: Involvement of spinal alpha 2-adrenoceptors. J. Pain 2004, 5, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.H.; Huh, J.E.; Lee, J.D.; Choi do, Y.; Park, D.S. Antinociceptive effect and the mechanism of bee venom acupuncture (apipuncture) on inflammatory pain in the rat model of collagen-induced arthritis: Mediation by alpha2-adrenoceptors. Brain Res. 2006, 1073–1074, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.B.; Kang, M.S.; Han, H.J.; Beitz, A.J.; Lee, J.H. Visceral antinociception produced by bee venom stimulation of the Zhongwan acupuncture point in mice: Role of alpha(2) adrenoceptors. Neurosci. Lett. 2001, 308, 133–137. [Google Scholar] [CrossRef]
- Kim, H.W.; Kwon, Y.B.; Han, H.J.; Yang, I.S.; Beitz, A.J.; Lee, J.H. Antinociceptive mechanisms associated with diluted bee venom acupuncture (apipuncture) in the rat formalin test: Involvement of descending adrenergic and serotonergic pathways. Pharmacol. Res. 2005, 51, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Li, D.X.; Yoon, H.; Go, D.; Quan, F.S.; Min, B.I.; Kim, S.K. Serotonergic mechanism of the relieving effect of bee venom acupuncture on oxaliplatin-induced neuropathic cold allodynia in rats. BMC Complement. Altern. Med. 2014, 14, 471. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Roh, D.H.; Park, J.H.; Lee, H.J.; Lee, J.H. Activation of spinal alpha2-adrenoceptors using diluted bee venom stimulation reduces cold allodynia in neuropathic pain rats. Evid. Based Complement. Altern. Med. 2012, 2012, 784713. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Roh, D.H.; Yoon, S.Y.; Moon, J.Y.; Kim, H.W.; Lee, H.J.; Beitz, A.J.; Lee, J.H. Repetitive treatment with diluted bee venom reduces neuropathic pain via potentiation of locus coeruleus noradrenergic neuronal activity and modulation of spinal NR1 phosphorylation in rats. J. Pain 2012, 13, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.C.; Casapullo, A.; Santomauro, C.; D’Auria, M.V.; Riccio, R.; Gomez-Paloma, L. The molecular mechanism of bee venom phospholipase A2 inactivation by bolinaquinone. Chembiochem 2006, 7, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Kinnunen, P.K. Modulation of the activity of secretory phospholipase A2 by antimicrobial peptides. Antimicrob. Agents Chemother. 2003, 47, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.K.; Moon, M.H.; Bae, B.C.; Lee, Y.J.; Seol, J.W.; Park, S.Y. Bee venom phospholipase A2 prevents prion peptide induced-cell death in neuronal cells. Int. J. Mol. Med. 2011, 28, 867–873. [Google Scholar] [PubMed]
- Lopez-Vales, R.; Ghasemlou, N.; Redensek, A.; Kerr, B.J.; Barbayianni, E.; Antonopoulou, G.; Baskakis, C.; Rathore, K.I.; Constantinou-Kokotou, V.; Stephens, D.; et al. Phospholipase A2 superfamily members play divergent roles after spinal cord injury. FASEB J. 2011, 25, 4240–4252. [Google Scholar] [CrossRef] [PubMed]
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix metalloproteinases: Role in arthritis. Front. Biosci. 2006, 11, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, H.; Lee, G.; Jang, H.; Kim, S.-S.; Yoon, H.; Kang, G.-H.; Hwang, D.-S.; Kim, S.K.; Chung, H.-S.; et al. Phospholipase A2 inhibits cisplatin-induced acute kidney injury by modulating regulatory T cells via CD206 mannose receptor. Kidney Int. 2015, 10. [Google Scholar] [CrossRef]
