Multi-Toxic Endpoints of the Foodborne Mycotoxins in Nematode Caenorhabditis elegans
Abstract
:1. Introduction
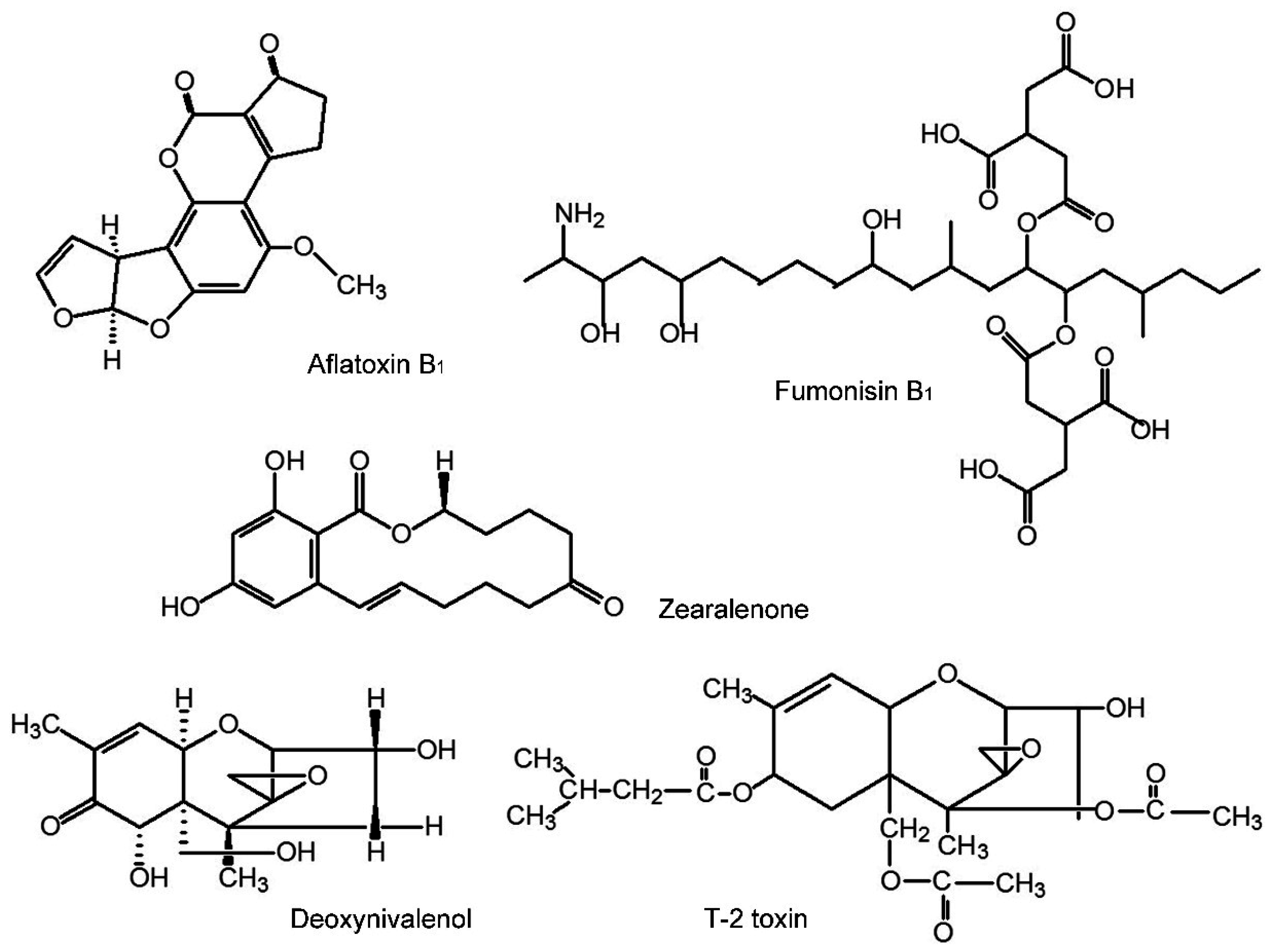
2. Results
2.1. Lethality
| Mycotoxins | AFB1 | DON | FB1 | T-2 | ZEA |
|---|---|---|---|---|---|
| LC50 mg/L | 20.47 | 656.67 | 235.62 | 1.38 | 75.79 |
| 95%CI | 12.67–45.21 | 435.96–1145.66 | 79.07–640.142 | 1.01–1.76 | 5.83–985.03 |
2.2. Toxic Effects on Growth
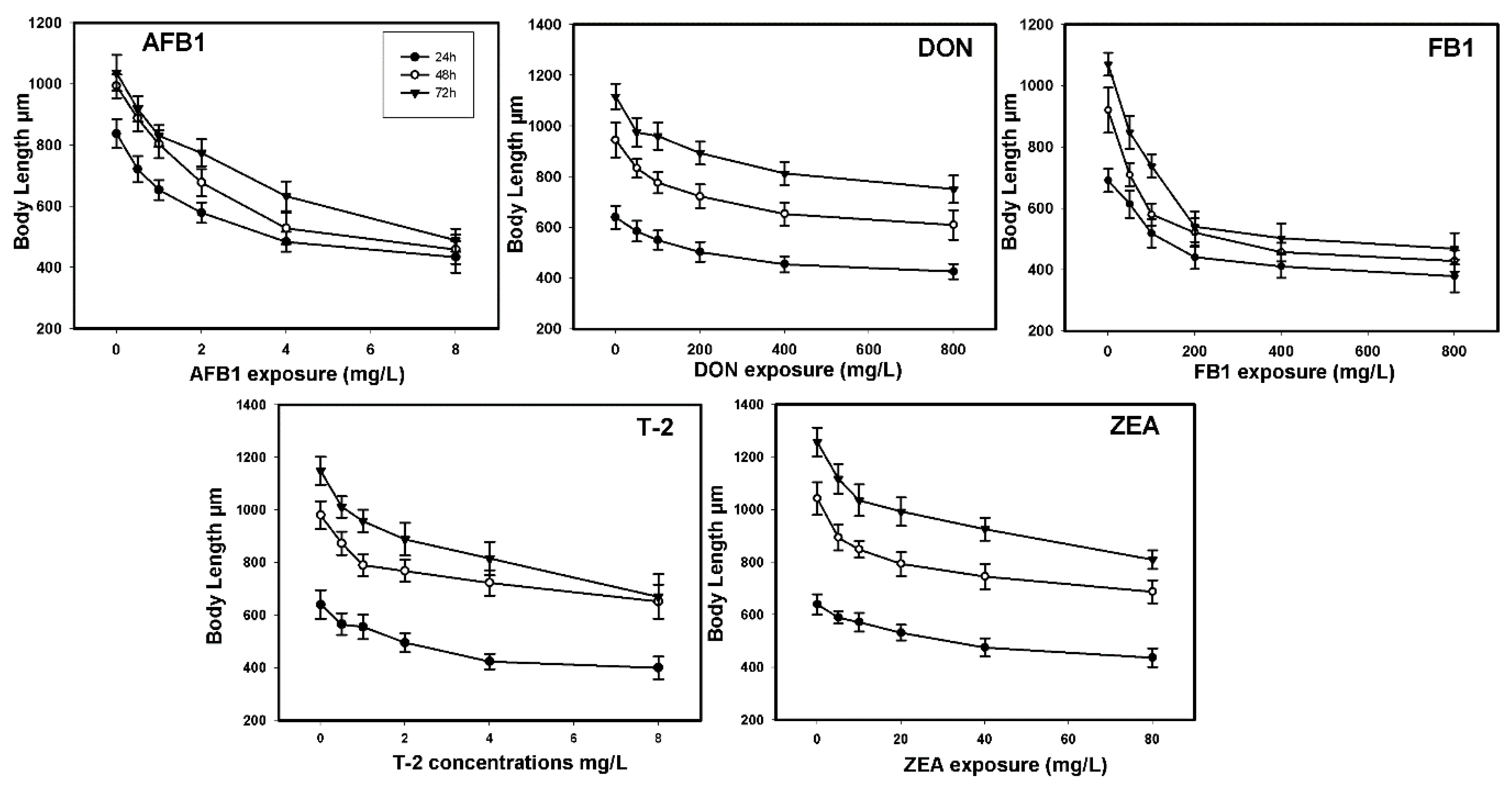
| Mycotoxins | Growth | Reproduction | ||||
|---|---|---|---|---|---|---|
| EC50 mg/L | Interval of Confidence (95%) | Ratio LC50/EC50 | EC50 mg/L | Interval of Confidence (95%) | Ratio LC50/EC50 | |
| AFB1 | 7.31 | 5.19–12.50 | 2.80 | 1.69 | 1.38–2.04 | 12.11 |
| DON | 533.07 | 412.63–686.02 | 0.49 | 487.28 | 311.15–515.90 | 0.55 |
| FB1 | 361.59 | 261.36–569.77 | 0.65 | 25.63 | 19.63–31.77 | 9.19 |
| T-2 | 16.96 | 9.13–59.81 | 0.08 | 1.82 | 1.33–2.44 | 0.76 |
| ZEA | 314.19 | 129.74–860.21 | 0.24 | 26.05 | 19.23–37.17 | 2.90 |

2.3. Toxic Effects of Reproduction

2.4. Influence on Lifespan
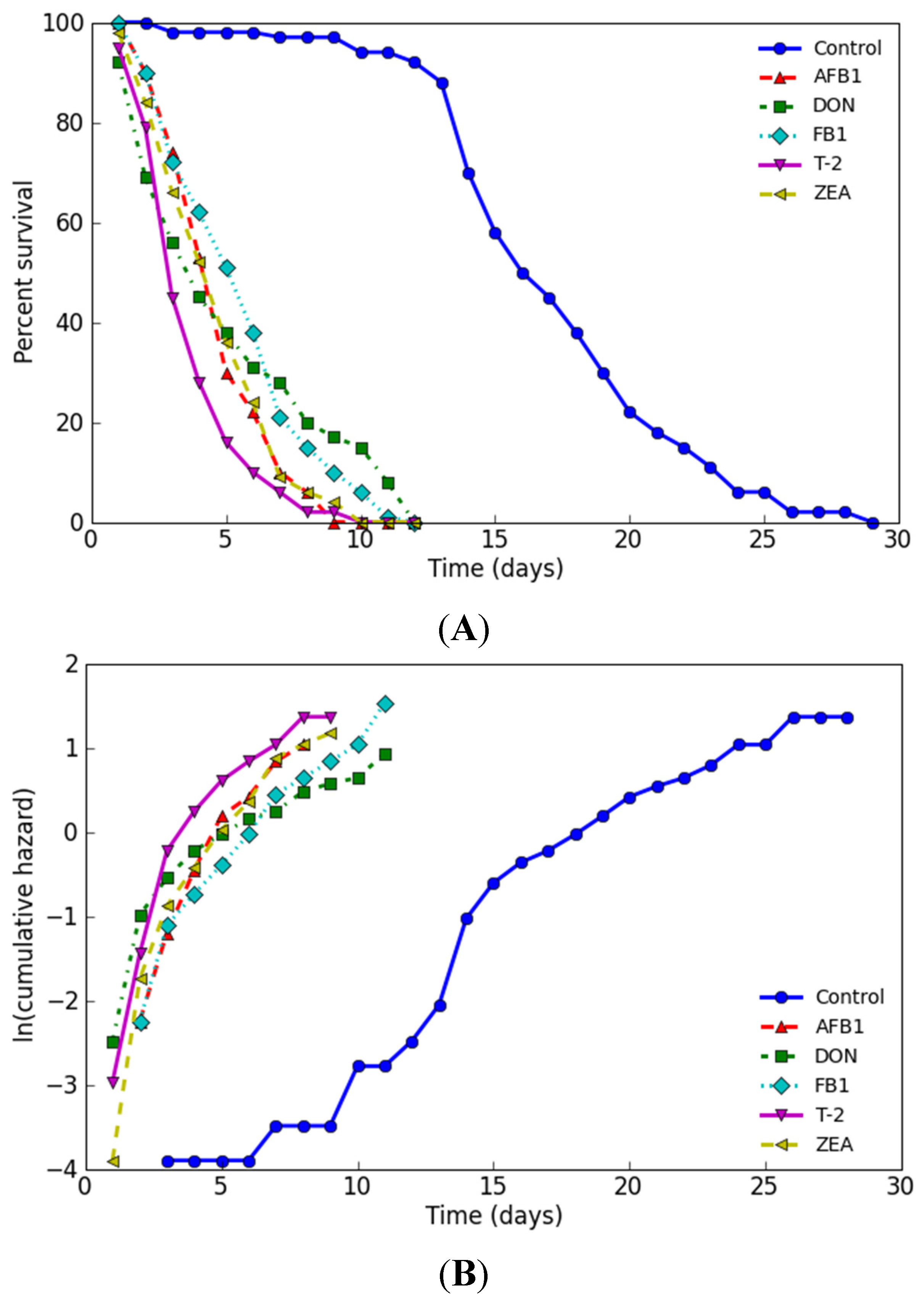
| Name | Dose (mg/L) | No. of Subjects | Estimated Mean | Mortality (Days) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Days | Std. | 95% C.I. | 25% | 50% | 75% | 90% | 100% | |||
| Control | 0 | 100 | 17.26 | 0.47 | 16.34–18.18 | 14 | 16 | 20 | 24 | 29 |
| AFB1 | 2.05 | 100 | 4.85 | 0.19 | 4.48–5.22 | 3 | 5 | 6 | 7 | 9 |
| DON | 65.67 | 100 | 5.19 | 0.35 | 4.50–5.88 | 2 | 4 | 8 | 11 | 12 |
| FB1 | 23.56 | 100 | 5.66 | 0.26 | 5.15–6.17 | 3 | 6 | 7 | 9 | 12 |
| T-2 | 0.14 | 100 | 3.83 | 0.19 | 3.46–4.20 | 3 | 4 | 5 | 6 | 10 |
| ZEA | 7.58 | 100 | 4.79 | 0.22 | 4.36–5.22 | 3 | 5 | 6 | 7 | 10 |
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Mycotoxins Exposures
4.3. Lethalality Assay
4.4. Measurement of Growth Endpoint
4.5. Measurement of Reproductive Endpoint
4.6. Life-Span Experiment
