Emergent Toxins in North Atlantic Temperate Waters: A Challenge for Monitoring Programs and Legislation
Abstract
:1. Introduction
1.1. Harmful Algal Blooms: General Description
1.2. Emergent Toxins
1.2.1. Tetrodotoxin
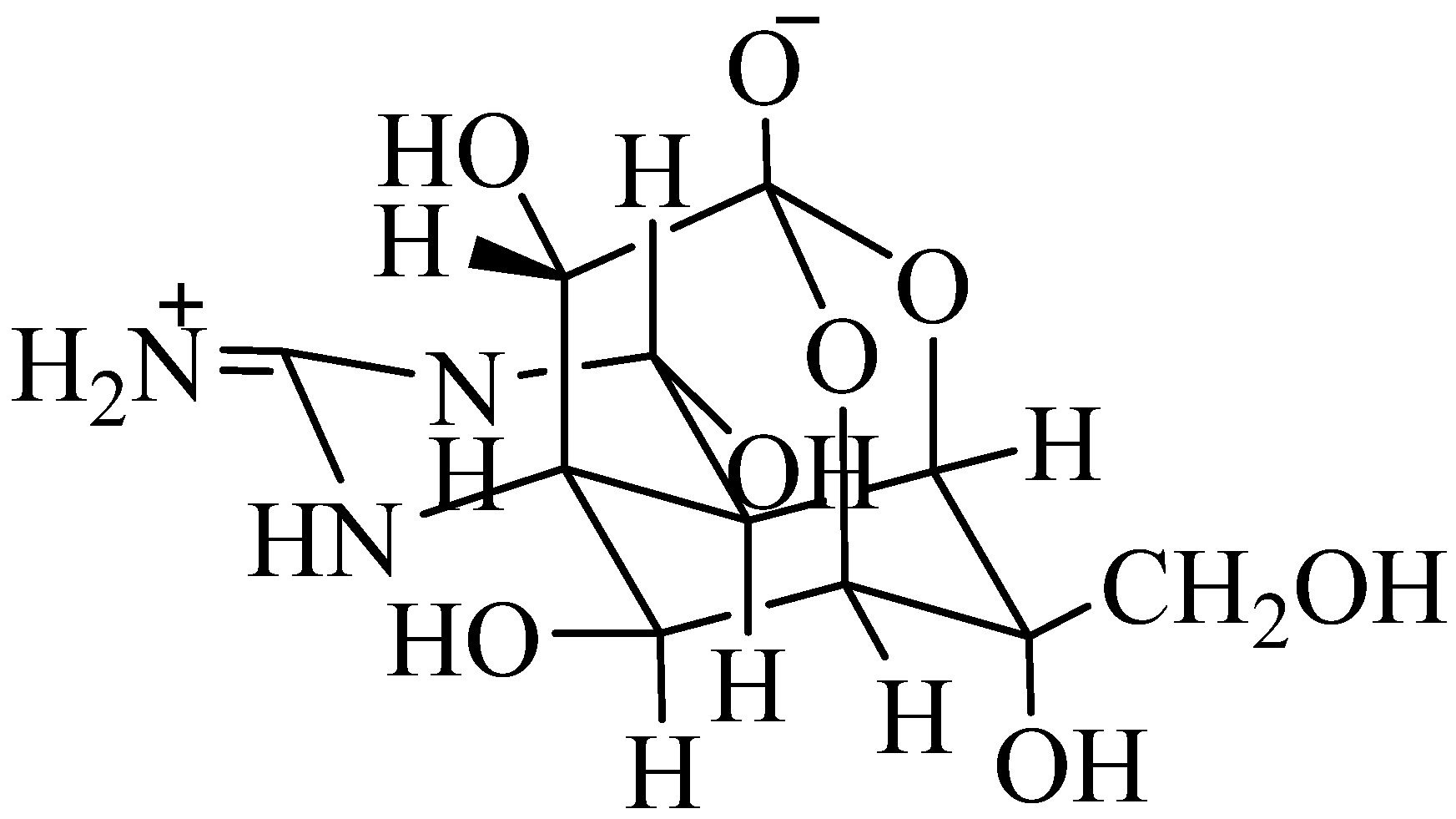
1.2.2. Palytoxin
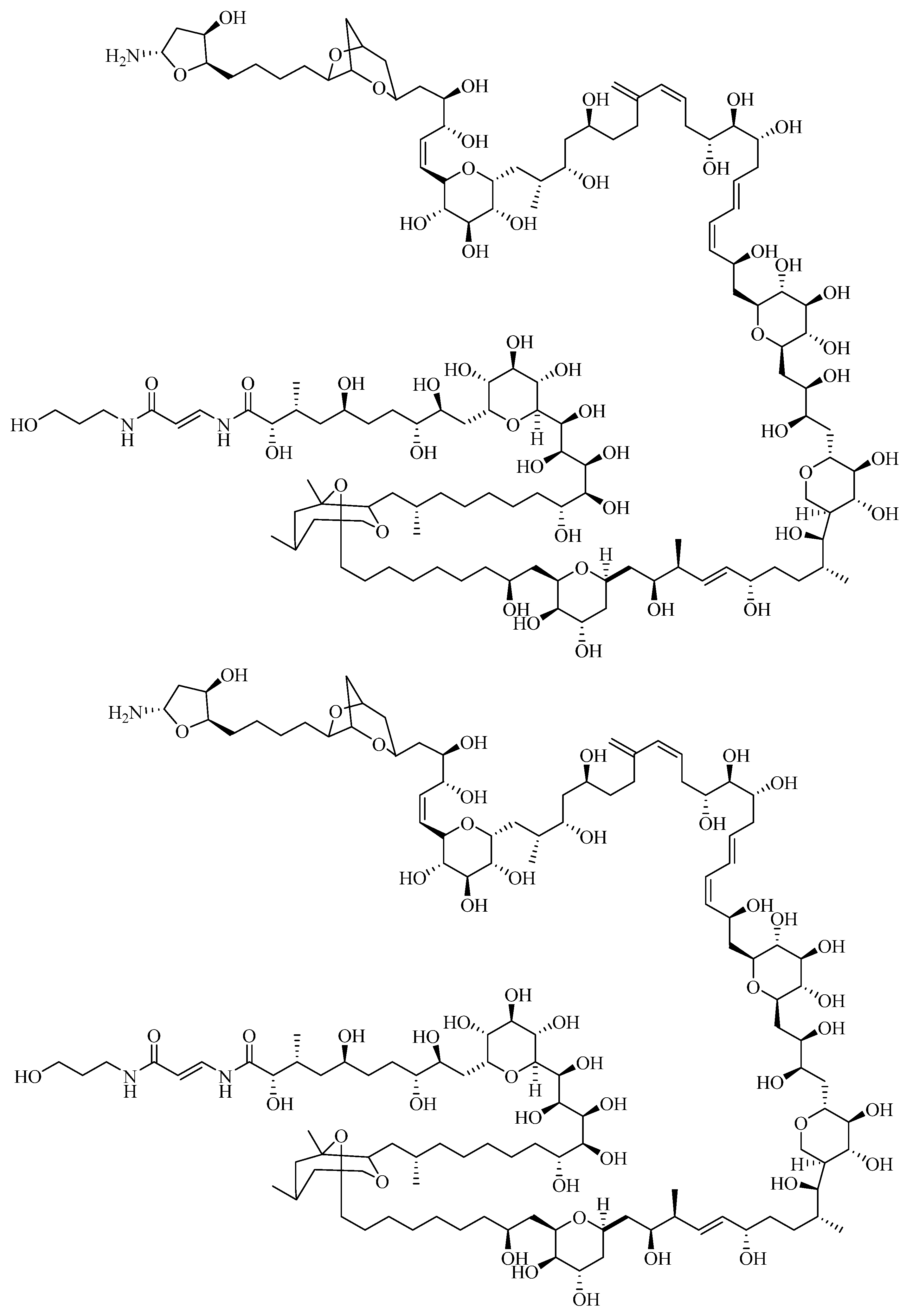
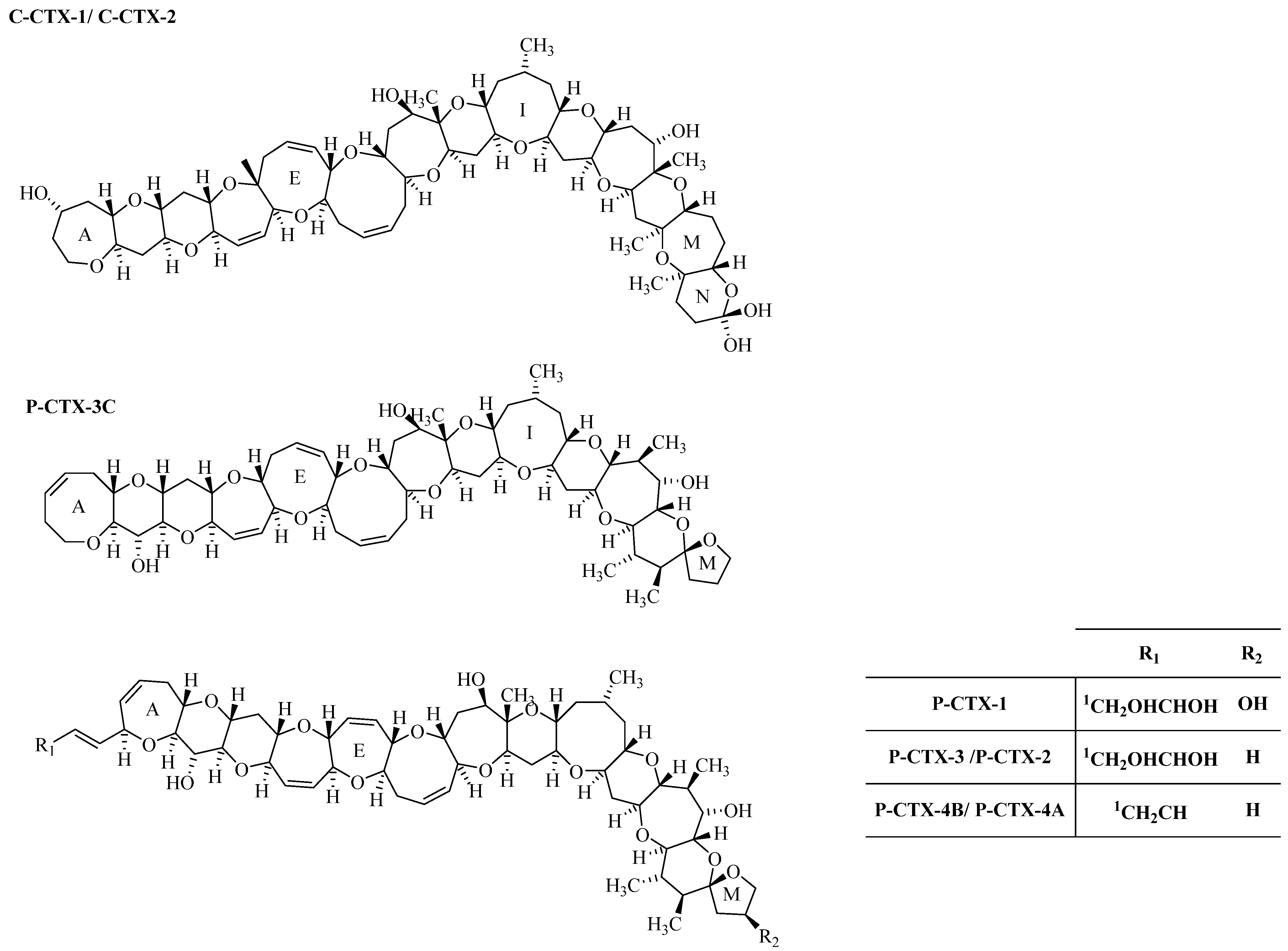
1.2.3. Ciguatoxin
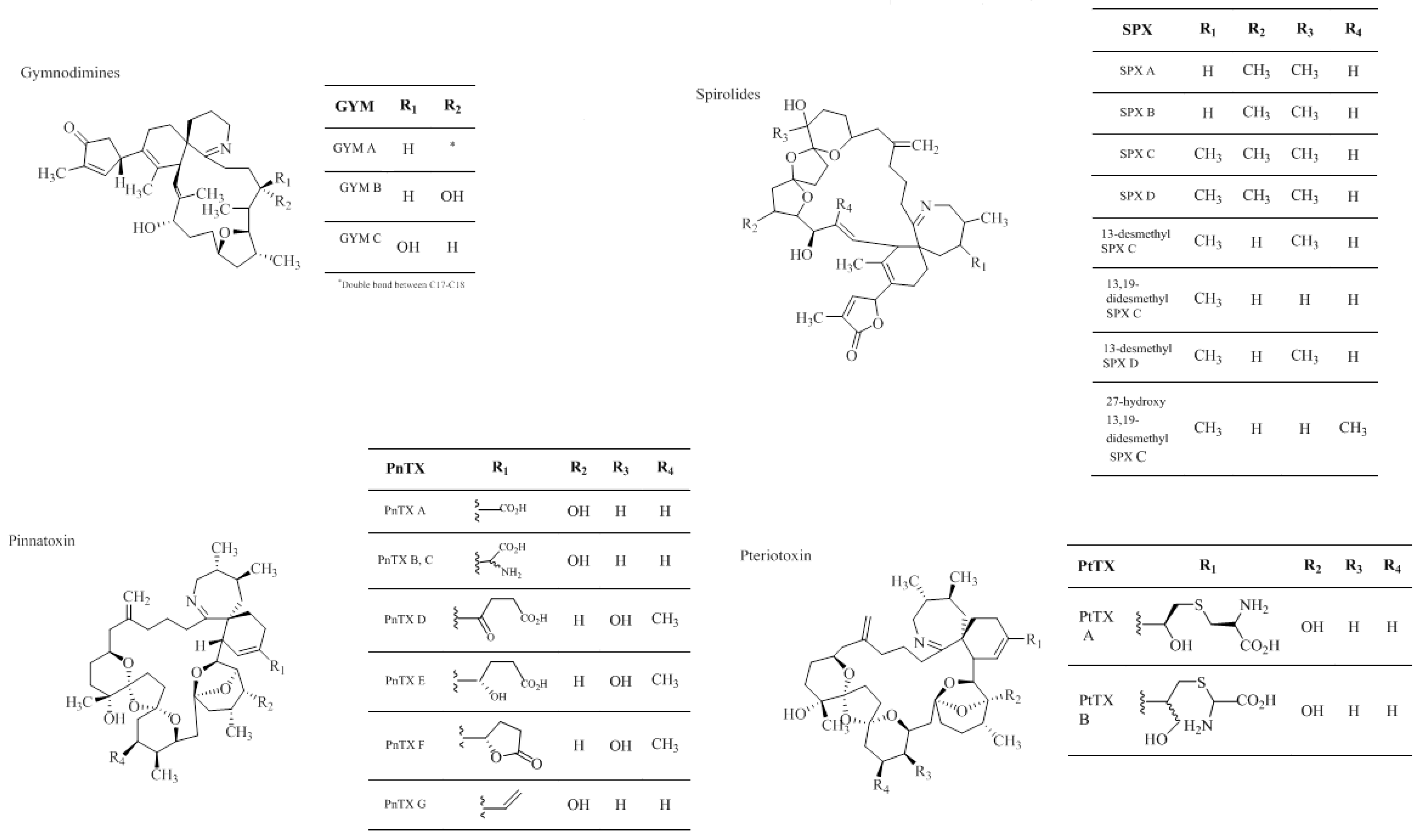
1.2.4. Cyclic Imines
| Toxin | Report location | Year | Vector/uptake route | No poisoning cases | Ref |
|---|---|---|---|---|---|
| TTX | Egypt/Israel | 2005/2007/2008 | Lagocephalus sceleratus (ingestion) | 13 | [136] |
| Spain | 2007 | Charonia lampas (ingestion) | 1 | [19] | |
| PTX | Italy | 2005/2006 | Ostreopsis ovate (aerosol) | 228 | [138] |
| Spain | 2010 | Ostreopsis sp. (aerosol) | 2 | [139] | |
| France | 2006–2009 | Ostreopsis sp. (aerosol/Dermic) | 47 | [29] | |
| CTX | Canary Islands | 2004 | Seriola rivoliana (ingestion) | 5 | [140] |
| Madeira Island | 2008 | Seriola sp. (ingestion) | 11 | [39] | |
| CI | - | - | - | - | - |
2. Analytical Methods
3. Monitoring and Legislation Challenges
| Assay | CTX | PTX | CI | TTX | Refs |
|---|---|---|---|---|---|
| MBA | LODP-CTX-1 = 0.2 μg/kg SM LODC-CTX-1 = 3.0 μg/kg | LD50 = 150–720 ng/µL | LOD13–desMeC = 5.6 μg/kg SM LODGYM A = 77 μg/kg SM. i.p. LD50PnTx E, F and G = 12.7–57 μg/kg SM | LODTTX = 0.2 µg | [114,129,142,143,144,155,156,157,158] |
| Citotoxicity assay | |||||
| Haemolysis assays | LOD = 50 µg/mL | LOD = 1.6 ng/kg SM 0.005 pg/µL–1 pg/mL | - | LOD = 5.0 µg/mL | [73,74,146,159,160] |
| Fluorimetric method | LOQC-CTX-1 = 0.039 ng/g | - | - | - | - |
| Receptor-binding assays | LOQP-CTX-3Ceq = 15.5 fg/cul for algal samples 0.155 ng/g in fish samples | - | - | - | [161] |
| RBA with Neuroblastoma | LOQC-CTX-1 = 0.039 ng/g | - | - | - | [162] |
| Fluorescence polarization | |||||
| Microsphere flow cytometry | - | - | LOQGYMA = 50–80 μg/kg LOQ13–desMeC = 50–85 μg/kg SM LOQ13,19–didesMeC = 40 μg/kg SM LOD13–desMeC = 10 μg/kg SM | - | [163,164,165,166,167,168] |
| Chemiluminescence method | - | - | LODSpirolides = 50 μg/kg SM | - | - |
| Assays with MCF-7 cells | - | LOD = 0.5 ng/mL | - | - | [169] |
| Assays with neuroblastoma cells | - | LOD = 5 ng/mL | - | LOD = 3.2–160 ng/mL | [145,170,171] |
| Immunoassays | |||||
| Immunobead assay (MIA) | LODP-CTX-1 = 32 ng/kg fish flesh | - | - | - | [147,172,173] |
| CIEIA | - | - | - | LOD = 10 ng/mL | [149] |
| ELISA | LOD = 0.28 ng/mL | LOD = 0.5 pg/mL | - | LOD = 5–50 ng/mL | [148,150,160,174,175] |
| Surface plasmon resonance (SPR) | - | - | - | 100 µg/kg | [176] |
| Chemical methods | |||||
