Preliminary Estimation of Deoxynivalenol Excretion through a 24 h Pilot Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Performance
| Analyte | Composite diet | Beer | Urine | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| REC± RSD (%) | LOD (µg/kg) | LOQ (µg/kg) | REC ± RSD (%) | LOD (µg/kg) | LOQ (µg/kg) | REC ± RSD (%) | LOD (µg/kg) | LOQ (µg/kg) | ||||
| Low level a | High level c | Low level b | High level c | Low level a | High level c | |||||||
| DOM-1 | 87 ± 7 | 93 ± 5 | 0.6 | 1.2 | 73 ± 6 | 77 ± 8 | 0.1 | 0.2 | 84 ± 2 | 86 ± 4 | 0.2 | 0.5 |
| DON | 89 ± 5 | 91 ± 6 | 0.6 | 1.2 | 75 ± 9 | 83 ± 9 | 0.05 | 0.1 | 96 ± 4 | 94 ± 8 | 0.1 | 0.2 |
| 3-ADON | 95 ± 4 | 90 ± 7 | 0.6 | 1.2 | 82 ± 6 | 80 ± 5 | 2 | 4 | 92 ± 5 | 94 ± 5 | 0.2 | 0.5 |
| FUS-X | 84 ± 5 | 89 ± 3 | 2.5 | 5 | 98 ± 8 | 93 ± 9 | 8 | 16 | 95 ± 3 | 90 ± 6 | 2 | 4 |
| DAS | 103 ± 3 | 99 ± 6 | 2.5 | 5 | 78 ± 6 | 82 ± 5 | 4 | 8 | 89 ± 4 | 84 ± 8 | 1 | 2 |
| NIV | 79 ± 6 | 82 ± 5 | 1.2 | 2.5 | 77 ± 12 | 81 ± 9 | 0.5 | 1 | 87 ± 3 | 93 ± 6 | 0.5 | 1 |
| NEO | 97 ± 8 | 92 ± 5 | 2.5 | 5 | 83 ± 8 | 88 ± 6 | 2 | 4 | 93 ± 5 | 94 ± 5 | 0.2 | 0.5 |
| HT-2 | 93 ± 7 | 89 ± 8 | 1.2 | 2.5 | 97 ± 9 | 93 ± 4 | 2 | 4 | 96 ± 4 | 91 ± 8 | 1 | 2 |
| T-2 | 84 ± 9 | 90 ± 6 | 2.5 | 5 | 108 ± 7 | 97 ± 8 | 4 | 8 | 102 ± 6 | 94 ± 9 | 0.5 | 1 |
| ZAN | 85 ± 5 | 90 ± 4 | 5 | 10 | 68 ± 9 | 73 ± 9 | 8 | 16 | 72 ± 7 | 74 ± 8 | 4 | 8 |
| α-ZAL | 72 ± 8 | 79 ± 8 | 5 | 10 | 70 ± 6 | 78 ± 7 | 4 | 8 | 79 ± 5 | 82 ± 5 | 4 | 8 |