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cole, R.J.; Cox, R.H. Handbook of Toxic Fungal Metabolites; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- Wogan, G.N. Mycotoxins. Annu. Rev. Pharmacol. 1975, 15, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Ciegler, A. Mycotoxins: Occurrence, chemistry, biological activity. Lloydia 1975, 38, 21–35. [Google Scholar] [PubMed]
- Groopman, J.D.; Donahue, K.F. Aflatoxin, a human carcinogen: determination in foods and biological samples by monoclonal antibody affinity chromatography. J. Assoc. Off. Anal. Chem. 1988, 71, 861–867. [Google Scholar] [PubMed]
- Newberne, P.M. Mycotoxins: Toxicity, carcinogenicity, and the influence of various nutritional conditions. Environ. Health Perspect. 1974, 9, 1–32. [Google Scholar] [PubMed]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.P.; Salunkhe, D.K. Mycotoxins and phytotoxins; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Stoev, S.D. Foodborne mycotoxicoses, risk assessment and underestimated hazard of masked mycotoxins and joint mycotoxin effects or interaction. Environ. Toxicol. Pharmacol. 2015, 39, 794–809. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Aflatoxins. In Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; Monogr Eval Carcinog Risks Hum: Lyon, France, 2002; Volume 82, pp. 171–300. [Google Scholar]
- Kensler, T.W.; Roebuck, B.D.; Wogan, G.N.; Groopman, J.D. Aflatoxin: A 50-year odyssey of mechanistic and translational toxicology. Toxicol. Sci. 2011, 120 (Suppl 1), S28–S48. [Google Scholar] [CrossRef] [PubMed]
- Wogan, G.N.; Kensler, T.W.; Groopman, J.D. Present and future directions of translational research on aflatoxin and hepatocellular carcinoma. A review. Food Addit. Contam. Part A 2012, 29, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Fumonisins (addendum). In Safety Evaluation of Certain Food Additives and Contaminants; World Health Organization: Geneva, Switzerland, 2012; pp. 325–754. [Google Scholar]