- Zhu, J.X.; Zhu, X.Y.; Owyang, C.; Li, Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J. Physiol. 2001, 530, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Maleki, N.; Nayebi, A.M.; Garjani, A. Effects of central and peripheral depletion of serotonergic system on carrageenan-induced paw oedema. Int. Immunopharmacol. 2005, 5, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Kushikata, T.; Kudo, M.; Hirota, K. Antinociceptive effects of neurotropin in a rat model of central neuropathic pain: DSP-4 induced noradrenergic lesion. Neurosci. Lett. 2011, 503, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Farquhar-Smith, P. Chemotherapy-induced neuropathic pain. Curr. Opin. Support. Palliat. Care 2011, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kannarkat, G.; Lasher, E.E.; Schiff, D. Neurologic complications of chemotherapy agents. Curr. Opin. Neurol. 2007, 20, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Lehky, T.J.; Leonard, G.D.; Wilson, R.H.; Grem, J.L.; Floeter, M.K. Oxaliplatin-induced neurotoxicity: Acute hyperexcitability and chronic neuropathy. Muscle Nerve 2004, 29, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Desoize, B.; Madoulet, C. Particular aspects of platinum compounds used at present in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 317–325. [Google Scholar] [CrossRef]
- Binder, A.; Stengel, M.; Maag, R.; Wasner, G.; Schoch, R.; Moosig, F.; Schommer, B.; Baron, R. Pain in oxaliplatin-induced neuropathy––Sensitisation in the peripheral and central nociceptive system. Eur. J. Cancer 2007, 43, 2658–2663. [Google Scholar] [CrossRef] [PubMed]
- Ling, B.; Coudore-Civiale, M.A.; Balayssac, D.; Eschalier, A.; Coudore, F.; Authier, N. Behavioral and immunohistological assessment of painful neuropathy induced by a single oxaliplatin injection in the rat. Toxicology 2007, 234, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Ling, B.; Coudore, F.; Decalonne, L.; Eschalier, A.; Authier, N. Comparative antiallodynic activity of morphine, pregabalin and lidocaine in a rat model of neuropathic pain produced by one oxaliplatin injection. Neuropharmacology 2008, 55, 724–728. [Google Scholar] [CrossRef] [PubMed]
- De Felipe, C.; Herrero, J.F.; O’Brien, J.A.; Palmer, J.A.; Doyle, C.A.; Smith, A.J.; Laird, J.M.; Belmonte, C.; Cervero, F.; Hunt, S.P. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature 1998, 392, 394–397. [Google Scholar] [PubMed]
- Honore, P.; Rogers, S.D.; Schwei, M.J.; Salak-Johnson, J.L.; Luger, N.M.; Sabino, M.C.; Clohisy, D.R.; Mantyh, P.W. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 2000, 98, 585–598. [Google Scholar] [CrossRef]
- Back, S.K.; Sung, B.; Hong, S.K.; Na, H.S. A mouse model for peripheral neuropathy produced by a partial injury of the nerve supplying the tail. Neurosci. Lett. 2002, 322, 153–156. [Google Scholar] [CrossRef]
- Kim, H.; Keum, D.J.; Kwak, J.; Chung, H.S.; Bae, H. Bee venom phospholipase A2 protects against acetaminophen-induced acute liver injury by modulating regulatory T cells and IL-10 in mice. PLoS ONE 2014, 9, e114726. [Google Scholar] [CrossRef] [PubMed]
- Lamont, L.A.; Tranquilli, W.J.; Grimm, K.A. Physiology of pain. Vet. Clin. N. Am. Small Anim. Pract. 2000, 30, 703–728. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Morimura, K.; Porreca, F. Descending pain modulation and chronification of pain. Curr. Opin. Support. Palliat. Care 2014, 8, 143–151. [Google Scholar] [PubMed]
- Crespi, F. Apamin increases 5-HT cell firing in raphe dorsalis and extracellular 5-HT levels in amygdala: A concomitant in vivo study in anesthetized rats. Brain Res. 2009, 1281, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Korenman, E.M.; Devor, M. Ectopic adrenergic sensitivity in damaged peripheral nerve axons in the rat. Exp. Neurol. 1981, 72, 63–81. [Google Scholar] [CrossRef]
- Lee, D.H.; Liu, X.; Kim, H.T.; Chung, K.; Chung, J.M. Receptor subtype mediating the adrenergic sensitivity of pain behavior and ectopic discharges in neuropathic Lewis rats. J. Neurophysiol. 1999, 81, 2226–2233. [Google Scholar] [PubMed]