| β-ZAL | 79 ± 6 | 77 ± 6 | 5 | 10 | 73 ± 8 | 79 ± 8 | 4 | 8 | 77 ± 8 | 74 ± 6 | 4 | 8 |
| ZON | 87 ± 8 | 84 ± 7 | 2.5 | 5 | 71 ± 5 | 78 ± 6 | 8 | 16 | 81 ± 5 | 84 ± 7 | 3 | 6 |
| α-ZOL | 83 ± 9 | 80 ± 7 | 2.5 | 5 | 78 ± 6 | 83 ± 4 | 2 | 4 | 88 ± 2 | 93 ± 5 | 1 | 2 |
| β-ZOL | 77 ± 6 | 78± 9 | 2.5 | 5 | 74 ± 8 | 73 ± 8 | 4 | 8 | 80 ± 6 | 84 ± 9 | 2 | 4 |
2.2. Deoxynivalenol Reduction during Cooking

2.3. Deoxynivalenol Content in Food
| Time of consumption | Food | Consumption (g/day) | Mean DON ± SD (µg/kg) | Mean DON intake (µg) |
|---|---|---|---|---|
| 8 am | Toast | 45 | 190.6 ± 2.3 | 8.7 |
| 11 am | Breadsticks | 30 | 49.7 ± 4.6 | 1.5 |
| 2 pm | Pasta | 67 a | 58.2 ± 2.7 | 3.9 |
| 7 pm | Wheat beer | 500 | 36.4 ± 1.8 | 18.1 |
| 8 pm | Beer | 330 | 32.1 ± 6.2 | 10.5 |
| 10 pm | Whole-wheat pasta | 24 a | 272.4 ± 5.9 | 6.5 |
| Σ DON Intake: 49.2 µg | ||||
| Composite b | 166 | 120 ± 7.3 | 19.9 µg |
2.4. DON Content in Urine
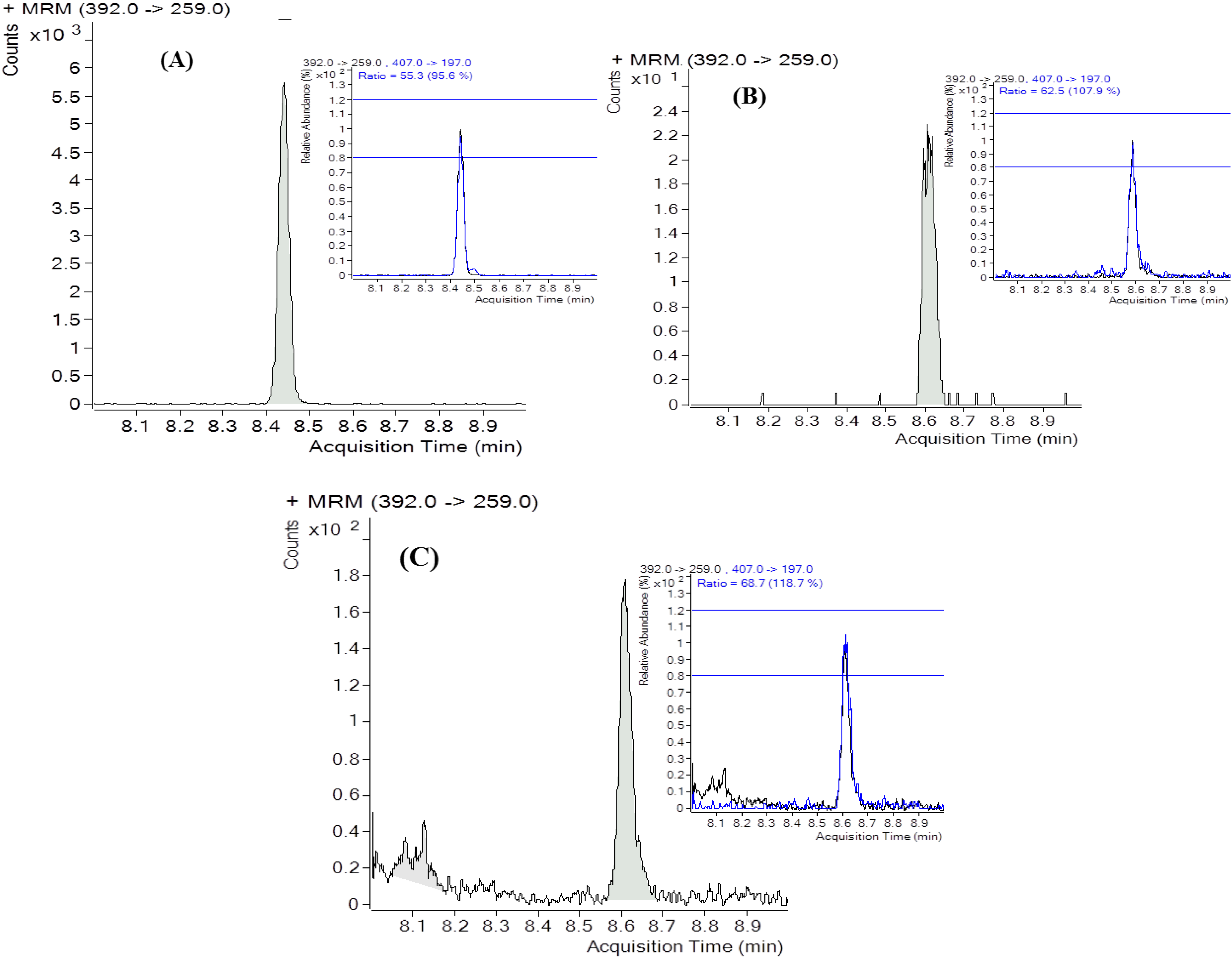
2.5. Exposure Estimates
3. Experimental Section
3.1. Materials
3.2. Standard Preparation
3.3. Study Design and Sampling
3.4. Composite Diet Sample
3.5. Sample Preparation
3.6. GC-MS/MS Method
3.7. Calculation of DON Daily Intake
3.8. Creatinine Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- FAO. Food Balance Sheet. Available online: http://faostat3.fao.org/faostat-gateway/go/to/home/E (accessed on 1 November 2014).