- International Agency for Research on Cancer (IARC). Fumonisin B1. In Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; Monogr Eval Carcinog Risks Hum: Lyon, France, 2002; pp. 301–366. [Google Scholar]
- Marasas, W.F.; Riley, R.T.; Hendricks, K.A.; Stevens, V.L.; Sadler, T.W.; Gelineau-van Waes, J.; Missmer, S.A.; Cabrera, J.; Torres, O.; Gelderblom, W.C.; et al. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: A potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J. Nutr. 2004, 134, 711–716. [Google Scholar] [PubMed]
- Shephard, G.S.; van der Westhuizen, L.; Sewram, V. Biomarkers of exposure to fumonisin mycotoxins: A review. Food Addit. Contam. 2007, 24, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Huang, T.; Yu, J.; Tang, L.; Gao, W.; Wang, J.S. Fumonisin B1 contamination of home-grown corn in high-risk areas for esophageal and liver cancer in China. Food Addit. Contam. 2007, 24, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Zhang, Y.; Xie, Y.; Zhang, H.; Tang, L.; Wang, J.S. Co-contamination of aflatoxin B1 and fumonisin B1 in food and human dietary exposure in three areas of China. Food Addit. Contam. Part A 2011, 28, 461–470. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Fumonisin B1. In Environmental Health Criteria 219; World Health Organization: Geneva, Switzerland, 2000; pp. 1–134. [Google Scholar]
- Missmer, S.A.; Suarez, L.; Felkner, M.; Wang, E.; Merrill, A.H., Jr.; Rothman, K.J.; Hendricks, K.A. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ. Health Perspect 2006, 114, 237–241. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Toxins derived from Fusarium graminearum, F. culmorum and F. crookwellense: Zearalenone, Deoxynivalenol and Fusarenone X. In Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxin; Monogr Eval Carcinog Risks Hum: Lyon, France, 1993; Volume 56, pp. 397–445. [Google Scholar]