| HPLC-FLD/LC-FLD | LOD = 0.5–1.0 ng | LOD = 0.75 ng | - | LOD = 0.07 pmol–0.4 pmol | [73,177,178,179,180,181,182] |
| HPLC/MS | LODP-CTX-1 = 4 ng/g | - | - | LOD = 2 ng/mL | [183,184] |
| HPLC-UV/LC-UV | - | LOD = 0.1–2 μg | LODGYM = 5 ng/mL | LOD = 10 ng/mL | [185,186] |
| Toxin group | Reference material | ARfD μg/kg bw | LD50 mice μg/kg bw | Legal limits in EU | Antidote | Refs |
|---|---|---|---|---|---|---|
| PSP | Yes (NRCC/Cifga) | 0.5 STX eq. | 10 | 0.8 µg SXT eq/g SM | N.A. | [191] |
| OA | Yes (NRCC/Cifga) | 0.3 | 192 | 0.16 µg OA eq/g SM | N.A. | [192,193] |
| TTX | Lacking analogues (Cifga) | 2 * | 9 | 2 μg of TTX eq/g SM * | N.A. | [194,195] |
| PTX | No certified material available | 0.2 | 0.15–0.72 | 30 µg PLT eq/kg SM ** | N.A. | [151,191] |
| CTX | No certified material available | N.A. | 0.25 | 0.01 μg P-CTX-1 eq/kg fish *** | N.A. | [80,129,156] |
| CI | Lacking analogues (NRCC/Cifga) | N.A. | 5–8 | 400 μg CI/kg SM **** | N.A. | [152,196] |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smayda, T.J. What is a bloom? A commentary. Limnol. Oceanogr. 1997, 42, 1132–1136. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. Algal blooms are not a simple toxic broth. Search 1993, 24, 179. [Google Scholar]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful Algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Glibert, P.; Seitzinger, S.; Heil, C.A.; Burkholder, J.M.; Parrow, M.W.; Codispoti, L.A.; Kelly, V. The role of eutrophication in coastal proliferation of harmful algal blooms: New perspectives and new approaches. Oceanography 2005, 18, 198–209. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Heisler, J.P.; Gilbert, J.; Burkholder, J.; Anderson, D.; Cochlan, W.; Dennison, W.; Dortch, Q.; Gobler, C.J.; Heil, C.; Humphries, E.; et al. Eutrophication and harmful algal blooms: Scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Burkholder, J.M.; Glibert, P.M.; Skelton, H.M. Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae 2008, 8, 77–93. [Google Scholar] [CrossRef]
- Thomas, A.; Carr, M.; Strub, P. Chlorophyll variability in eastern boundary currents. Geophys. Res. Lett. 2001, 28, 3421–3424. [Google Scholar] [CrossRef]
- Benemann, J.R. Microalgae aquaculture feeds. J. Appl. Phycol. 1992, 4, 233–245. [Google Scholar] [CrossRef]
- Peperzak, L. Future increase in harmful algal blooms in the North Sea due to climate change. Water Sci. Technol. 2005, 51, 31–36. [Google Scholar] [PubMed]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health 2008, 7, S4. [Google Scholar] [CrossRef] [PubMed]
