Microcystins Alter Chemotactic Behavior in Caenorhabditis elegans by Selectively Targeting the AWA Sensory Neuron
Abstract
:1. Introduction
2. Results and Discussion
2.1. Statistical Evaluation of Chemotaxis Using a Generalized Linear Model
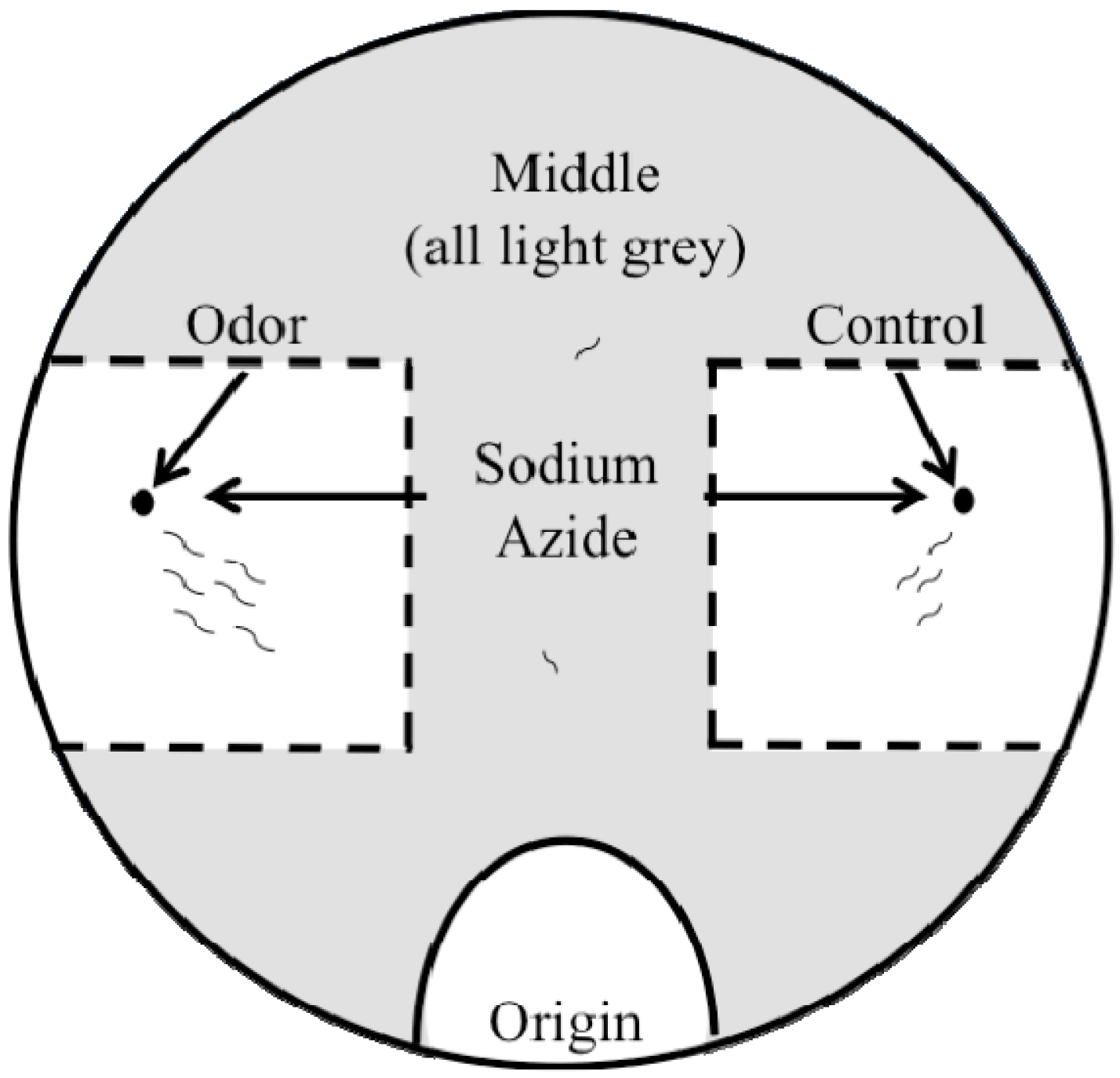

2.2. Outlier Chemotaxis Assays
2.3. MC-LR Impairs AWA Function, but not AWC Function
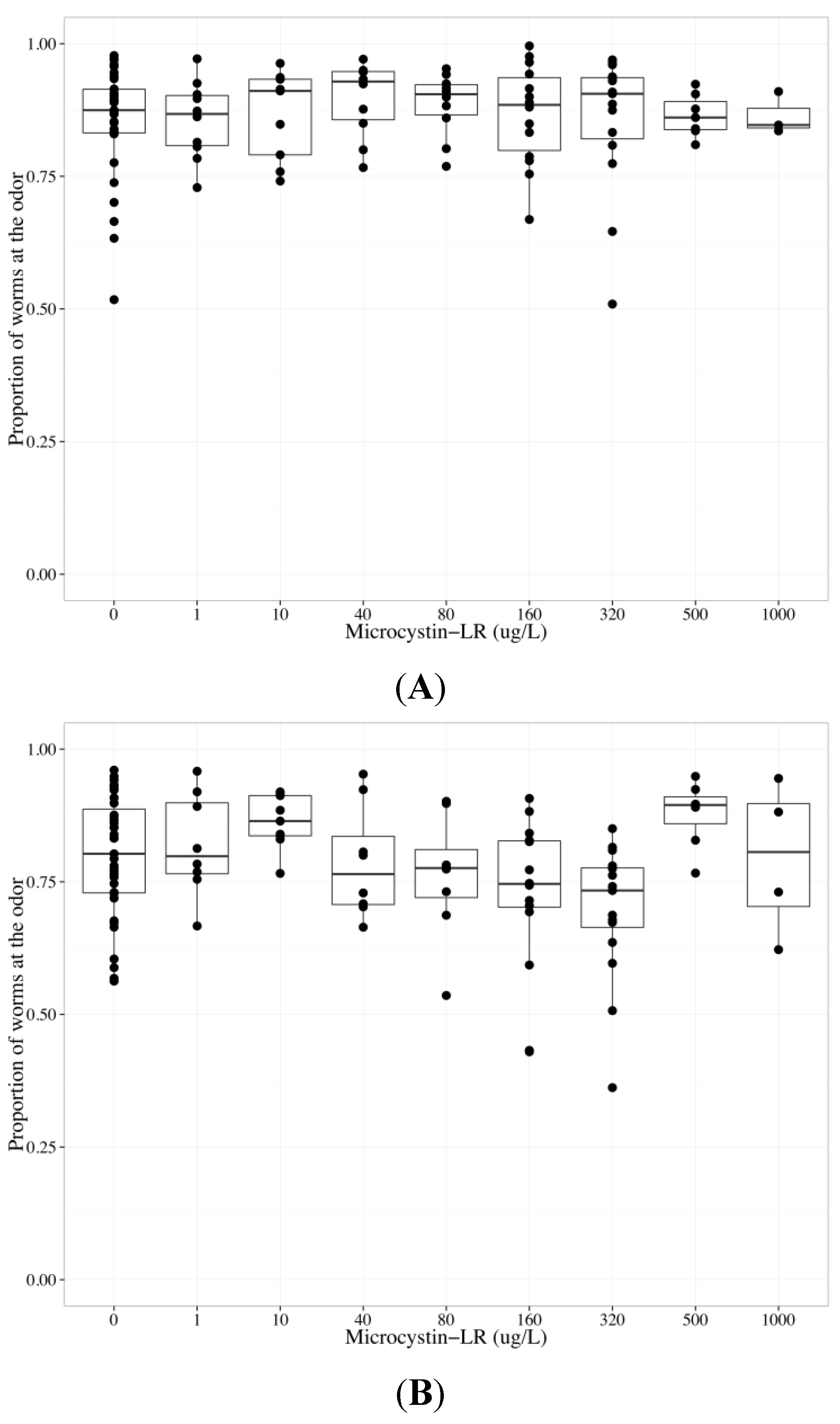
| Chemotaxis endpoints | Neuron | Coefficient | Parameter estimate | Standard error | p-value |
|---|---|---|---|---|---|
| Odor | Both | Concentration | −0.00190 | 0.000543 | 0.000571 *** |
| Both | Neuron | 0.433 | 0.138 | 0.00200 ** | |
| Both | Concentration*Neuron | 0.00180 | 0.000858 | 0.0370 * | |
| AWC | Concentration | −0.000101 | 0.000682 | 0.883 | |
| AWA | Concentration | −0.00190 | 0.000528 | 0.000501 *** | |
| Middle | AWC | Concentration | 0.0000272 | 0.000604 | 0.964 |
| AWA | Concentration | 0.00170 | 0.000570 | 0.00356 ** | |
| Control | AWC | Concentration | 0.000139 | 0.000851 | 0.871 |
| AWA | Concentration | 0.00161 | 0.000561 | 0.00498 ** |
2.4. MC-LF Impairs AWA Function, but not AWC Function
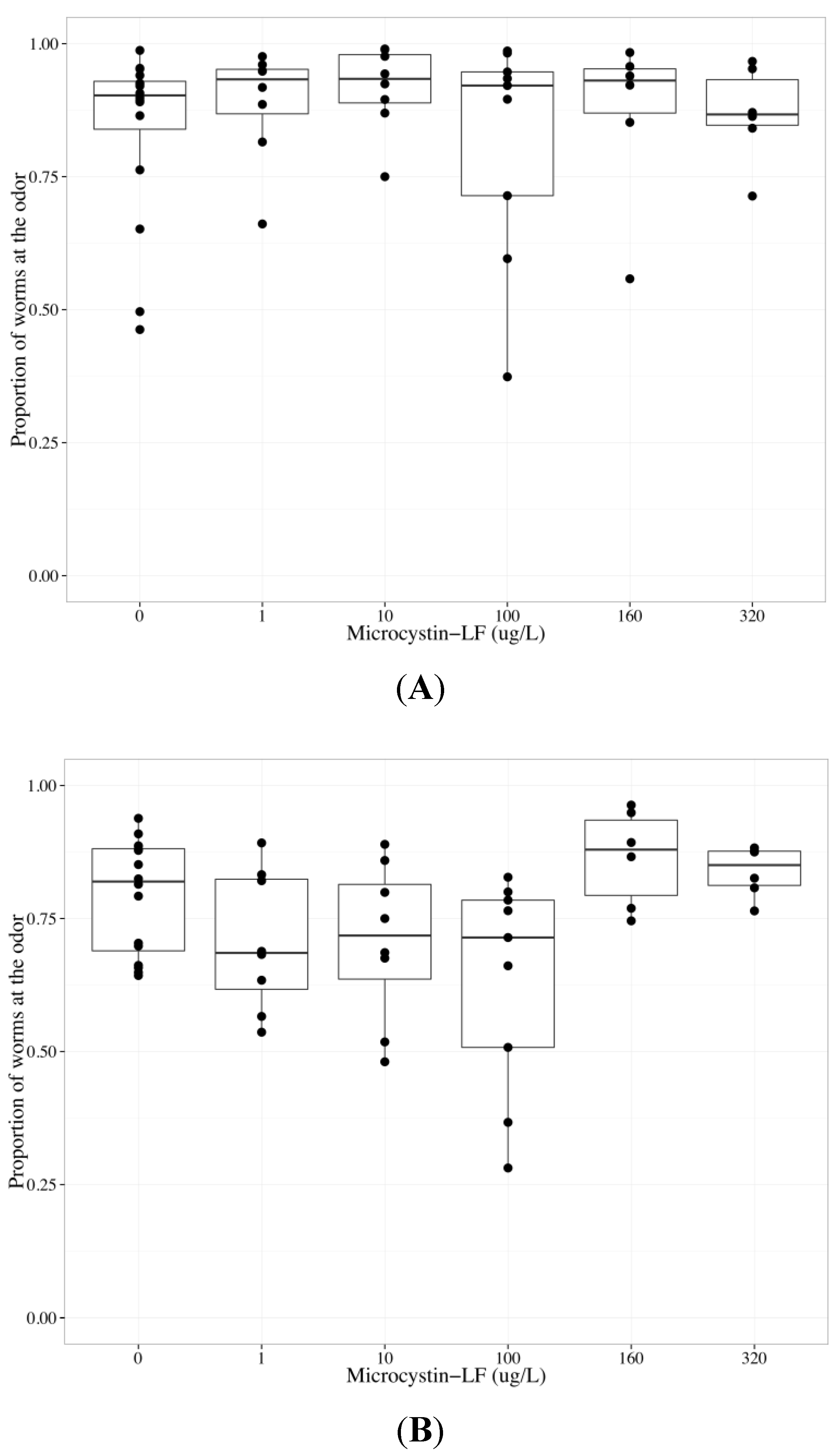
| Chemotaxis endpoint | Neuron | Coefficient | Parameter estimate | Standard error | p-value |
|---|---|---|---|---|---|
| Odor | Both | Concentration | −0.00593 | 0.00342 | 0.0873 |
| Both | Neuron | 0.970 | 0.223 | 4.04 × 10−5 *** | |
| AWC | Concentration | −0.00216 | 0.00460 | 0.641 | |
| AWA | Concentration | −0.00593 | 0.00280 | 0.0403 * | |
| Middle | AWC | Concentration | 0.000643 | 0.00291 | 0.826 |
| AWA | Concentration | 0.00714 | 0.00197 | 0.00082 *** | |
| Control | AWC | Concentration | 0.00267 | 0.00551 | 0.631 |
| AWA | Concentration | 0.00375 | 0.00327 | 0.259 |
2.5. MC-LF may be More Potent than MC-LR at Impairing AWA Function
| Chemotaxis endpoint | Toxin | Coefficient | Parameter estimate | Standard error | p-value |
|---|---|---|---|---|---|
| Odor | Both | Concentration | −0.00204 | 0.000524 | 0.000152 *** |
| Both | Toxin | 0.381 | 0.141 | 0.00763 ** |
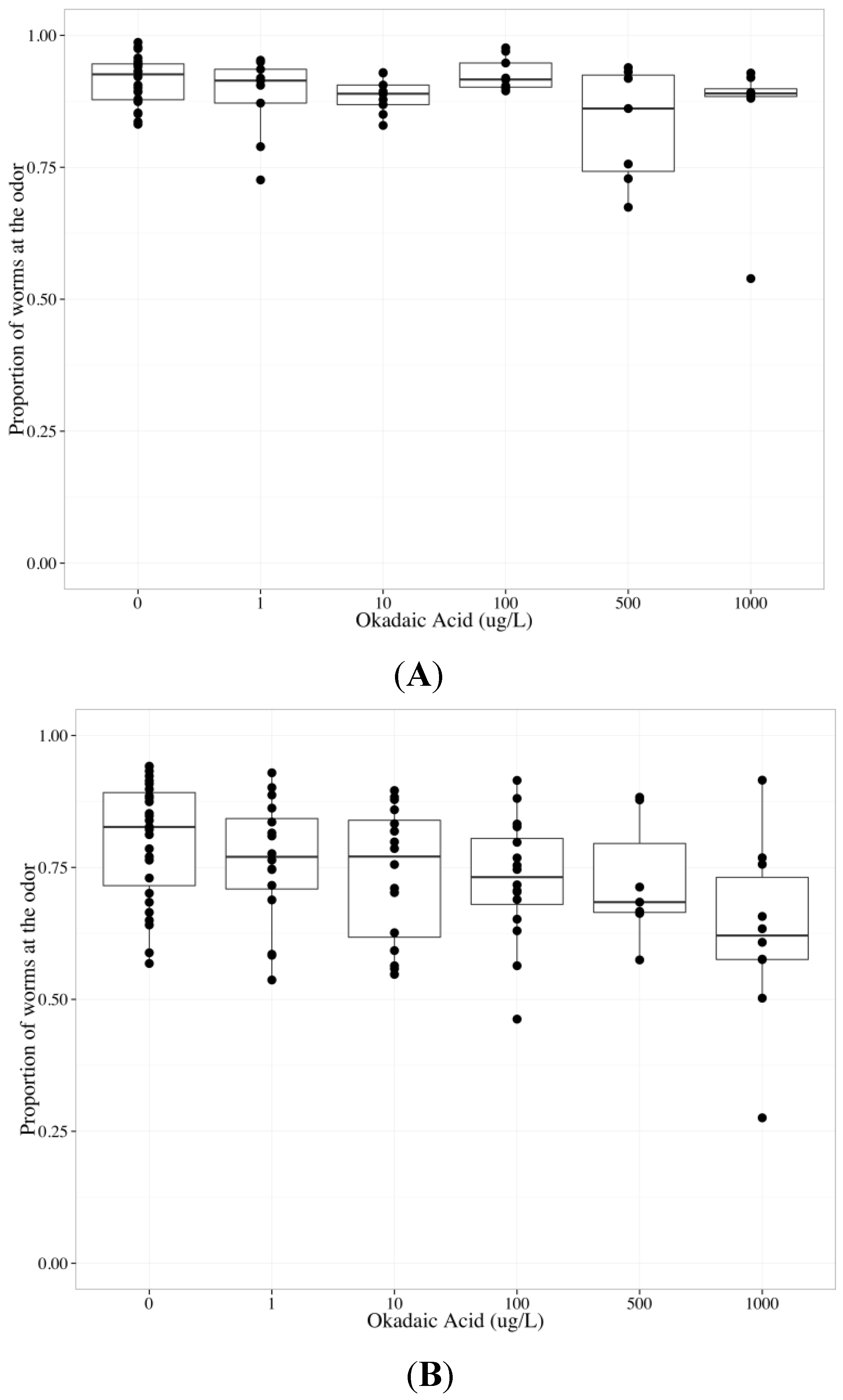
2.6. Tautomycin Does not Impair AWC or AWA function, While Okadaic Acid Impairs Both
| Chemotaxis endpoint | Neuron | Coefficient | Parameter estimate | Standard error | p-value |
|---|---|---|---|---|---|
| Odor | Both | Concentration | −0.000206 | 0.000231 | 0.375 |
| Both | Neuron | 0.413 | 0.165 | 0.0136 * | |
| AWC | Concentration | −3.14 × 10−5 | 4.23 × 10−4 | 0.941 | |
| AWA | Concentration | −0.000343 | 0.000226 | 0.134 | |