- Kuiper-Goodman, T.; Scott, P.M.; Watanabe, H. Risk assessment of the mycotoxin zearalenone. Regul. Toxicol. Pharmacol. 1987, 7, 253–306. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.J.; Gu, J.H.; Yuan, Y.; Liu, X.Z.; Zheng, W.L.; Huang, Q.Y.; Liu, Z.P.; Bian, J.C. Zearalenone inhibits testosterone biosynthesis in mouse Leydig cells via the crosstalk of estrogen receptor signaling and orphan nuclear receptor Nur77 expression. Toxicol. Vitro. 2014, 28, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Sato, N.; Ishii, K.; Sakai, K.; Tsunoda, H. Biological and chemical detection of trichothecene mycotoxins of Fusarium species. Appl. Microbiol. 1973, 25, 699–704. [Google Scholar] [PubMed]
- International Agency for Research on Cancer (IARC). Toxins derived from Fusarium sporotrichioides: T-2 toxin. In Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxin; Monogr Eval Carcinog Risks Hum: Lyon, France, 1993; Volume 56, pp. 467–489. [Google Scholar]
- Wan, D.; Wang, X.; Wu, Q.; Lin, P.; Pan, Y.; Sattar, A.; Huang, L.; Ahmad, I.; Zhang, Y.; Yuan, Z. Integrated Transcriptional and Proteomic Analysis of Growth Hormone Suppression Mediated by Trichothecene T-2 Toxin in Rat GH3 Cells. Toxicol. Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [PubMed]
- Anderson, G.L.; Boyd, W.A.; Williams, P.L. Assessment of sublethal endpoints for toxicity testing with the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 2001, 20, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Boyd, W.A.; McBride, S.J.; Rice, J.R.; Snyder, D.W.; Freedman, J.H. A high-throughput method for assessing chemical toxicity using a Caenorhabditis elegans reproduction assay. Toxicol. Appl. Pharmacol. 2010, 245, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Boyd, W.A.; Smith, M.V.; Freedman, J.H. Caenorhabditis elegans as a model in developmental toxicology. Methods Mol. Biol. 2012, 889, 15–24. [Google Scholar] [PubMed]