- Leem, J.W.; Gwak, Y.S.; Nam, T.S.; Paik, K.S. Involvement of alpha2-adrenoceptors in mediating sympathetic excitation of injured dorsal root ganglion neurons in rats with spinal nerve ligation. Neurosci. Lett. 1997, 234, 39–42. [Google Scholar] [CrossRef]
- Zhang, J.M.; Song, X.J.; LaMotte, R.H. An in vitro study of ectopic discharge generation and adrenergic sensitivity in the intact, nerve-injured rat dorsal root ganglion. Pain 1997, 72, 51–57. [Google Scholar] [CrossRef]
- Hord, A.H.; Denson, D.D.; Stowe, B.; Haygood, R.M. Alpha-1 and alpha-2 adrenergic antagonists relieve thermal hyperalgesia in experimental mononeuropathy from chronic constriction injury. Anesth. Analg. 2001, 92, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- Tracey, D.J.; Cunningham, J.E.; Romm, M.A. Peripheral hyperalgesia in experimental neuropathy: Mediation by alpha 2-adrenoreceptors on post-ganglionic sympathetic terminals. Pain 1995, 60, 317–327. [Google Scholar] [CrossRef]
- Austin, P.J.; Kim, C.F.; Perera, C.J.; Moalem-Taylor, G. Regulatory T cells attenuate neuropathic pain following peripheral nerve injury and experimental autoimmune neuritis. Pain 2012, 153, 1916–1931. [Google Scholar] [CrossRef] [PubMed]
- Flatters, S.J.; Bennett, G.J. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain 2004, 109, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Joseph, E.K.; Levine, J.D. Comparison of oxaliplatin- and cisplatin-induced painful peripheral neuropathy in the rat. J. Pain 2009, 10, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Sugawara, T.; Fujishita, K.; Shinozaki, Y.; Matsukawa, T.; Suzuki, T.; Koizumi, S. The astrocyte-targeted therapy by bushi for the neuropathic pain in mice. PLoS ONE 2011, 6, e23510. [Google Scholar] [CrossRef] [PubMed]
- Scullion, G.A.; Kendall, D.A.; Sunter, D.; Marsden, C.A.; Pardon, M.C. Central noradrenergic depletion by DSP-4 prevents stress-induced memory impairments in the object recognition task. Neuroscience 2009, 164, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.D.; Campisi, J.; Sharkey, C.M.; Kennedy, S.L.; Nickerson, M.; Greenwood, B.N.; Fleshner, M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 2005, 135, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.E.; Lu, J.; Guo, T.; Saper, C.B.; Franks, N.P.; Maze, M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology 2003, 98, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Zarrindast, M.R.; Homayoun, H.; Khavandgar, S.; Fayaz-Dastgerdi, M. The effects of simultaneous administration of alpha(2)-adrenergic agents with L-NAME or L-arginine on the development and expression of morphine dependence in mice. Behav. Pharmacol. 2002, 13, 117–125. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Lee, Y.; Kim, W.; Lee, K.; Bae, H.; Kim, S.K. Analgesic Effects of Bee Venom Derived Phospholipase A2 in a Mouse Model of Oxaliplatin-Induced Neuropathic Pain. Toxins 2015, 7, 2422-2434. https://doi.org/10.3390/toxins7072422
Li D, Lee Y, Kim W, Lee K, Bae H, Kim SK. Analgesic Effects of Bee Venom Derived Phospholipase A2 in a Mouse Model of Oxaliplatin-Induced Neuropathic Pain. Toxins. 2015; 7(7):2422-2434. https://doi.org/10.3390/toxins7072422
Chicago/Turabian StyleLi, Dongxing, Younju Lee, Woojin Kim, Kyungjin Lee, Hyunsu Bae, and Sun Kwang Kim. 2015. "Analgesic Effects of Bee Venom Derived Phospholipase A2 in a Mouse Model of Oxaliplatin-Induced Neuropathic Pain" Toxins 7, no. 7: 2422-2434. https://doi.org/10.3390/toxins7072422
APA StyleLi, D., Lee, Y., Kim, W., Lee, K., Bae, H., & Kim, S. K. (2015). Analgesic Effects of Bee Venom Derived Phospholipase A2 in a Mouse Model of Oxaliplatin-Induced Neuropathic Pain. Toxins, 7(7), 2422-2434. https://doi.org/10.3390/toxins7072422