| Middle | AWC | Concentration | 0.000313 | 0.000353 | 0.378 |
| AWA | Concentration | 0.000182 | 0.000194 | 0.353 | |
| Control | AWC | Concentration | −0.000127 | 0.000488 | 0.796 |
| AWA | Concentration | 0.000371 | 0.000279 | 0.188 |

| Chemotaxis endpoint | Neuron | Coefficient | Parameter estimate | Standard error | p-value |
|---|---|---|---|---|---|
| Odor | Both | Concentration | −0.000442 | 0.000137 | 0.00148 ** |
| Both | Neuron | 1.03 | 0.120 | 6.57 × 10−15 *** | |
| AWC | Concentration | −0.000475 | 0.000188 | 0.0141 * | |
| AWA | Concentration | −0.000424 | 0.000189 | 0.0274 * | |
| Middle | AWC | Concentration | 0.000625 | 0.000144 | 4.89 × 10−5 *** |
| AWA | Concentration | 0.000674 | 0.000144 | 1.06 × 10−5 *** | |
| Control | AWC | Concentration | 0.000282 | 0.000271 | 0.303 |
| AWA | Concentration | 0.000145 | 0.000236 | 0.54 |
3. Experimental Section
3.1. Strains
3.2. Materials
3.3. Protein Phosphatase Inhibitors
3.4. 24-Hour Inhibitor Exposure
3.5. Chemotaxis Assay
3.6. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miller, M.A.; Kudela, R.M.; Mekebri, A.; Crane, D.; Oates, S.C.; Tinker, M.T.; Staedler, M.; Miller, W.A.; Toy-Choutka, S.; Dominik, C.; et al. Evidence for a novel marine harmful algal bloom: Cyanotoxin (microcystin) transfer from land to sea otters. PLoS ONE 2010, 5, e12576. [Google Scholar] [CrossRef]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef]
- Elleman, T.C.; Falconer, I.R.; Jackson, A.R.; Runnegar, M.T. Isolation, characterization and pathology of the toxin from a Microcystis aeruginosa (=Anacystis cyanea) bloom. Aust. J. Biol. Sci. 1978, 31, 209–218. [Google Scholar]
- Falconer, I.R.; Beresford, A.M.; Runnegar, M.T. Evidence of liver damage by toxin from a bloom of the blue-green alga, Microcystis aeruginosa. Med. J. Aust. 1983, 1, 511–514. [Google Scholar]
- Eriksson, J.E.; Paatero, G.I.; Meriluoto, J.A.; Codd, G.A.; Kass, G.E.; Nicotera, P.; Orrenius, S. Rapid microfilament reorganization induced in isolated rat hepatocytes by microcystin-LR, a cyclic peptide toxin. Exp. Cell Res. 1989, 185, 86–100. [Google Scholar] [CrossRef]
- Azevedo, S.M.; Carmichael, W.W.; Jochimsen, E.M.; Rinehart, K.L.; Lau, S.; Shaw, G.R.; Eaglesham, G.K. Human intoxication by microcystins during renal dialysis treatment in Caruaru-Brazil. Toxicology 2002, 181–182, 441–446. [Google Scholar] [CrossRef]