- Graves, A.L.; Boyd, W.A.; Williams, P.L. Using transgenic Caenorhabditis elegans in soil toxicity testing. Arch. Environ. Contam. Toxicol. 2005, 48, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.C.; Williams, P.L.; Benedetto, A.; Au, C.; Helmcke, K.J.; Aschner, M.; Meyer, J.N. Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicol. Sci. 2008, 106, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Reynolds, R.M.; Morran, L.T.; Tolman-Thompson, J.; Phillips, P.C. Experimental Evolution Reveals Antagonistic Pleiotropy in Reproductive Timing but Not Life Span in Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Kaletta, T.; Hengartner, M.O. Finding function in novel targets: C. Elegans as a model organism. Nat. Rev. Drug Discov. 2006, 5, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Burden, N.; Chapman, K.; Sewell, F.; Robinson, V. Pioneering better science through the 3Rs: An introduction to the national centre for the replacement, refinement, and reduction of animals in research (NC3Rs). J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 198–208. [Google Scholar] [PubMed]
- Singh, J. The national centre for the replacement, refinement, and reduction of animals in research. J. Pharmacol. Pharmacother. 2012, 3, 87–89. [Google Scholar] [PubMed]
- Middendorf, P.J.; Dusenbery, D.B. Fluoroacetic acid is a potent and specific inhibitor of reproduction in the nematode Caenorhabditis elegans. J. Nematol. 1993, 25, 573–577. [Google Scholar] [PubMed]
- Luder, H.U. Onset of human aging estimated from hazard functions associated with various causes of death. Mech. Ageing Dev. 1993, 67, 247–259. [Google Scholar] [CrossRef]