- Touzet, N.; Farrell, H.; Ní Rathaille, A.; Rodriguez, P.; Alfonso, A.; Botana, L.M.; Raine, R. Dynamics of co-occurring Alexandrium minutum (Global Clade) and A. tamarense (West European) (Dinophyceae) during a summer bloom in Cork Harbour, Ireland (2006). Deep Sea Res. II 2010, 57, 268–278. [Google Scholar] [CrossRef]
- Schmidt, L.; Hansen, P. Allelopathy in the prymnesiophyte Chrysochromulina polylepis: Effect of cell concentration, growth phase and pH. Mar. Ecol. Prog. Ser. 2001, 216, 67–81. [Google Scholar] [CrossRef]
- Cembella, A. Chemical ecology of eukaryotic microalgae in marine ecosystems. Phycologia 2003, 42, 420–447. [Google Scholar] [CrossRef]
- Anderson, D.; Cembella, A.; Hallegraeff, G.M. Physiology and bloom dynamics of toxic Alexandrium species, with emphasis on life cycle transitions. In Physiological Ecology of Harmful Algal Blooms; Anderson, D., Cembella, A., Hallegraeff, G.M., Eds.; Springer-Verlag: Berlin, Germany, 1998; pp. 29–48. [Google Scholar]
- Calbet, A.; Vaque, D.; Felipe, J.; Vila, M.; Sala, M.; Alcaraz, M.; Estrada, M. Relative grazing impact of microzooplankton and mesozooplankton on a bloom of the toxic dinoflagellate Alexandrium minutum. Mar. Ecol. Prog. Ser. 2003, 259, 303–309. [Google Scholar] [CrossRef]
- Correia, F.S. Um caso raro de intoxicação alimentar colectiva. Bol. Inst. Super. Hig. Doutor Ricardo Jorge 1946, 3, 216–221. [Google Scholar]
- Rodriguez, P.; Alfonso, A.; Vale, C.; Alfonso, C.; Vale, P.; Tellez, A.; Botana, L.M. First Toxicity Report of Tetrodotoxin and 5,6,11-trideoxyTTX in the Trumpet Shell Charonia lampas lampas in Europe. Anal. Chem. 2008, 80, 5622–5629. [Google Scholar] [CrossRef] [PubMed]
- Moestrup, Ø. Haptophyt Algae; Clarendon Press: Oxford, UK, 1994; Volume 51. [Google Scholar]
- Davidson, K.; Miller, P.; Wilding, T.A.; Shutler, J.; Bresnan, E.; Kennington, K.; Swan, S. A large and prolonged bloom of Karenia mikimotoi in Scottish waters in 2006. Harmful Algae 2009, 8, 349–361. [Google Scholar] [CrossRef]
- Yang, C.Z.; Albright, L.J. Effects of the harmful diatom Chaetoceros concavicornis on respiration of rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 1992, 14, 105–114. [Google Scholar] [CrossRef]
- Conley, D.J.; Björck, S.; Bonsdorff, E.; Carstensen, J.; Destouni, G.; Gustafsson, B.G.; Hietanen, S.; Kortekaas, M.; Kuosa, H.; Meier, H.E.M.; et al. Critical review: Hypoxia-related processes in the Baltic Sea. Environ. Sci. Technol. 2009, 43, 3412–3420. [Google Scholar] [CrossRef] [PubMed]
- National Oceanic and Atmospheric Administration, NOAA. Dead Zones: Hypoxia in the Gulf of Mexico. Available online: http://www.noaanews.noaa.gov/stories2009/pdfs/new%20fact%20sheet%20dead%20zones_final.pdf (accessed on 31 March 2014).
