Presence of the Neurotoxin BMAA in Aquatic Ecosystems: What Do We Really Know?
Abstract
:1. Introduction
| Publication year | Quantification method | Derivatization method | n tested samples | % positive samples | [BMAA] in positive samples µg/g DW | Ref | |
|---|---|---|---|---|---|---|---|
| average | median | ||||||
| 2005 | LC-FLD | AQC § | 30 | 97 | 968 | 265 | [13] |
| 2008 | LC-FLD | AQC | 12 | 100 | 103 | 76 | [24] |
| 2008 | LC-FLD | AQC | 7 | 100 | 10 | 7.3 | [46] |
| 2008 | GC-MS | EZ:faast | 27 | 96 | 130 | 3.5 | [44] |
| 2008 | LC-MS/MS * | none | 34 | 0 | - | - | [47] |
| 2009 | LC-MS/MS | none | 21 | 43 | 13 | 6.0 | [48] |
| 2010 | LC-MS/MS ^ | none | 30 | 0 | - | - | [49] |
| 2010 | LC-MS/MS | AQC | 21 | 100 | 0.01 | 0.01 | [23] |
| 2011 | LC-MS | EZ:faast | 20 | 80 | 1.4 | 0.49 | [45] |
| 2011 | CE-UV | none | 8 | 100 | 402 | 277 | [50] |
| 2012 | LC-FLD | AQC | 18 | 100 | 14 | 9.0 | [42] |
| 2012 | LC-FLD | AQC | 16 | 100 | 0.29 | 0.24 | [42] |
| 2012 | LC-MS/MS # | AQC | 8 | 0 | - | - | [43] |