- Pouria, S.; de Andrade, A.; Barbosa, J.; Cavalcanti, R.L.; Barreto, V.T.; Ward, C.J.; Preiser, W.; Poon, G.K.; Neild, G.H.; Codd, G.A. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet 1998, 352, 21–26. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Azevedo, S.M.; An, J.S.; Molica, R.J.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ. Health Perspect 2001, 109, 663–668. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality. Addendum to Vol. 2. Health Criteria and other Supporting Information, 2nd ed.; World Health Organization: Geneva, Switzerland, 1998; pp. 1–283. [Google Scholar]
- Runnegar, M.; Berndt, N.; Kong, S.M.; Lee, E.Y.; Zhang, L. In vivo and in vitro binding of microcystin to protein phosphatases 1 and 2A. Biochem. Biophys. Res. Commun. 1995, 216, 162–169. [Google Scholar]
- Honkanen, R.E.; Zwiller, J.; Moore, R.E.; Daily, S.L.; Khatra, B.S.; Dukelow, M.; Boynton, A.L. Characterization of microcystin-LR, a potent inhibitor of type 1 and type 2A protein phosphatases. J. Biol. Chem. 1990, 265, 19401–19404. [Google Scholar]
- Siddoway, B.A.; Altimimi, H.F.; Hou, H.; Petralia, R.S.; Xu, B.; Stellwagen, D.; Xia, H. An essential role for inhibitor-2 regulation of protein phosphatase-1 in synaptic scaling. J. Neurosci. 2013, 33, 11206–11211. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, Y.; Fang, L.; Mu, Y.; Wu, J.; Zhang, X. Roles of phosphotase 2A in nociceptive signal processing. Mol. Pain 2013, 9. [Google Scholar] [CrossRef]
- Farrell, K.F.; Krishnamachari, S.; Villanueva, E.; Lou, H.; Alerte, T.N.; Peet, E.; Drolet, R.E.; Perez, R.G. Non-motor parkinsonian pathology in aging A53T alpha-synuclein mice is associated with progressive synucleinopathy and altered enzymatic function. J. Neurochem. 2014, 128, 536–546. [Google Scholar] [CrossRef]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.L.; Terro, F. Tau protein phosphatases in Alzheimer’s disease: The leading role of PP2A. Ageing Res. Rev. 2013, 12, 39–49. [Google Scholar] [CrossRef]
- Fischer, A.; Hoeger, S.J.; Stemmer, K.; Feurstein, D.J.; Knobeloch, D.; Nussler, A.; Dietrich, D.R. The role of organic anion transporting polypeptides (OATPs/SLCOs) in the toxicity of different microcystin congeners in vitro: A comparison of primary human hepatocytes and OATP-transfected HEK293 cells. Toxicol. Appl. Pharmacol. 2010, 245, 9–20. [Google Scholar] [CrossRef]
- Fischer, W.J.; Altheimer, S.; Cattori, V.; Meier, P.J.; Dietrich, D.R.; Hagenbuch, B. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol. Appl. Pharmacol. 2005, 203, 257–263. [Google Scholar] [CrossRef]
- Bronger, H.; König, J.; Kopplow, K.; Steiner, H.H.; Ahmadi, R.; Herold-Mende, C.; Keppler, D.; Nies, A.T. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Res. 2005, 65, 11419–11428. [Google Scholar] [CrossRef]