- Morgan, K.L.; Larkin, S.L.; Adams, C.M. Firm-level economic effects of HABS: A tool for business loss assessment. Harmful Algae 2009, 8, 212–218. [Google Scholar] [CrossRef]
- Garcés, E.; Masó, M.; Camp, M. A recurrent and localized dinoflagellate bloom in a Mediterranean beach. J. Plankton Res. 1999, 21, 2373–2391. [Google Scholar] [CrossRef]
- Degobbis, D.; Umani, S.F.; Franco, P.; Malej, A.; Precali, R.; Smodlaka, N. Changes in the northern Adriatic ecosystem and hypertrophic appearance of gelatinous aggregates. Sci. Total Environ. 1995, 165, 43–48. [Google Scholar] [CrossRef]
- Hoagland, P.; Scatasta, S. Ecology on harmful algae. In The Economic Effect of Harmful Algal Blooms; Graneli, E., Turner, T., Eds.; Springer: Berlin, Germany, 2006; Volume 189, pp. 391–402. [Google Scholar]
- Tichadou, L.; Glaizal, M.; Armengaud, A.; Grossel, H.; Lemée, R.; Kantin, R.; Lasalle, J.-L.; Drouet, G.; Rambaud, L.; Malfait, P.; et al. Health impact of unicellular algae of the Ostreopsis genus blooms in the Mediterranean Sea: Experience of the French Mediterranean coast surveillance network from 2006 to 2009. Clin. Toxicol. 2010, 48, 839–844. [Google Scholar] [CrossRef]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A. A multi-nutrient model for the description of stoichiometric modulation of predation in micro- and mesozooplankton. J. Plankton Res. 2006, 28, 597–611. [Google Scholar] [CrossRef]
- Jones, R.H.; Flynn, K.J. Nutritional status and diet composition affect the value of diatoms as copepod prey. Science 2005, 307, 1457–1459. [Google Scholar] [CrossRef] [PubMed]
- Geraci, J.R.; Anderson, D.M.; Timperi, R.J.; Aubin, D.J.S.; Early, G.A.; Prescott, J.H.; Mayo, C.A. Humpback whales (Megaptera novaeangliae) fatally poisoned by dinoflagellate toxin. Can. J. Fish. Aquat. Sci. 1989, 46, 1895–1898. [Google Scholar] [CrossRef]
- Scholin, C.A.; Gulland, F.; Doucette, G.J.; Benson, S.; Busman, M.; Chavez, F.P.; Cordaro, J.; DeLong, R.; Vogetaere, A.D.; Harvey, J.; et al. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature 2000, 403, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.; Yentsch, C.M. Red tide and paralytic shellfish poisoning. Oceanus 1978, 2, 41–49. [Google Scholar]
- Vale, P.; Sampayo, M.A.M. Domoic acid in Portuguese shellfish and fish. Toxicon 2001, 39, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Vale, P.; Sampayo, M.A.D.M. First confirmation of human diarrhoeic poisonings by okadaic acid esters after ingestion of razor clams (Solen marginatus) and green crabs (Carcinus maenas) in Aveiro lagoon, Portugal and detection of okadaic acid esters in phytoplankton. Toxicon 2002, 40, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Sabrah, M.M.; El-Ganainy, A.A.; Zaky, M.A. Biology and toxicity of the pufferfish Lagocephalus sceleratus (Gmelin, 1789) from the Gulf of Suez. Egypt. J. Aquat. Res. 2006, 32, 283–287. [Google Scholar]
- Otero, P.; Pérez, S.; Alfonso, A.; Vale, C.; Rodríguez, P.; Gouveia, N.N.; Gouveia, N.; Delgado, J.; Vale, P.; Hirama, M.; et al. First toxin profile of ciguateric fish in Madeira Archipelago (Europe). Anal. Chem. 2010, 82, 6032–6039. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Barreiro, A.; Rodriguez, P.; Otero, P.; Azevedo, J.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. New invertebrate vectors for PST, spirolides and okadaic acid in the North Atlantic. Mar. Drugs 2013, 11, 1936–1960. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Azevedo, J.; Rodriguez, P.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. New gastropod vectors and tetrodotoxin potential expansion in temperate waters of the Atlantic Ocean. Mar. Drugs 2012, 10, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.P.; Wethey, D.S. Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nat. Commun. 2012, 3, 704. [Google Scholar] [CrossRef] [PubMed]
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Burkholder, J.M. Implications of harmful microalgae and heterotrophic dinoflagellates in management of sustainable marine fisheries. Ecol. Appl. 1998, 8, 37–62. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Forino, M.; Magno, G.S.; Tartaglione, L.; Grillo, C.; Melchiorre, N. The Genoa 2005 outbreak. Determination of putative palytoxin in Mediterranean Ostreopsis ovata by a new liquid chromatography tandem mass spectrometry method. Anal. Chem. 2006, 78, 6153–6159. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Grillo, C.; Melchiorre, N. Putative palytoxin and its new analogue, ovatoxin-a, in Ostreopsis ovata collected along the Ligurian coasts during the 2006 toxic outbreak. J. Am. Soc. Mass Spectrom. 2008, 19, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Fleming, L.E.; Katz, D.; Bean, J.A.; Hammond, R. Epidemiology of seafood poisoning. In Foodborn Disease Handbook: Seafood and Environmental Toxins; Hui, Y.H., Kitts, D., Stanfield, S., Eds.; Marcel Dekker: New York, NY, USA, 2001; Volume 4, pp. 288–306. [Google Scholar]
- Casabianca, S.; Casabianca, A.; Riobo, P.; Franco, J.M.; Vila, M.; Penna, A. Quantification of the toxic dinoflagellate Ostreopsis spp. by qPCR assay in marine aerosol. Environ. Sci. Technol. 2013, 47, 3788–3795. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, A. Study on chemical purification of tetrodotoxin—Purification of spheroidine. J. Chem. Soc. Jpn. 1950, 71, 590–592. [Google Scholar]
- Tahara, Y.; Hirata, Y. Studies on the puffer fish toxin. J. Pharm. Soc. Jpn. 1909, 29, 587–625. [Google Scholar]
- Goto, T.; Kishi, Y.; Takahashi, S.; Hirata, Y. Tetrodotoxin. Tetrahedron 1965, 21, 2059–2088. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Ikuma, S.; Kawamura, M.; Tachikawa, R.; Sakai, K. Tetrodotoxin. VII. On the structure of tetrodotoxin and its derivatives. Chem. Pharm. Bull. 1964, 12, 1356–1374. [Google Scholar]
- Woodward, R.B. The structure of tetrodotoxin. Pure Appl. Chem. 1964, 9, 49–74. [Google Scholar] [CrossRef]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Arakawa, O. Tetrodotoxin—Distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs 2008, 6, 220–242. [Google Scholar] [CrossRef] [PubMed]
- Chau, R.; Kalaitzis, J.A.; Neilan, B.A. On the origins and biosynthesis of tetrodotoxin. Aquat. Toxicol. 2011, 104, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Pratheepa, V.; Vasconcelos, V. Microbial diversity associated with tetrodotoxin production in marine organisms. Environ. Toxicol. Pharm. 2013, 36, 1046–1054. [Google Scholar] [CrossRef]
- Noguchi, T.; Narita, H.; Maruyama, J.; Hashimoto, K. Tetrodotoxin in the starfish Astropecten polyacanthus, in association with toxification of a trumpet shell, “Boshubora”, Charonia sauliae. Bull. Jpn. Soc. Sci. Fish. 1982, 48, 1173–1177. [Google Scholar] [CrossRef]
- Noguchi, T.; Ebesu, J.S.M. Puffer poisoning: Epidemiology and treatment. J. Toxicol. Toxin Rev. 2001, 20, 1–10. [Google Scholar] [CrossRef]
- Hommel, D.; Hulin, A.; Saignavong, S.; Desbordes, J.M. Intoxication par poisson-coffre (tetradotoxine). A propos d'une intoxication familiale. Méd. DAfrique Noire 1992, 39, 146–148. [Google Scholar]
- Ravaonindrina, N.; Andriamaso, T.H.; Rasolofonirina, N. Puffer fish poisoning in Madagascar: Four case reports. Arch. Inst. Pasteur Madag. 2001, 67, 61–64. [Google Scholar]
- Moore, R.E.; Scheuer, P.J. Palytoxin—New marine toxin from a coelenterate. Science 1971, 172, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.E.; Bartolini, G. Structure of palytoxin. J. Am. Chem. Soc. 1981, 103, 2491–2494. [Google Scholar] [CrossRef]
- Deeds, J.R.; Schwartz, M.D. Human risk associated with palytoxin exposure. Toxicon 2010, 56, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Florio, C.; Lorenzon, P.; de Bortpo, M.; et al. 1851–1859, Stereostructure and biological activity of 42-hydroxy-palytoxin: A new palytoxin analogue from Hawaiian Palythoa subspecies. Chem. Res. Toxicol. 2009, 22, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Ramos, V.; Vasconcelos, V. Palytoxin and analogs: Biological and ecological effects. Mar. Drugs 2010, 8, 2021–2037. [Google Scholar] [CrossRef] [PubMed]
- Ukena, T.; Satake, M.; Usami, M.; Oshima, Y.; Naoki, H.; Fujita, T.; Kan, Y.; Yasumoto, T. Structure elucidation of ostreocin D, a palytoxin analog isolated from the dinoflagellate Ostreopsis siamensis. Biosci. Biotechnol. Biochem. 2001, 65, 2585–2588. [Google Scholar] [CrossRef] [PubMed]
- Uemura, D.; Ueda, K.; Hirata, Y.; Naoki, H.; Iwashita, T. Further-studies on palytoxin. II. Structure of palytoxin. Tetrahedron Lett. 1981, 22, 2781–2784. [Google Scholar] [CrossRef]
- Tan, C.H.; Lau, C.O. Seafood and freshwater toxins. In Pharmacology, Physiology and Detection; Botana, L.M., Ed.; Marcel Dekker: New York, NY, USA, 2000; p. 533. [Google Scholar]
- García-Altares, M.; Tartaglione, L.; Dell’Aversano, C.; Carnicer, O.; De la Iglesia, P.; Forino, M.; Diogène, J.; Ciminiello, P. The novel ovatoxin-g and isobaric palytoxin (so far referred to as putative palytoxin) from Ostreopsis cf. ovata (NW Mediterranean Sea): Structural insights by LC-high resolution MSn. Anal. Bioanal. Chem. 2015, 407, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, S.; Ten-Hage, L.; Turquet, J.; Quod, J.P.; Bernard, C.; Hennion, M.C. First evidence of palytoxin analogs from an Ostreopsis mascarenensis (Dinophyceae) benthic bloom in Southwestern Indian Ocean. J. Phycol. 2004, 40, 1042–1051. [Google Scholar] [CrossRef]