| 2012 | LC-MS/MS ~ | none | 8 | 0 | - | - | [43] |
| 2012 | LC-FLD | AQC | 8 | 38 | 28 | 22 | [43] |
| 2014 | LC-MS/MS | AQC | 10 | 100 | 4.4 | 3.2 | [51] |
2. The Role of Analytical Methods in the BMAA Controversy
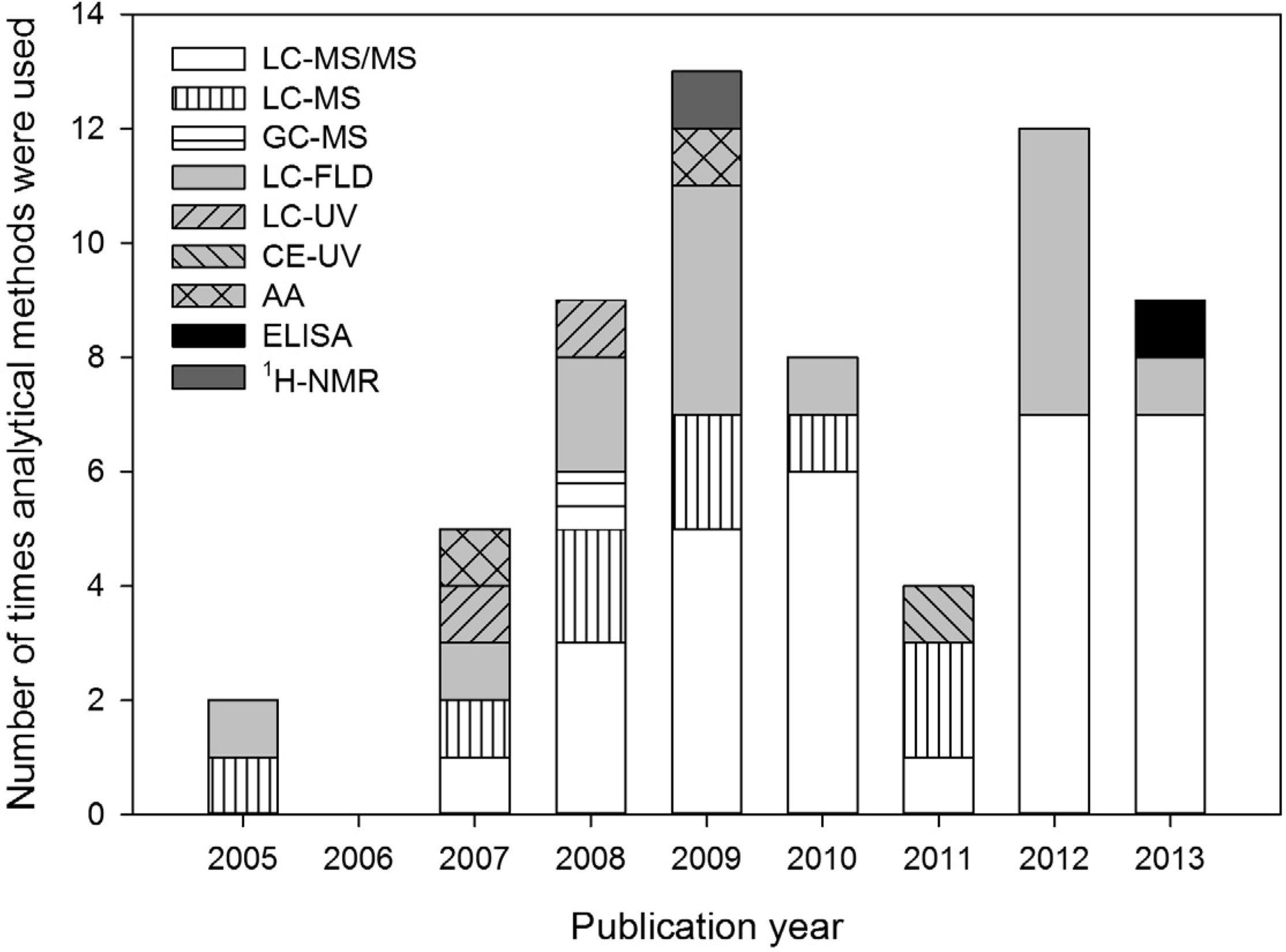
3. Review of Reported Methods and Results
3.1. Sample Origin and Storage
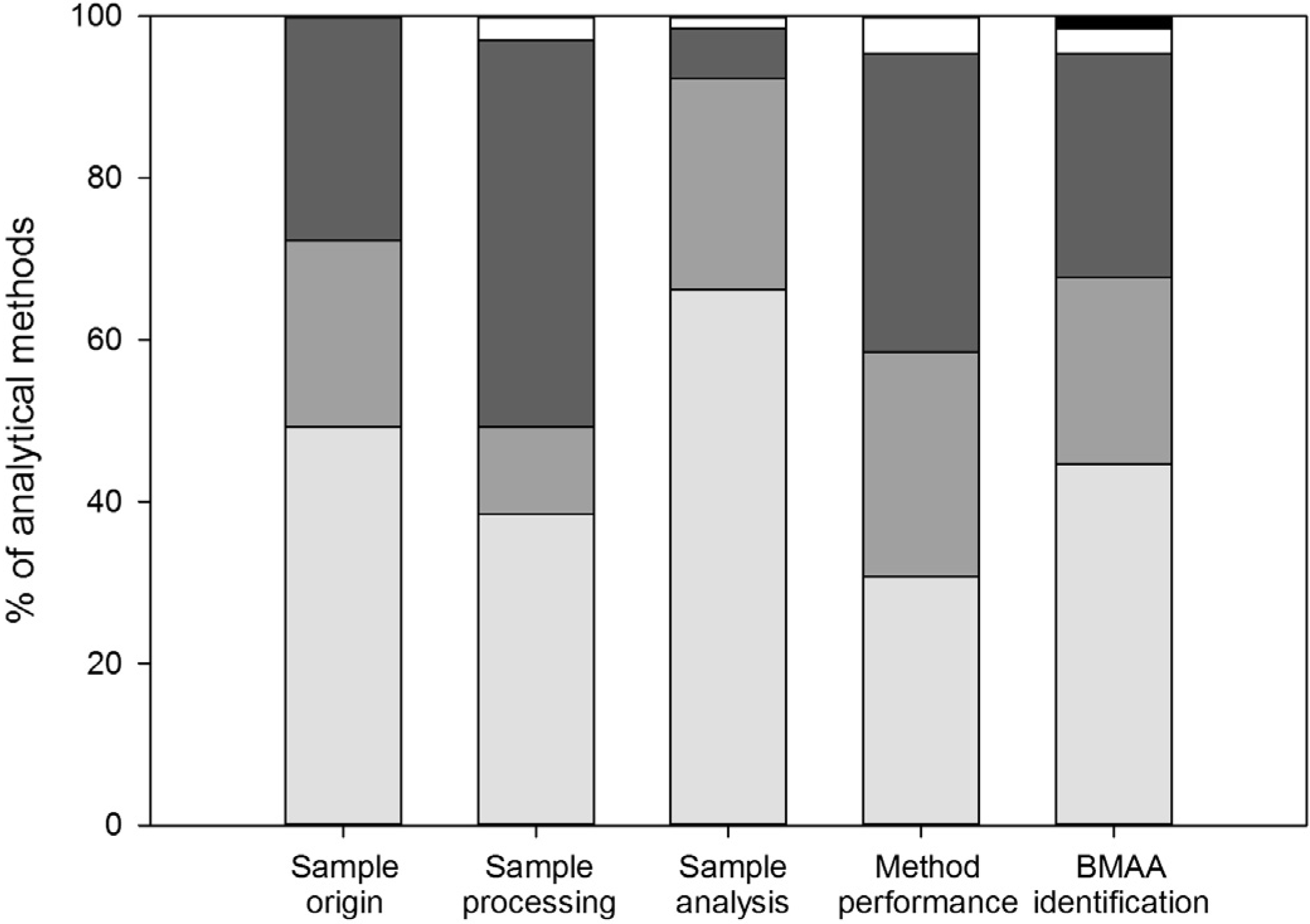
3.2. Sample Processing
3.3. Sample Analysis
3.4. Method Performance
3.5. BMAA Identification
4. Bias through Selective Literature References and Lack of Discussion
4.1. Selective Use of References
4.2. Discussion of Quality and Limitations of the Study
5. Conclusions
5.1. Presence of BMAA in Aquatic Ecosystems

5.2. Improving the Science
Acknowledgments
Conflict of Interest
Appendix
- Appendix information 1: Method abbreviations
- Appendix information 2: Article discussion ‘Distinguishing the cyanobacterial neurotoxin β-N-methylamino-l-alanine (BMAA) from its structural isomer 2,4-diaminobutyric acid (2,4-DAB) [55]’
- Appendix information 3: Article discussion ‘Distinguishing the cyanobacterial neurotoxin β-N-methylamino-l-alanine (BMAA) from other diamino acids [56]’
- Appendix information 4: Article discussion ‘Cyanobacteria and BMAA exposure from desert dust: A possible link to sporadic ALS among Gulf War veterans [60]’
- Appendix information 5: Article discussion ‘Reactivity of β-methylamino-l-alanine in complex sample matrixes complicating detection and quantification by mass spectrometry [57]’
- Appendix information 6: Article discussion ‘Nitrogen starvation results in the production of β-N-methylamino-l-alanine [76]’
- Appendix information 7: Reporting quality of methods and results
- Appendix information 8: Criteria used for classification in Figure 4
Appendix information 1: Method abbreviations
Appendix information 2: Article discussion ‘Distinguishing the cyanobacterial neurotoxin β-N-methylamino-l-alanine (BMAA) from its structural isomer 2,4-diaminobutyric acid (2,4-DAB) [55]’
LC Separation of BMAA and DAB
- Figure 7 in [55]: No method given.