- Haschek, W.M.; Voss, K.A. Chapter 39—Mycotoxins. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology, 3rd ed.; Haschek, W.M., Rousseaux, C.G., Wallig, M.A., Eds.; Academic Press: Boston, MA, USA, 2013; pp. 1187–1258. [Google Scholar]
- Alassane-Kpembi, I.; Kolf-Clauw, M.; Gauthier, T.; Abrami, R.; Abiola, F.A.; Oswald, I.P.; Puel, O. New Insights into mycotoxin mixtures: The toxicity of low doses of type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol. Appl. Pharmacol. 2013, 272, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; van Immerseel, F.; Croubels, S. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure Assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Cnudde, F. Safe Foods—Promoting Food Safety through a New Integrated Risk Analysis Approach for Foods. 2005, 30, 194–195. [Google Scholar]
- RASFF. Rapid Alert System for Food and Feed. Available online: http://ec.europa.eu/food/safety/rasff/index_en.htm (accessed on 6 November 2014).
- Lindblad, M.; Gidlund, A.; Sulyok, M.; Börjesson, T.; Krska, R.; Olsen, M.; Fredlund, E. Deoxynivalenol and other selected Fusarium toxins in swedish wheat—Occurrence and correlation to specific Fusarium species. Int. J. Food Microbiol. 2013, 167, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, I.; Do, W.; Nam, W.; Li, H.; Jang, H.; Lee, C. Incidence and levels of deoxynivalenol, fumonisins and zearalenone contaminants in animal feeds used in Korea in 2012. Toxins 2014, 6, 20–32. [Google Scholar] [CrossRef]
- Visconti, A.; Haidukowski, E.M.; Pascale, M.; Silvestri, M. Reduction of deoxynivalenol during durum wheat processing and spaghetti cooking. Toxicol. Lett. 2004, 153, 181–189. [Google Scholar] [CrossRef] [PubMed]
- EC No 1881/2006. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs (Text with EEA Relevance)
- EC No 1126/2007. Commission Regulation (EC) No 1126/2007 of 28 September 2007 amending Regulation (EC) no 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products (Text with EEA Relevance)
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Safety evaluation of certain mycotoxins in food. Food and Agriculture Organization: Rome, Italy, 2001; pp. 281–320. [Google Scholar]
- WHO (World Health Organisation). Guidelines for the Study of Dietary Intakes of Chemical Contaminants. World Health Organisation: Geneva, Switzerland, 1985; p. 101. [Google Scholar]
- Sakuma, H.; Watanabe, Y.; Furusawa, H.; Yoshinari, T.; Akashi, H.; Kawakami, H.; Saito, S.; Sugita-Konishi, Y. Estimated dietary exposure to mycotoxins after taking into account the cooking of staple foods in Japan. Toxins 2013, 5, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Šarkanj, B.; Warth, B.; Uhlig, S.; Abia, W.A.; Sulyok, M.; Klapec, T.; Krska, R.; Banjari, I. Urinary analysis reveals high deoxynivalenol exposure in pregnant women from Croatia. Food Chem. Toxicol. 2013, 62, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzo, M.; Gambacorta, L.; Visconti, A. Assessment of multi-mycotoxin exposure in Southern Italy by urinary multi-biomarker determination. Toxins 2014, 6, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Mirocha, C.J.; Pathre, S.V.; Robison, T.S. Comparative metabolism of zearalenone and transmission into bovine milk. Food Cosmet. Toxicol. 1981, 19, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Sulyok, M.; Berthiller, F.; Schuhmacher, R.; Krska, R. New insights into the human metabolism of the Fusarium mycotoxins deoxynivalenol and zearalenone. Toxicol. Lett. 2013, 220, 88–94. [Google Scholar] [CrossRef]
- EC No 401/2006. Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs (text with EEA relevance)
- Turner, P.C.; Hopton, R.P.; White, K.L.; Fisher, J.; Cade, J.E.; Wild, C.P. Assessment of deoxynivalenol metabolite profiles in UK adults. Food Chem. Toxicol. 2011, 49, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.G. Fusarium mycotoxin content of UK organic and conventional wheat. Food Addit. Contam. A 2009, 26, 496–506. [Google Scholar] [CrossRef]
- González-Osnaya, L.; Corteé, C.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Occurrence of deoxynivalenol and T-2 toxin in bread and pasta commercialised in Spain. Food Chem. 2011, 124, 156–161. [Google Scholar] [CrossRef]