- Westholm, D.E.; Salo, D.R.; Viken, K.J.; Rumbley, J.N.; Anderson, G.W. The blood-brain barrier thyroxine transporter organic anion-transporting polypeptide 1c1 displays atypical transport kinetics. Endocrinology 2009, 150, 5153–5162. [Google Scholar]
- Huber, R.D.; Gao., B.; Sidler Pfändler, M.A.; Zhang-Fu, W.; Leuthold, S.; Hagenbuch, B.; Folkers, G.; Meier, P.J.; Stieger, B. Characterization of two splice variants of human organic anion transporting polypeptide 3A1 isolated from human brain. Am. J. Physiol. Cell Physiol. 2007, 292, C795–C806. [Google Scholar]
- Kullak-Ublick, G.A.; Ismair, M.G.; Stieger, B.; Landmann, L.; Huber, R.; Pizzagalli, F.; Fattinger, K.; Meier, P.J.; Hagenbuch, B. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology 2001, 120, 525–533. [Google Scholar]
- Baganz, D.; Staaks, G.; Pflugmacher, S.; Steinberg, C.E. Comparative study of microcystin-LR-induced behavioral changes of two fish species, Danio rerio and Leucaspius delineatus. Environ. Toxicol. 2004, 19, 564–570. [Google Scholar] [CrossRef]
- Cazenave, J.; Nores, M.L.; Miceli, M.; Díaz, M.P.; Wunderlin, D.A.; Bistoni, M.A. Changes in the swimming activity and the glutathione S-transferase activity of Jenynsia multidentata fed with microcystin-RR. Water Res. 2008, 42, 1299–1307. [Google Scholar] [CrossRef]
- Li, G.; Yan, W.; Cai, F.; Li, C.; Chen, N.; Wang, J. Spatial learning and memory impairment and pathological change in rats induced by acute exposure to microcystin-LR. Environ. Toxicol 2014, 29, 261–268. [Google Scholar] [CrossRef]
- Wang, J.; Lin, F.; Cai, F.; Yan, W.; Zhou, Q.; Xie, L. Microcystin-LR inhibited hippocampal long-term potential via regulation of the glycogen synthase kinase-3beta pathway. Chemosphere 2013, 93, 223–229. [Google Scholar] [CrossRef]
- Feurstein, D.; Stemmer, K.; Kleinteich, J.; Speicher, T.; Dietrich, D.R. Microcystin congener- and concentration-dependent induction of murine neuron apoptosis and neurite degeneration. Toxicol. Sci. 2011, 124, 424–431. [Google Scholar] [CrossRef]
- Feurstein, D.; Holst, K.; Fischer, A.; Dietrich, D.R. Oatp-associated uptake and toxicity of microcystins in primary murine whole brain cells. Toxicol. Appl. Pharmacol. 2009, 234, 247–255. [Google Scholar] [CrossRef]
- Gaillard, I.; Rouquier, S.; Giorgi, D. Olfactory receptors. Cell Mol. Life Sci. 2004, 61, 456–469. [Google Scholar] [CrossRef]