- Meyer, D.; Williams, P.L. Toxicity testing of neurotoxic pesticides in Caenorhabditis elegans. J. Toxicol. Environ. Health B Crit. Rev. 2014, 17, 284–306. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, R.; Dusenbery, D.B.; Williams, P.L. Comparison of lethality, reproduction, and behavior as toxicological endpoints in the nematode Caenorhabditis elegans. J. Toxicol. Environ. Health A 1999, 58, 451–462. [Google Scholar] [PubMed]
- Williams, P.L.; Anderson, G.L.; Johnstone, J.L.; Nunn, A.D.; Tweedle, M.F.; Wedeking, P. Caenorhabditis elegans as an alternative animal species. J. Toxicol. Environ. Health A 2000, 61, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.C.; Goldstone, J.V.; Boyd, W.A.; Freedman, J.H.; Meyer, J.N. Caenorhabditis elegans generates biologically relevant levels of genotoxic metabolites from aflatoxin B1 but not benzo[a]pyrene in vivo. Toxicol. Sci. 2010, 118, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Gowrinathan, Y.; Pacan, J.C.; Hawke, A.; Zhou, T.; Sabour, P.M. Toxicity assay for deoxynivalenol using Caenorhabditis elegans. Food Addit. Contam. Part A 2011, 28, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- McKean, C.; Tang, L.; Billam, M.; Tang, M.; Theodorakis, C.W.; Kendall, R.J.; Wang, J.S. Comparative acute and combinative toxicity of aflatoxin B1 and T-2 toxin in animals and immortalized human cell lines. J. Appl. Toxicol. 2006, 26, 139–147. [Google Scholar] [CrossRef] [PubMed]
- McKean, C.; Tang, L.; Tang, M.; Billam, M.; Wang, Z.; Theodorakis, C.W.; Kendall, R.J.; Wang, J.S. Comparative acute and combinative toxicity of aflatoxin B1 and fumonisin B1 in animals and human cells. Food Chem. Toxicol. 2006, 44, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.; Harrington, D. Counting Processes and Survival Analysis; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Yang, J.S.; Nam, H.J.; Seo, M.; Han, S.K.; Choi, Y.; Nam, H.G.; Lee, S.J.; Kim, S. Oasis: Online application for the survival analysis of lifespan assays performed in aging research. PLoS One 2011, 6, e23525. [Google Scholar] [CrossRef] [PubMed]