- Mercado, J.A.; Viera, M.; Escallona de Mota, G.; Tosteson, T.R.; González, I.; Silva, W. An extraction procedure modification changes the toxicity, chromatographic profile and pharmacologic action of Ostreopsis lenticularis extracts. Toxicon 1994, 32, 256. [Google Scholar] [CrossRef]
- Riobó, P.; Paz, B.; Franco, J.M. Analysis of palytoxin-like in Ostreopsis cultures by liquid chromatography with precolumn derivatization and fluorescence detection. Anal. Chim. Acta 2006, 566, 217–223. [Google Scholar] [CrossRef]
- Onuma, Y.; Satake, M.; Ukena, T.; Roux, J.; Chanteau, S.; Rasolofonirina, N.; Ratsimaloto, M.; Naoki, H.; Yasumoto, T. Identification of putative palytoxin as the cause of clupeotoxism. Toxicon 1999, 37, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Gleibs, S.; Mebs, D. Distribution and sequestration of palytoxin in coral reef animals. Toxicon 1999, 37, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.D.U.; Ohizumi, Y. Chemistry and pharmacology of palytoxin. In Handbook of Natural Toxins: Marine Toxins and Venoms; Tu, A.T., Ed.; Marcel Drecker: New York, NY, USA, 1988; pp. 241–258. [Google Scholar]
- Wiles, J.S.; Vick, J.A.; Christensen, M.K. Toxicological evaluation of palytoxin in several animal species. Toxicon 1974, 12, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Taniyama, S.; Arakawa, O.; Terada, M.; Nishio, S.; Takatani, T.; Mahmud, Y.; Noguchi, T. Ostreopsis sp., a possible origin of palytoxin (PTX) in parrotfish Scarus ovifrons. Toxicon 2003, 42, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, T.; Nakajima, I.; Bagnis, R.; Adachi, R. Finding of a dinoflagellate as a likely culprit of ciguatera. Bull. Jpn. Soc. Sci. Fish. 1977, 43, 1021–1026. [Google Scholar] [CrossRef]
- Lewis, R.J.; Sellin, M.; Poli, M.A.; Norton, R.S.; MacLeod, J.K.; Sheil, M.M. Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, Muraenidae). Toxicon 1991, 29, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J. The changing face of ciguatera. Toxicon 2001, 39, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Legrand, A.M.; Ishibashi, Y.; Fukui, M.; Yasumoto, T. Structures and configurations of ciguatoxin from the moray eel Gymnothorax javanicus and its likely precursor from the dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1990, 112, 4380–4386. [Google Scholar] [CrossRef]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Fukui, M.; Legrand, A.M.; Cruchet, P.; Yasumoto, T. Isolation and structures of new ciguatoxin analogs, 2,3-DihydroxyCTX3C and 51-HydroxyCTX3C, accumulated in tropical reef fish. Tetrahedron Lett. 1998, 39, 1197–1198. [Google Scholar] [CrossRef]
- Satake, M.; Murata, M.; Yasumoto, T. Gambierol: A new toxic polyether compound isolated from the marine dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1993, 115, 361–362. [Google Scholar] [CrossRef]
- Satake, M.; Murata, M.; Yasumoto, T. The structure of CTX3C, a ciguatoxin congener isolated from cultured Gambierdsicus toxicus. Tetrahedron Lett. 1993, 34, 1975–1978. [Google Scholar] [CrossRef]
- Pottier, I.; Vernoux, J.P.; Jones, A.; Lewis, R.J. Characterization of multiple Caribbean ciguatoxins and congeners in individual specimens of horse-eye jack (Caranx latus) by high performance liquid chromatography/mass spectrometry. Toxicon 2002, 40, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Vernoux, J.P.; Brereton, I.M. Structure of Caribbean ciguatoxin isolated from Caranx latus. J. Am. Chem. Soc. 1998, 120, 5914–5920. [Google Scholar] [CrossRef]
- Hamilton, B.; Hurbungs, M.; Jones, A.; Lewis, R.J. Multiple ciguatoxins present in Indian Ocean reef fish. Toxicon 2002, 40, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.; King, G. Ciguatera (fish poisoning); University of New South Wales: Sydney, Australia, 1996. [Google Scholar]
- Pottier, I.; Vernoux, J.P.; Jones, A.; Lewis, R.J. Analysis of toxin profiles in three different fish species causing ciguatera fish poisoning in Guadalupe, French West Indies. Food Addit. Contam. 2002, 19, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Z.; Deckey, R.; Jiao, G.L.; Ren, H.F.; Li, M. Caribbean maitotoxin elevates [Ca2+]i and activates non-selective cation channels in HIT-T15 cells. World J. Diabetes 2013, 4, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Krock, B.; Tillmann, U.; John, U.; Cembella, A. LC/MS-MS aboard ship: Tandem mass spectrometry in the search for phycotoxins and novel toxigenic plankton from the North Sea. Anal. Bioanal. Chem. 2008, 392, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Molgó, J.; Girard, E.; Benoit, E. Cyclic imines: An insight into this emerging group of bioactive marine toxins. In Phycotoxins: Chemistry and Biochemistry; Botana, L.M., Ed.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 319–335. [Google Scholar]
- EFSA Report on Data Collection: Future Directions; EFSA Journal: Parma, Italy, 2010; p. 1533.
- Otero, A.; Chapela, M.J.; Atassanova, M.; Vieites, J.M.; Cabado, A.G. Cyclic imines: Chemistry and mechanism of action: A review. Chem. Res. Toxicol. 2011, 24, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Curtis, J.M.; Oshima, Y.; Quilliam, M.A.; Walter, J.A.; Watson-Wright, W.M.; Wright, J.L.C. Spirolides B and D, two novel macrocycles isolated from the digestive glands of shellfish. J. Chem. Soc. Chem. Commun. 1995, 20, 2159–2161. [Google Scholar] [CrossRef]
- Cembella, A.D.; Lewis, N.I.; Quilliam, M.A. Spirolide composition of micro-extracted pooled cells isolated from natural plankton assemblages and from cultures of the dinoflagellate Alexandrium ostenfeldii. Nat. Toxins 1999, 7, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Arsenaulf, E.; Cembella, A.; Quilliam, M. Investigations into the toxicology and pharmacology of spirolides, a novel group of shellfish toxins, in harmful algal blooms. In Proceedings of the Ninth International Conference on Harmful Algal Blooms, Hobbart, Australia, 7–11 February 2000; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J., Lewis, R.J., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2001; pp. 383–390. [Google Scholar]
- Hu, T.; Curtis, J.M.; Walter, J.A.; Wright, J.L.C. Characterization of biologically inactive spirolides E and F: Identification of the spirolide pharmacophore. Tetrahedron Lett. 1996, 37, 7671–7674. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Boschetti, L.; Rubini, S.; Cangini, M.; Pigozzi, S.; Poletti, R. Complex toxin profile of Mytilus galloprovincialis from the Adriatic sea revealed by LC-MS. Toxicon 2010, 55, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Burton, I.W.; Cembella, A.D.; Curtis, J.M.; Quilliam, M.A.; Walter, J.A.; Wright, J.L. Characterization of spirolides A, C, and 13-desmethyl C, new marine toxins isolated from toxic plankton and contaminated shellfish. J. Nat. Prod. 2001, 64, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Satake, M.; Mackenzie, A.L.; Kaspar, H.F.; Yasumoto, T. Gimnodimine, a new marine toxin of unprecedented structure isolated from New Zealand oysters and the dinoflagellate, Gymnodinium sp. Tetrahedron Lett. 1995, 36, 7093–7096. [Google Scholar] [CrossRef]
- Stewart, M.; Blunt, J.W.; Munro, M.H.; Robinson, W.T.; Hannah, D.J. The absolute stereochemistry of the New Zealand shellfish toxin gymnodimine. Tetrahedron Lett. 1997, 38, 4889–4890. [Google Scholar] [CrossRef]
- Bire, R.; Krys, S.; Fremy, J.M.; Dragacci, S.; Stirling, D.; Kharrat, R. First evidence on occurrence of gymnodimine in clams from Tunisia. J. Nat. Toxins 2002, 11, 269–275. [Google Scholar] [PubMed]
- Miles, C.O.; Wilkins, A.L.; Stirling, D.J.; MacKenzie, A.L. New analogue of gymnodimine from a Gymnodinium species. J. Agric. Food Chem. 2000, 48, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Miles, C.O.; Wilkins, A.L.; Stirling, D.J.; MacKenzie, A.L. Gymnodimine C, an isomer of gymnodimine B, from Karenia selliformis. J. Agric. Food Chem. 2003, 51, 4838–4840. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.Z.; Huang, F.L.; Chen, S.C.; Tan, X.F.; Zuo, J.B.; Peng, J.; Xie, R.W. The Isolation and Bioactivities of Pinnatoxin. Chin. J. Mar. Drugs 1990, 9, 33–35. [Google Scholar]
- Chou, T. Relative stereochemistry of pinnatoxin A, a potent shellfish poison from Pinna muricata. Tetrahedron Lett. 1996, 37, 4023–4026. [Google Scholar] [CrossRef]
- Takada, N.; Umemura, N.; Suenaga, K.; Chou, T.; Nagatsu, A.; Haino, T.; Yamada, K.; Uemura, D. Pinnatoxins B and C, the most toxic components in the pinnatoxin series from the Okinawan bivalve Pinna muricata. Tetrahedron Lett. 2001, 42, 3491–3494. [Google Scholar] [CrossRef]
- Rhodes, L.; Adamson, J.; Suzuki, T.; Briggs, L.; Garthwaite, I. Toxic marine epiphytic dinoflagellates, Ostreopsis siamensis and Coolia monotis (Dinophyceae), in New Zealand. N. Z. J. Mar. Freshw. Res. 2000, 34, 371–383. [Google Scholar] [CrossRef]
- Rundberget, T.; Aasen, J.A.B.; Selwood, A.I.; Miles, C.O. Pinnatoxins and spirolides in Norwegian blue mussels and seawater. Toxicon 2011, 58, 700–711. [Google Scholar] [CrossRef] [PubMed]
- McNabb, P.; Rhodes, L.; Selwood, A. Results of Analyses for Brevetoxins and Pinnatoxins in Rangaunu Harbour Oysters, 1993–2008. In Prepared for New Zealand Food Safety Authority, Northland, New Zealand, 2008; Cawthron Report No. 1453. p. 18.