- Aschner, M.; Levin, E.D.; Suñol, C.; Olopade, J.O.; Helmcke, K.J.; Avila, D.S.; Sledge, D.; Ali, R.H.; Upchurch, L.; Donerly, S.; et al. Gene-environment interactions: Neurodegeneration in non-mammals and mammals. Neurotoxicology 2010, 31, 582–588. [Google Scholar] [CrossRef]
- Prasad, B.C.; Reed, R.R. Chemosensation: Molecular mechanisms in worms and mammals. Trends Genet. 1999, 15, 150–153. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar]
- Gray, J.M.; Hill, J.J.; Bargmann, C.I. A circuit for navigation in Caenorhabditis elegans. Proc.Natl. Acad. Sci. USA 2005, 102, 3184–3191. [Google Scholar] [CrossRef]
- Bargmann, C.I. Chemosensation in C. elegans. WormBook 2006. [Google Scholar] [CrossRef]
- Bargmann, C.I.; Hartwieg, E.; Horvitz, H.R. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 1993, 74, 515–527. [Google Scholar] [CrossRef]
- Chen, L.; Fu, Y.; Ren, M.; Xiao, B.; Rubin, C.S. A RasGRP, C. elegans RGEF-1b, couples external stimuli to behavior by activating LET-60 (Ras) in sensory neurons. Neuron 2011, 70, 51–65. [Google Scholar] [CrossRef]
- Lee, J.I.; O’Halloran, D.M.; Eastham-Anderson, J.; Juang, B.T.; Kaye, J.A.; Scott Hamilton, O.; Lesch, B.; Goga, A.; L’Etoile, N.D. Nuclear entry of a cGMP-dependent kinase converts transient into long-lasting olfactory adaptation. Proc. Natl. Acad. Sci. USA 2010, 107, 6016–6021. [Google Scholar] [CrossRef]
- Chou, J.H.; Bargmann, C.I.; Sengupta, P. The Caenorhabditis elegans odr-2 gene encodes a novel Ly-6-related protein required for olfaction. Genetics 2001, 157, 211–224. [Google Scholar]
- Sassa, T.; Ueda-Ohba, H.; Kitamura, K.; Harada, S.; Hosono, R. Role of Caenorhabditis elegans protein phosphatase type 1, CeGLC-7 beta, in metaphase to anaphase transition during embryonic development. Exp. Cell Res. 2003, 287, 350–360. [Google Scholar] [CrossRef]
- Asencio, C.; Davidson, I.F.; Santarella-Mellwig, R.; Ly-Hartig, T.B.; Mall, M.; Wallenfang, M.R.; Mattaj, I.W.; Gorjánácz, M. Coordination of kinase and phosphatase activities by Lem4 enables nuclear envelope reassembly during mitosis. Cell 2012, 150, 122–135. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Yin, L.; Pu, Y.; Wang, D. Using the nematode Caenorhabditis elegans as a model animal for assessing the toxicity induced by microcystin-LR. J. Environ. Sci. 2009, 21, 395–401. [Google Scholar] [CrossRef]
- Li, Y.; Ye, H.; Du, M.; Zhang, Y.; Ye, B.; Pu, Y.; Wang, D. Induction of chemotaxis to sodium chloride and diacetyl and thermotaxis defects by microcystin-LR exposure in nematode Caenorhabditis elegans. J. Environ. Sci. 2009, 21, 971–979. [Google Scholar] [CrossRef]