- Schrader, S.; Kramer, S.; Oldenburg, E.; Weinert, J. Uptake of deoxynivalenol by earthworms from Fusarium-infected wheat straw. Mycotoxin Res. 2009, 25, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, R.M.; Donkin, S.G.; Chin, K.J.; Grenache, D.G.; Bhatt, H.; Politz, S.M. Altered Expression of an L1-Specific, O-Linked Cuticle Surface Glycoprotein in Mutants of the Nematode Caenorhabditis elegans. J. Cell Biol. 1991, 115, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, R.; Dusenbery, D.B.; Williams, P.L. A comparison of metal-induced lethality and behavioral responses in the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 2000, 19, 3061–3067. [Google Scholar] [CrossRef]
- Apfeld, J.; Kenyon, C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature 1999, 402, 804–809. [Google Scholar] [PubMed]
- Hosono, R. Sterilization and growth inhibition of Caenorhabditis elegans by 5-fluorodeoxyuridine. Exp. Gerontol. 1978, 13, 369–374. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Xue, K.S.; Sun, X.; Tang, L.; Wang, J.-S. Multi-Toxic Endpoints of the Foodborne Mycotoxins in Nematode Caenorhabditis elegans. Toxins 2015, 7, 5224-5235. https://doi.org/10.3390/toxins7124876
Yang Z, Xue KS, Sun X, Tang L, Wang J-S. Multi-Toxic Endpoints of the Foodborne Mycotoxins in Nematode Caenorhabditis elegans. Toxins. 2015; 7(12):5224-5235. https://doi.org/10.3390/toxins7124876
Chicago/Turabian StyleYang, Zhendong, Kathy S. Xue, Xiulan Sun, Lili Tang, and Jia-Sheng Wang. 2015. "Multi-Toxic Endpoints of the Foodborne Mycotoxins in Nematode Caenorhabditis elegans" Toxins 7, no. 12: 5224-5235. https://doi.org/10.3390/toxins7124876
APA StyleYang, Z., Xue, K. S., Sun, X., Tang, L., & Wang, J.-S. (2015). Multi-Toxic Endpoints of the Foodborne Mycotoxins in Nematode Caenorhabditis elegans. Toxins, 7(12), 5224-5235. https://doi.org/10.3390/toxins7124876