- Selwood, A.; Miles, C.; Wilkins, A.; van Ginkel, R.; Munday, R.; Rise, F.; McNabb, P. Isolation, structural determination and acute toxicity of pinnatoxins E, F and G. J. Agric. Food Chem. 2010, 58, 6532–6542. [Google Scholar] [CrossRef] [PubMed]
- Nézan, E.; Chomérat, N. Vulcanodinium rugosum gen. et sp. nov. (Dinophyceae), un nouveau dinoflagellé marin de la côte méditerranéenne française. Cryptogam. Algol. 2011, 32, 3–18. [Google Scholar] [CrossRef]
- Rhodes, L.; Smith, K.; Selwood, A.; McNabb, P.; Molenaar, S.; Munday, R.; Wilkinson, C.; Hallegraeff, G. Production of pinnatoxins E, F and G by scrippsielloid dinoflagellates isolated from Franklin Harbour, South Australia. N. Z. J. Mar. Freshw. Res. 2011, 45, 703–709. [Google Scholar] [CrossRef]
- Rhodes, L.; Smith, K.; Selwood, A.; McNabb, P.; van Ginkel, R.; Holland, P.; Munday, R. Production of pinnatoxins by a peridinoid dinoflagellate isolated from Northland, New Zealand. Harmful Algae 2010, 9, 384–389. [Google Scholar] [CrossRef]
- Satta, C.T.; Anglès, S.; Lugliè, A.; Guillén, J.; Sechi, N.; Camp, J.; Garcés, E. Studies on dinoflagellate cyst assemblages in two estuarine Mediterranean bays: A useful tool for the discovery and mapping of harmful algal species. Harmful Algae 2013, 24, 65–79. [Google Scholar] [CrossRef]
- Smith, K.; Rhodes, L.; Suda, S.; Selwood, A. A dinoflagellate producer of pinnatoxin G, isolated from sub-tropical Japanese waters. Harmful Algae 2011, 10, 702–705. [Google Scholar] [CrossRef]
- Zeng, N.; Gu, H.; Smith, K.; Rhodes, L.; Selwood, A.; Yang, W. The first report of Vulcanodinium rugosum (Dinophyceae) from the South China Sea with a focus on the life cycle. N. Z. J. Mar. Freshw. Res. 2012, 46, 511–521. [Google Scholar] [CrossRef]
- Gill, S.; Murphy, M.; Clausen, J.; Richard, D.; Quilliam, M.; MacKinnon, S.; LaBlanc, P.; Mueller, R.; Pulido, O. Neural injury biomarkers of novel shellfish toxins, spirolides: A pilot study using immunochemical and transcriptional analysis. NeuroToxicology 2003, 24, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.; Loreal, H.; Toyofuku, H.; Hess, P.; Karunasagar, I.; Ababouch, L. Assessment and Management of Biotoxin Risks in Bivalve Mollusks; No. 551; FAO: Rome, Italy, 2011; p. 337. [Google Scholar]
- Cembella, A.; Krock, B. Cyclic Imine Toxins: Chemistry, Biogeography, Biosynthesis and Pharmacology; CRC Press (Taylor and Francys Group): Boca Raton, FL, USA, 2008. [Google Scholar]
- Otero, P.; Alfonso, A.; Alfonso, C.; Vieytes, M.R.; Louzao, M.C.; Botana, A.M.; Botana, L.M. New protocol to obtain spirolides from Alexandrium ostenfeldii cultures with high recovery and purity. Biomed. Chromatogr. 2010, 24, 878–886. [Google Scholar] [PubMed]
- Tubaro, A.; Durando, P.; Del Favero, G.; Ansaldi, F.; Icardi, G.; Deeds, J.R.; Sosa, S. Case definitions for human poisonings postulated to palytoxins exposure. Toxicon 2011, 57, 478–495. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; de Haro, L. Clinical marine toxicology: A European perspective for clinical toxicologists and poison centres. Toxins 2013, 5, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Aligizaki, K.; Nikolaidis, G. Morphological identification of two tropical dinoflagellates of the genera Gambierdiscus and Sinophysis in the Mediterranean Sea. J. Biol. Res. Thessalon. 2008, 9, 75–82. [Google Scholar]
- Touzet, N.; Franco, J.M.; Raine, R. Morphogenetic diversity and biotoxin composition of Alexandrium (Dinophyceae) in Irish coastal waters. Harmful Algae 2008, 7, 782–797. [Google Scholar] [CrossRef]
- Munday, R. Toxicology of cyclic imines: Gymnodimine, spirolides, pinnatoxins, pteriatoxins, prorocentrolide, spiro-prorocentrimine, and symbioimines. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection, 2nd ed.; Botana, L.M., Ed.; CRC Press (Taylor and Francys Group): Boca Raton, FL, USA, 2008; Volume 173, pp. 581–594. [Google Scholar]
- Aasen, J.A.B.; MacKinnon, S.L.; LeBlanc, P.; Walter, J.A.; Hovgaard, P.; Aune, T.; Quilliam, M.A. Detection and identification of spirolides in Norwegian shellfish and plankton. Chem. Res. Toxicol. 2005, 18, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Magno, S.; Tartaglione, L.; Cangini, M.; Pompei, M.; Guerrini, F.; Boni, L.; Pistocchi, R. Toxin profile of Alexandrium ostenfeldii (Dinophyceae) from the Northern Adriatic Sea revealed by liquid chromatography-mass spectrometry. Toxicon 2006, 47, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Amzil, Z.; Sibat, M.; Royer, F.; Masson, N.; Abadie, E. Report on the first detection of pectenotoxin-2, spirolide a and their derivatives in French shellfish. Mar. Drugs 2007, 5, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, G.; Uribe, E.; Ávalos, P.; Mariño, C.; Blanco, J. First identification of azaspiracid and spirolides in Mesodesma donacium and Mulinia edulis from Northern Chile. Toxicon 2010, 55, 638–641. [Google Scholar] [CrossRef] [PubMed]
- David, H.; Laza-Martinez, A.; Orive, E.; Silva, A.; Moita, T.; Mateus, M.; de Pablo, H. First bloom of Ostreopsis cf. ovata in the continental Portuguese coast. Harmful Algae News 2012, 45, 12–13. [Google Scholar]
- Lasram, F.B.; Mouillot, D. Increasing southern invasion enhances congruence between endemic and exotic Mediterranean fish fauna. Biol. Invasions 2009, 11, 697–711. [Google Scholar] [CrossRef]
- Bentur, Y.; Ashkar, J.; Lurie, Y.; Levy, Y.; Azzam, Z.S.; Litmanovich, M.; Golik, M.; Gurevych, B.; Golani, D.; Eisenman, A. Lessepsian migration and tetrodotoxin poisoning due to Lagocephalus sceleratus in the eastern Mediterranean. Toxicon 2008, 52, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Katikou, P.; Georgantelis, D.; Sinouris, N.; Petsi, A.; Fotaras, T. First report on toxicity assessment of the Lessepsian migrant pufferfish Lagocephalus sceleratus (Gmelin, 1789) from European waters (Aegean Sea, Greece). Toxicon 2009, 54, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Durando, P.; Ansaldi, F.; Oreste, P.; Moscatelli, P.; Marensi, L.; Grillo, C.; Gasparini, R.; Icardi, G. Ostreopsis ovata and human health: Epidemiological and clinical features of respiratory syndrome outbreaks from a two-year syndromic surveillance, 2005–2006 in north-west Italy. Euro Surveill. 2007, 12, 191–193. [Google Scholar]
- Vila, M.; Arin, L.; Battochi, C.; Bravo, I.; Fragac, S.; Penna, A.; Reñé, A.; Riobó, P.; Rodriguez, F.; Sala, M.M.; et al. Management of Ostreopsis blooms in recreational waters along the Catalan Coast (NW Mediterranean Sea): Cooperation between a research project and a monitoring program. Cryptogam. Algol. 2012, 33, 143–152. [Google Scholar] [CrossRef]
- Peréz-Arellano, J.L.; Luzardo, O.P.; Brito, A.P.; Cabrera, M.H.; Zumbado, M.; Carranza, C.; Alfonso, A.M.; Dickey, R.W.; Boada, L.D. Ciguatera fish poisoning, Canary Islands. Emerg. Infect. Dis. 2015, 11, 1981–1982. [Google Scholar] [CrossRef]
- Lewis, R.J.; Sellin, M. Recovery of ciguatoxin from fish flesh. Toxicon 1993, 31, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Munday, R.; Towers, N.R.; Mackenzie, L.; Beuzenberg, V.; Holland, P.T.; Miles, C.O. Acute toxicity of gymnodimine to mice. Toxicon 2004, 44, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Mahmud, Y. Current methodologies for detection of tetrodotoxin. J. Toxicol. Toxin Rev. 2001, 20, 35–50. [Google Scholar] [CrossRef]