- Xing, X.; Guo, Y.; Wang, D. Using the larvae nematode Caenorhabditis elegans to evaluate neurobehavioral toxicity to metallic salts. Ecotoxicol. Environ. Saf. 2009, 72, 1819–1823. [Google Scholar] [CrossRef]
- Lezcano, N.; Sedán, D.; Lucotti, I.; Giannuzzi, L.; Vittone, L.; Andrinolo, D.; Mundiña-Weilenmann, C. Subchronic microcystin-LR exposure increased hepatic apoptosis and induced compensatory mechanisms in mice. J. Biochem. Mol. Toxicol. 2012, 26, 131–138. [Google Scholar] [CrossRef]
- Wang, M.; Wang, D.; Lin, L.; Hong, H. Protein profiles in zebrafish (Danio rerio) brains exposed to chronic microcystin-LR. Chemosphere 2010, 81, 716–724. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Chen, Y.; Zhu, Y. Microcystin-RR induces apoptosis in fish lymphocytes by generating reactive oxygen species and causing mitochondrial damage. Fish Physiol. Biochem. 2008, 34, 307–312. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Chen, Y.; Zhu, Y. Influence of intracellular Ca(2+), mitochondria membrane potential, reactive oxygen species, and intracellular ATP on the mechanism of microcystin-LR induced apoptosis in Carassius auratus lymphocytes in vitro. Environ. Toxicol. 2007, 22, 559–564. [Google Scholar] [CrossRef]
- Ding, W.X.; Shen, H.M.; Ong, C.N. Pivotal role of mitochondrial Ca(2+) in microcystin-induced mitochondrial permeability transition in rat hepatocytes. Biochem. Biophys. Res. Commun. 2001, 285, 1155–1161. [Google Scholar] [CrossRef]
- Agam, K.; Frechter, S.; Minke, B. Activation of the Drosophila TRP and TRPL channels requires both Ca2+ and protein dephosphorylation. Cell Calcium 2004, 35, 87–105. [Google Scholar] [CrossRef]
- Kamp, T.J.; Hell, J.W. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ. Res. 2000, 87, 1095–1102. [Google Scholar] [CrossRef]
- Colbert, H.A.; Smith, T.L.; Bargmann, C.I. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J. Neurosci. 1997, 17, 8259–8269. [Google Scholar]
- Sengupta, P.; Chou, J.H.; Bargmann, C.I. odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell 1996, 84, 899–909. [Google Scholar] [CrossRef]
- Larsch, J.; Ventimiglia, D.; Bargmann, C.; Albrecht, D.R. High-throughput imaging of neuronal activity in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2013, 110, E4266–E4273. [Google Scholar]
- Coburn, C.M.; Bargmann, C.I. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron 1996, 17, 695–706. [Google Scholar] [CrossRef]
- Sarafi-Reinach, T.R.; Melkman, T.; Hobert, O.; Sengupta, P. The lin-11 LIM homeobox gene specifies olfactory and chemosensory neuron fates in C. elegans. Development 2001, 128, 3269–3281. [Google Scholar]