- Juan, C.; Ritieni, A.; Mañes, J. Occurrence of Fusarium mycotoxins in Italian cereal and cereal products from organic farming. Food Chem. 2013, 141, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrasco, Y.; Moltó, J.C.; Berrada, H.; Mañes, J. A survey of trichothecenes, zearalenone and patulin in milled grain-based products using GC-MS/MS. Food Chem. 2014, 146, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.C.; Hopton, R.P.; Lecluse, Y.; White, K.L.M.; Fisher, J.; Lebailly, P. Determinants of urinary deoxynivalenol and de-epoxy deoxynivalenol in male farmers from Normandy, France. J. Agric. Food Chem. 2010, 58, 5206–5212. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Sulyok, M.; Fruhmann, P.; Berthiller, F.; Schuhmacher, R.; Hametner, C.; Adam, G.; Fröhlich, J.; Krska, R. Assessment of human deoxynivalenol exposure using an LC-MS/MS based biomarker method. Toxicol. Lett. 2012, 211, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.C.; Taylor, E.F.; White, K.L.M.; Cade, J.E.; Wild, C.P. A comparison of 24 h urinary deoxynivalenol with recent v. average cereal consumption for UK adults. Br. J. Nutr. 2009, 102, 1276–1279. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.C.; White, K.L.M.; Burley, V.J.; Hopton, R.P.; Rajendram, A.; Fisher, J.; Cade, J.E.; Wild, C.P. A comparison of deoxynivalenol intake and urinary deoxynivalenol in UK adults. Biomarkers 2010, 15, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.W.; Richardson, A.J.; Duncan, G.; Holtrop, G. Annual variation of dietary deoxynivalenol exposure during years of different Fusarium prevalence: A pilot biomonitoring study. Food Addit. Contam. A 2014, 31, 1579–1585. [Google Scholar] [CrossRef]
- Rubert, J.; Soriano, J.M.; Mañes, J.; Soler, C. Rapid mycotoxin analysis in human urine: A pilot study. Food Chem. Toxicol. 2011, 49, 2299–2304. [Google Scholar] [CrossRef] [PubMed]
- Schoen, T.; Blum, J.; Paccaud, F.; Burnier, M.; Bochud, M.; Conen, D. Factors associated with 24-hour urinary volume: The Swiss salt survey. BMC Nephrology 2013, 14, 246. [Google Scholar] [CrossRef] [PubMed]
- Warth, B.; Petchkongkaew, A.; Sulyok, M.; Krska, R. Utilising an LC-MS/MS-based multi-biomarker approach to assess mycotoxin exposure in the Bangkok metropolitan area and surrounding provinces. Food Addit. Contam. A 2014, 31, 2040–2046. [Google Scholar] [CrossRef]
- Sirot, V.; Fremy, J.; Leblanc, J. Dietary exposure to mycotoxins and health risk assessment in the second French Total Diet Study. Food Chem. Toxicol. 2013, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schothorst, R.C.; Jekel, A.A.; Van Egmond, H.P.; de Mul, A.; Boon, P.E.; Van Klaveren, J.D. Determination of trichothecenes in duplicate diets of young children by capillary gas chromatography with mass spectrometric detection. Food Addit. Contam. 2005, 22, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Gareis, M.; Zimmermann, C.; Schothorst, R. Collection of Occurrence Data of Fusarium Toxin in Food and Assessment of Dietary Intake by the Population of EU Member States. Final Report SCOOP Task 2003, 3, 10. [Google Scholar]
- FAO/WHO. Safety Evaluation of Certain Mycotoxins in Food; World Health Organisation: Geneva, Switzerland, 2001; pp. 281–387. [Google Scholar]
- Cavret, S.; Lecoeur, S. Fusariotoxin transfer in animal. Food Chem. Toxicol. 2006, 44, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrasco, Y.; Berrada, H.; Font, G.; Mañes, J. Multi-mycotoxin analysis in wheat semolina using an acetonitrile-based extraction procedure and gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1270, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Njumbe Ediage, E.; Diana Di Mavungu, J.; Song, S.; Wu, A.; Van Peteghem, C.; De Saeger, S. A direct assessment of mycotoxin biomarkers in human urine samples by liquid chromatography tandem mass spectrometry. Anal. Chim. Acta 2012, 741, 58–69. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Carrasco, Y.; Mañes, J.; Berrada, H.; Font, G. Preliminary Estimation of Deoxynivalenol Excretion through a 24 h Pilot Study. Toxins 2015, 7, 705-718. https://doi.org/10.3390/toxins7030705
Rodríguez-Carrasco Y, Mañes J, Berrada H, Font G. Preliminary Estimation of Deoxynivalenol Excretion through a 24 h Pilot Study. Toxins. 2015; 7(3):705-718. https://doi.org/10.3390/toxins7030705
Chicago/Turabian StyleRodríguez-Carrasco, Yelko, Jordi Mañes, Houda Berrada, and Guillermina Font. 2015. "Preliminary Estimation of Deoxynivalenol Excretion through a 24 h Pilot Study" Toxins 7, no. 3: 705-718. https://doi.org/10.3390/toxins7030705
APA StyleRodríguez-Carrasco, Y., Mañes, J., Berrada, H., & Font, G. (2015). Preliminary Estimation of Deoxynivalenol Excretion through a 24 h Pilot Study. Toxins, 7(3), 705-718. https://doi.org/10.3390/toxins7030705