- Riobó, P.; Franco, J.M. Palytoxins: Biological and chemical determination. Toxicon 2011, 57, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Gallacher, S.; Birkbeck, T.H. A tissue culture assay for direct detection of sodium channel blocking toxins in bacterial culture supernates. FEMS Microbiol. Lett. 1992, 71, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Shimojo, R.Y.; Iwaoka, W.T. A rapid hemolysis assay for the detection of sodium channel-specific marine toxins. Toxicology 2000, 154, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Manger, R.L.; Leja, L.S.; Lee, S.Y.; Hungerford, J.M.; Hokama, Y.; Dickey, R.W.; Granade, H.R.; Lewis, R.; Yasumoto, T.; Wekell, M.M. Detection of sodium-channel toxins—Directed cytotoxicity assays of purified ciguatoxins, brevetoxins, saxitoxins, and seafood extracts. J. AOAC Int. 1995, 78, 521–527. [Google Scholar] [PubMed]
- Tsumuraya, T.; Takeuchi, K.; Yamashita, S.; Fujii, I.; Hirama, M. Development of a monoclonal antibody against the left wing of ciguatoxin CTX1B: Thiol strategy and detection using a sandwich ELISA. Toxicon 2012, 60, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Stokes, A.N.; Williams, B.L.; French, S.S. An improved competitive inhibition enzymatic immunoassay method for tetrodotoxin quantification. Biol. Proced. Online 2012, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Garet, E.; Cabado, A.G.; Vieites, J.M.; González-Fernández, A. Rapid isolation of single-chain antibodies by phage display technology directed against one of the most potent marine toxins: Palytoxin. Toxicon 2010, 55, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cravedi, J.P.; Di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; Farmer, P.; et al. EFSA Panel on Contaminants in the Food Chain (CONTAM); Scientific Opinion on marine biotoxins in shellfish—Palytoxin group. EFSA J. 2009, 1393, 1–38. [Google Scholar]
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cravedi, J.P.; Di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; Farmer, P.; et al. Scientific Opinion on marine biotoxins in shellfish—Cyclic imines (spirolides, gymnodimines, pinnatoxins and pteriatoxins). EFSA J. 2010, 1628, 1–39. [Google Scholar]
- Caillaud, A.; Iglesia, P.; Darius, H.T.; Pauillac, S.; Aligizaki, K.; Fraga, S.; Chinain, M.; Diogène, J. Update on methodologies available for ciguatoxin determination: Perspectives to confront the onset of ciguatera fish poisoning in Europe. Mar. Drugs 2010, 8, 1838–1907. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Noguchi, T. Distribution and origin of tetrodotoxin. J. Toxicol. Toxin Rev. 2001, 20, 11–33. [Google Scholar] [CrossRef]
- Dickey, R.W. Ciguatera toxins: Chemistry, toxicology, and detection. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2008; pp. 479–500. [Google Scholar]
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cravedi, J.P.; Di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; Farmer, P.; et al. EFSA Panel on contaminants in the food chain; Scientific opinion on marine biotoxins in shellfish—Emerging toxins: Ciguatoxin group. EFSA J. 2010, 1627, 1–38. [Google Scholar]
- Munday, R.; Selwood, A.I.; Rhodes, L. Acute toxicity of pinnatoxins E, F and G to mice. Toxicon 2012, 60, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Hess, P.; Abadie, E.; Hervé, F.; Berteaux, T.; Séchet, V.; Aráoz, R.; Molgó, J.; Zakarian, A.; Sibat, M.; Rundberget, T.; et al. Pinnatoxin G is responsible for atypical toxicity in mussels (Mytilus galloprovincialis) and clams (Venerupis decussata) from Ingril, a French Mediterranean lagoon. Toxicon 2013, 75, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Aligizaki, K.; Katikou, P.; Nikolaidis, G.; Panou, A. First episode of shellfish contamination by palytoxin-like compounds from Ostreopsis species (Aegean Sea, Greece). Toxicon 2008, 51, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Bignami, G.S. A rapid and sensitive hemolysis neutralization assay for palytoxin. Toxicon 1993, 31, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Darius, H.T.; Ponton, D.; Revel, T.; Cruchet, P.; Ung, A.; Fouc, M.T.; Chinain, M. Ciguatera risk assessment in two toxic sites of French Polynesia using the receptor-binding assay. Toxicon 2007, 50, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Dechraoui, M.Y.B.; Tiedeken, J.A.; Persad, R.; Wang, Z.; Granade, H.R.; Dickey, R.W.; Ramsdell, J.S. Use of two detection methods to discriminate ciguatoxins from brevetoxins: Application to great barracuda from Florida Keys. Toxicon 2005, 46, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Alfonso, A.; Alfonso, C.; Araoz, R.; Molgo, J.; Vieytes, M.R.; Botana, L.M. First direct fluorescence polarization assay for the detection and quantification of spirolides in mussel samples. Anal. Chim. Acta 2011, 701, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Fonfría, E.S.; Vilariño, N.; Molgó, J.; Aráoz, R.; Otero, P.; Espiña, B.; Louzao, M.C.; Alvarez, M.; Botana, L.M. Detection of 13,19-didesmethyl C spirolide by fluorescence polarization using Torpedo electrocyte membranes. Anal. Biochem. 2010, 403, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Fonfría, E.S.; Vilariño, N.; Espiña, B.; Louzao, M.C.; Álvarez, M.; Molgó, J.; Aráoz, R.; Botana, L.M. Feasibility of gymnodimine and 13-desmethyl C spirolide detection by fluorescence polarization using a receptor-based assay in shellfish matrixes. Anal. Chim. Acta 2010, 657, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.P.; Vilariño, N.; Molgó, J.; Aráoz, R.; Louzao, M.C.; Taylor, P.; Talley, T.; Botana, L.M. Development of a solid-phase receptor-based assay for the detection of cyclic imines using a microsphere-flow cytometry system. Anal. Chem. 2013, 85, 2340–2347. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.P.; Vilariño, N.; Molgó, J.; Aráoz, R.; Botana, L.M. High-throughput receptor-based assay for the detection of spirolides by chemiluminescence. Toxicon 2013, 75, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Vilariño, N.; Fonfría, E.S.; Molgó, J.; Aráoz, R.; Botana, L.M. Detection of gymnodimine-A and 13-desmethyl C spirolide phycotoxins by fluorescence polarization. Anal. Chem. 2009, 81, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Bellocci, M.; Ronzitti, G.; Milandri, A.; Melchiorre, N.; Grillo, C.; Poletti, R.; Yasumoto, T.; Rossini, G.P. A cytolytic assay for the measurement of palytoxin based on a cultured monolayer cell line. Anal. Biochem. 2008, 374, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Ledreux, A.; Krys, S.; Bernard, C. Suitability of the Neuro-2a cell line for the detection of palytoxin and analogues (neurotoxic phycotoxins). Toxicon 2009, 53, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Espiña, B.; Cagide, E.; Louzao, M.C.; Fernandez, M.M.; Vieytes, M.R.; Katikou, P.; Villar, A.; Jaen, D.; Maman, L.; Botana, L.M. Specific and dynamic detection of palytoxins by in vitro microplate assay with human neuroblastoma cells. Biosci. Rep. 2009, 29, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Hokama, Y.; Takenaka, W.E.; Nishimura, K.L.; Ebeso, J.S.M.; Bourke, R.; Sullivan, P.K. A simple membrane immunobead assay for detecting ciguatoxin and related polyethers from human ciguatera intoxication and natural reef fishes. J. AOAC Int. 1998, 81, 727–735. [Google Scholar] [PubMed]
- Campora, C.E.; Hokama, Y.; Yabusaki, K.; Isobe, M. Development of an enzyme-linked immunosorbent assay for the detection of ciguatoxin in fish tissue using chicken immunoglobulin Y. J. Clin. Lab. Anal. 2008, 22, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Wei, W.J.; Nan, L.; Lei, L.H.; Hui, H.C.; Fen, G.X.; Jun, L.Y.; Jing, Z.; Rong, J. Development of competitive indirect ELISA for the detection of tetrodotoxin and a survey of the distribution of tetrodotoxin in the tissues of wild puffer fish in the waters of south-east China. Food Addit. Contam. Part A 2010, 27, 1589–1597. [Google Scholar] [CrossRef]