- Gambaro, A.; Barbaro, E.; Zangrando, R.; Barbante, C. Simultaneous quantification of microcystins and nodularin in aerosol samples using high-performance liquid chromatography/negative electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 1497–1506. [Google Scholar] [CrossRef]
- Faassen, E.J.; Lurling, M. Occurrence of the microcystins MC-LW and MC-LF in Dutch surface waters and their contribution to total microcystin toxicity. Mar. Drugs 2013, 11, 2643–2654. [Google Scholar] [CrossRef]
- Vesterkvist, P.S.; Misiorek, J.O.; Spoof, L.E.; Toivola, D.M.; Meriluoto, J.A. Comparative cellular toxicity of hydrophilic and hydrophobic microcystins on Caco-2 cells. Toxins 2012, 4, 1008–1023. [Google Scholar] [CrossRef]
- Takai, A.; Sasaki, K.; Nagai, H.; Mieskes, G.; Isobe, M.; Isono, K.; Yasumoto, T. Inhibition of specific binding of okadaic acid to protein phosphatase 2A by microcystin-LR, calyculin-A and tautomycin: Method of analysis of interactions of tight-binding ligands with target protein. Biochem. J. 1995, 306, 657–665. [Google Scholar]
- Li, W.; Ju, J.; Osada, H.; Shen, B. Utilization of the methoxymalonyl-acyl carrier protein biosynthesis locus for cloning of the tautomycin biosynthetic gene cluster from Streptomyces spiroverticillatus. J. Bacteriol. 2006, 188, 4148–4152. [Google Scholar] [CrossRef]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef]
- Fladmark, K.E.; Brustugun, O.T.; Mellgren, G.; Krakstad, C.; Boe, R.; Vintermyr, O.K.; Schulman, H.; Doskeland, S.O. Ca2+/calmodulin-dependent protein kinase II is required for microcystin-induced apoptosis. J. Biol. Chem. 2002, 277, 2804–2811. [Google Scholar] [CrossRef]
- Krakstad, C.; Herfindal, L.; Gjertsen, B.T.; Bøe, R.; Vintermyr, O.K.; Fladmark, K.E.; Døskeland, S.O. CaM-kinaseII-dependent commitment to microcystin-induced apoptosis is coupled to cell budding, but not to shrinkage or chromatin hypercondensation. Cell Death Differ. 2006, 13, 1191–1202. [Google Scholar] [CrossRef]
- Bandyopadhyay, J.; Lee, J.; Lee, J.; Lee, J.I.; Yu, J.R.; Jee, C.; Cho, J.H.; Jung, S.; Lee, M.H.; Zannoni, S.; et al. Calcineurin, a calcium/calmodulin-dependent protein phosphatase, is involved in movement, fertility, egg laying, and growth in Caenorhabditis elegans. Mol. Biol. Cell 2002, 13, 3281–3293. [Google Scholar] [CrossRef]
- Kuhara, A.; Inada, H.; Katsura, I.; Mori, I. Negative regulation and gain control of sensory neurons by the C. elegans calcineurin TAX-6. Neuron 2002, 33, 751–763. [Google Scholar] [CrossRef]
- Yang, D.; Kim, K.H.; Phimister, A.; Bachstetter, A.D.; Ward, T.R.; Stackman, R.W.; Mervis, R.F.; Wisniewski, A.B.; Klein, S.L.; Kodavanti, P.R.; et al. Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ. Health Perspect. 2009, 117, 426–435. [Google Scholar] [CrossRef]
- Hart, A.C.; Chao, M.Y. From odors to behaviors in Caenorhabditis elegans. In The Neurobiology of Olfaction; Menini, A., Ed.; CRC press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Hart, A.C. Behavior. WormBook 2006. [Google Scholar] [CrossRef]
- Team, R.D.C. R: A Language and Environment for Statistical Computing; the R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Cole, R.D.; Anderson, G.L.; Williams, P.L. The nematode Caenorhabditis elegans as a model of organophosphate-induced mammalian neurotoxicity. Toxicol. Appl. Pharmacol. 2004, 194, 248–256. [Google Scholar] [CrossRef]
- Roegner, A.F.; Brena, B.; González-Sapienza, G.; Puschner, B. Microcystins in potable surface waters: Toxic effects and removal strategies. J. Appl. Toxicol. 2014, 34, 441–457. [Google Scholar] [CrossRef]
- Page, A.P.; Johnstone, I.L. The cuticle. WormBook 2007. [Google Scholar] [CrossRef]
- Giannuzzi, L.; Sedan, D.; Echenique, R.; Andrinolo, D. An acute case of intoxication with cyanobacteria and cyanotoxins in recreational water in Salto Grande Dam, Argentina. Mar. Drugs 2011, 9, 2164–2175. [Google Scholar] [CrossRef]
- Roegner, A.F.; Puschner, B. Aggregate culture: A more accurate predictor of microcystin toxicity for risk assessment. Toxicon 2014, 83, 1–14. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moore, C.E.; Lein, P.J.; Puschner, B. Microcystins Alter Chemotactic Behavior in Caenorhabditis elegans by Selectively Targeting the AWA Sensory Neuron. Toxins 2014, 6, 1813-1836. https://doi.org/10.3390/toxins6061813
Moore CE, Lein PJ, Puschner B. Microcystins Alter Chemotactic Behavior in Caenorhabditis elegans by Selectively Targeting the AWA Sensory Neuron. Toxins. 2014; 6(6):1813-1836. https://doi.org/10.3390/toxins6061813
Chicago/Turabian StyleMoore, Caroline E., Pamela J. Lein, and Birgit Puschner. 2014. "Microcystins Alter Chemotactic Behavior in Caenorhabditis elegans by Selectively Targeting the AWA Sensory Neuron" Toxins 6, no. 6: 1813-1836. https://doi.org/10.3390/toxins6061813
APA StyleMoore, C. E., Lein, P. J., & Puschner, B. (2014). Microcystins Alter Chemotactic Behavior in Caenorhabditis elegans by Selectively Targeting the AWA Sensory Neuron. Toxins, 6(6), 1813-1836. https://doi.org/10.3390/toxins6061813