- Wang, X.; Yu, R.; Luo, X.; Zhou, M.; Lin, X. Toxin-screening and identification of bacteria isolated from highly toxic marine gastropod Nassarius semiplicatus. Toxicon 2008, 52, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.; Barnes, P.; Haughey, S.A.; Higgins, C.; Kawatsu, K.; Vasconcelos, V.; Elliott, C.T. Development and single laboratory validation of an optical biosensor assay for tetrodotoxin detection as a tool to combat emerging risks in European seafood. Anal. Bioanal. Chem. 2013, 405, 7753–7763. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.; Holmes, M.; Alewood, P.; Jones, A. Ionspray mass spectrometry of ciguatoxin-1, maitotoxin-2 and -3, and related marine polyether toxins. Nat. Toxins 1994, 2, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Dickey, R.W.; Bencsath, F.A.; Granade, H.R.; Lewis, R.J. Liquid chromatographic mass spectrometric methods for the determination of marine polyether toxins. Bull. Exot. Pathol. Society 1992, 85, 514–515. [Google Scholar]
- Shoji, Y.; Yotsu-Yamashita, M.; Miyazawa, T.; Yasumoto, T. Electrospray ionization mass spectrometry of tetrodotoxin and its analogs: Liquid chromatography/mass spectrometry, tandem mass spectrometry, and liquid chromatography/tandem mass spectrometry. Anal. Biochem. 2001, 290, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Jang, J.; Yotsu-Yamashita, M. Hydrophilic interaction liquid chromatography electrospray ionization mass spectrometry of tetrodotoxin and its analogs. Anal. Biochem. 2006, 352, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Yotsu-Yamashita, M. Distribution of tetrodotoxin, saxitoxin, and their analogs among tissues of the puffer fish Fugu pardalis. Toxicon 2006, 48, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Mebs, D.; Arakawa, O.; Yotsu-Yamashita, M. Tissue distribution of tetrodotoxin in the red-spotted newt Notophthalmus viridescens. Toxicon 2010, 55, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.; Jones, A. Characterization of ciguatoxins and ciguatoxin congeners present in ciguateric fish by gradient reverse-phase high-performance liquid chromatography/mass spectrometry. Toxicon 1997, 37, 159–168. [Google Scholar] [CrossRef]
- Kawatsu, K.; Hamano, Y.; Yoda, T.; Terano, Y.; Shibata, T. Rapid and highly sensitive enzyme immunoassay for quantitative determination of tetrodotoxin. Jpn. J. Med. Sci. Biol. 1997, 50, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Marrouchi, R.; Dziri, F.; Belayouni, N.; Hamza, A.; Benoit, E.; Molgó, J.; Kharrat, R. Quantitative Determination of Gymnodimine-A by High Performance Liquid Chromatography in Contaminated Clams from Tunisia Coastline. Mar. Biotechnol. 2010, 12, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Jen, H.C.; Lin, S.J.; Lin, S.Y.; Huang, Y.W.; Liao, I.C.; Arakawa, O.; Hwang, D.F. Occurrence of tetrodotoxin and paralytic shellfish poisons in a gastropod implicated in food poisoning in southern Taiwan. Food Addit. Contam. 2007, 8, 902–909. [Google Scholar] [CrossRef]
- Caillaud, A.; Iglesia, P.; Barber, E.; Eixarch, H.; Mohammad-Noor, N.; Yasumoto, T.; Diogène, J. Monitoring of dissolved ciguatoxin and maitotoxin using solid-phase adsorption toxin tracking devices: Application to Gambierdiscus pacificus in culture. Harmful Algae 2011, 10, 433–446. [Google Scholar] [CrossRef]
- Rundberget, T.; Gustad, E.; Samdal, I.A.; Sandvik, M.; Miles, C.O. A convenient and cost-effective method for monitoring marine algal toxins with passive samplers. Toxicon 2009, 53, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Louzao, M.C.; Espiña, B.; Cagide, E.; Ares, I.R.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Cytotoxic effect of palytoxin on mussel. Toxicon 2010, 56, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, S.; Ilardi, E.A.; Tappin, N.D.C.; Zakarian, A. Marine toxins with spiroimine rings: Total synthesis of pinnatoxin A. Eur. J. Org. Chem. 2010, 2010, 5743–5765. [Google Scholar] [CrossRef]
- Alexander, J.; Auðunsson, G.A.; Benford, D.; Cockburn, A.; Cravedi, J.P.; Dogliotti, E.; Di Domenico, A.; Fernández-Cruz, M.L.; Fink-Gremmels, J.; Fürst, P.; et al. Scientific opinion of the panel on contaminants in the food chain on a request from the european commission on marine biotoxins in shellfish—saxitoxin group. EFSA J. 2009, 1019, 1–76. [Google Scholar]
- Alexander, J.; Auðunsson, G.A.; Benford, D.; Cockburn, A.; Cravedi, J.P.; Dogliotti, E.; Di Domenico, A.; Fernández-Cruz, M.L.; Fink-Gremmels, J.; Fürst, P.; et al. Opinion of the Scientific Panel on Contaminants in the Food chain on a request from the European Commission on marine biotoxins in shellfish—Okadaic acid and analogues. EFSA J. 2008, 589, 1–62. [Google Scholar]
- Aune, T.; Larsen, S.; Aasen, J.A.; Rehmann, N.; Satake, M.; Hess, P. Relative toxicity of dinophysistoxin-2 (DTX-2) compared with okadaic acid, based on acute intraperitoneal toxicity in mice. Toxicon 2007, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Islam, Q.T.; Razzak, M.A.; Islam, M.A.; Bari, M.I.; Basher, A.; Chowdhury, F.R.; Sayeduzzaman, A.B.M.; Ahasan, H.A.M.N.; Faiz, M.A.; Arakawa, O.; et al. Puffer fish poisoning in Bangladesh: Clinical and toxicological results from large outbreaks in 2008. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T. The manual for the methods of food sanitation tests. In Bureau of Environmental Health, Ministry of Health and Welfare; Japan Food Hygienic Association: Tokyo, Japan, 1978; Volume II, p. 232. [Google Scholar]
- CRLMB (Community Reference Laboratory for Marine Biotoxins). Report on toxicology working group meeting, Cesenatico, Italy, 24–25 October 2005. Available online: http://www.aesan.msps.es/en/CRLMB/web/home.shtml (accessed on 9 April 2014).
- Botelho, M.J.; Vale, C.; Mota, A.M.; Rodrigues, S.M.; Costa, P.R.; Simões Gonçalves, M.L.S. Matrix effect on paralytic shellfish toxins quantification and toxicity estimation in mussels exposed to Gymnodinium catenatum. Food Addit. Contam. Part A 2010, 27, 1724–1732. [Google Scholar] [CrossRef]
- Regulation (EC) No. 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying Down Specific Hygiene Rules for Food of Animal Origin OJ L 139, 30.4.2004. Available online: http://eur-lex.europa.eu (accessed on 15 April 2014).
- De Fouw, J.C.; van Egmond, H.P.; Speijers, G.J.A. Ciguatera fish poisoning: A review. Available online: http://www.rivm.nl/bibliotheek/rapporten/388802021.pdf (accessed on 10 May 2014).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.; Pratheepa, V.K.; Botana, L.M.; Vasconcelos, V. Emergent Toxins in North Atlantic Temperate Waters: A Challenge for Monitoring Programs and Legislation. Toxins 2015, 7, 859-885. https://doi.org/10.3390/toxins7030859
Silva M, Pratheepa VK, Botana LM, Vasconcelos V. Emergent Toxins in North Atlantic Temperate Waters: A Challenge for Monitoring Programs and Legislation. Toxins. 2015; 7(3):859-885. https://doi.org/10.3390/toxins7030859
Chicago/Turabian StyleSilva, Marisa, Vijaya K. Pratheepa, Luis M. Botana, and Vitor Vasconcelos. 2015. "Emergent Toxins in North Atlantic Temperate Waters: A Challenge for Monitoring Programs and Legislation" Toxins 7, no. 3: 859-885. https://doi.org/10.3390/toxins7030859
APA StyleSilva, M., Pratheepa, V. K., Botana, L. M., & Vasconcelos, V. (2015). Emergent Toxins in North Atlantic Temperate Waters: A Challenge for Monitoring Programs and Legislation. Toxins, 7(3), 859-885. https://doi.org/10.3390/toxins7030859