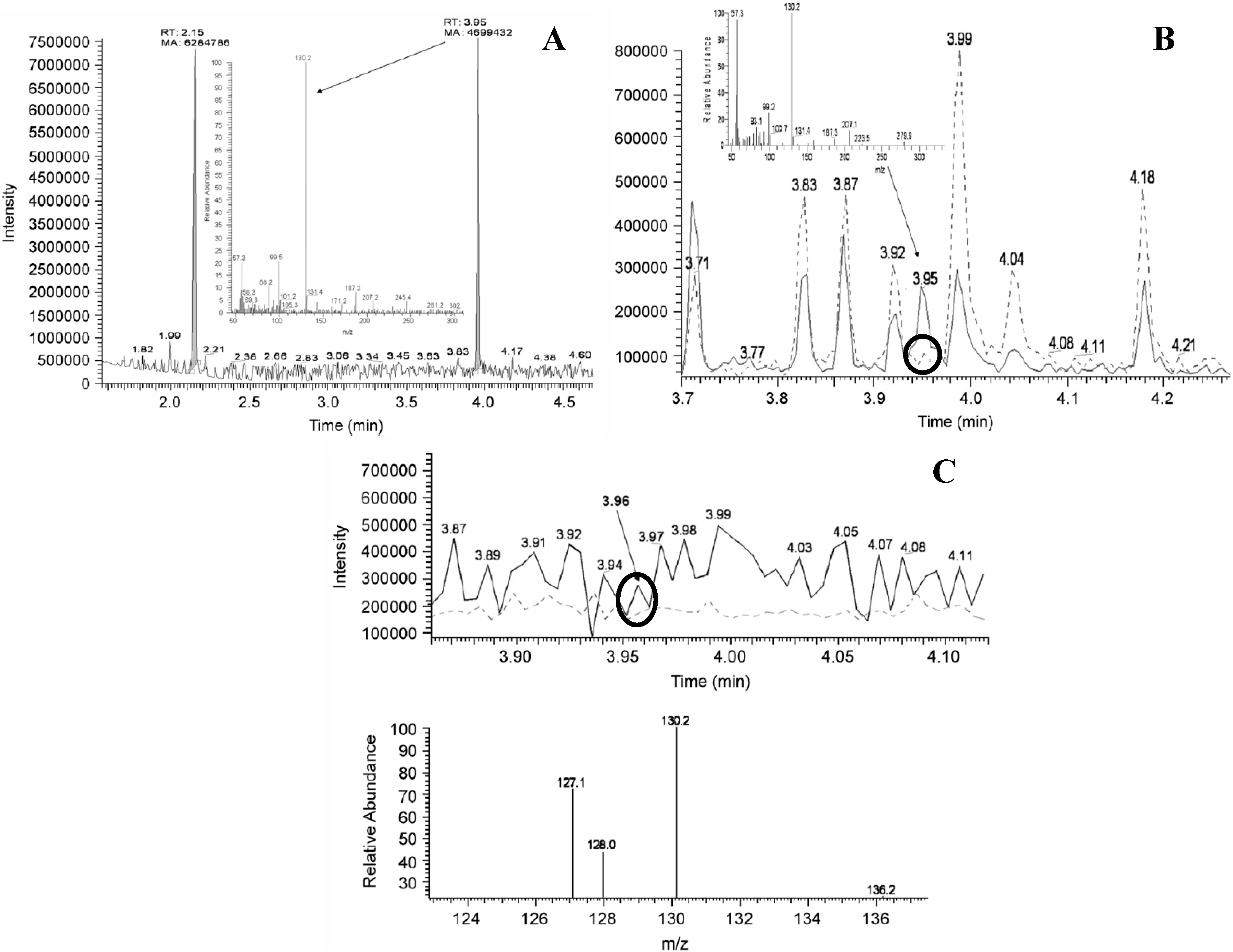
- Figure 8 in [55]: DAB not shown, no methods given but figure is identical to Figure 1 in a later published study [67]. Legend states that samples have been derivatized according to [44], but [44] is not a LC-MS but a GC-MS study in which a different derivatization procedure has been used. This is reflected by the different reported m/z for the BMAA derivative: 130.2 in [44] and 333 in this figure.
GC Separation of BMAA and DAB
Discussion
In Conclusion
Appendix information 3: Article discussion ‘Distinguishing the cyanobacterial neurotoxin β-N-methylamino-l-alanine (BMAA) from other diamino acids [56]’
Justification and Research Aim
Relevance of the Work
- Diamino acids with a different molecular weight from BMAA are not the most likely candidates to interfere in methods with mass-spectrometry detection [43]. Why are all but two tested diamino acids compounds with a different molecular weight?
Relation to Previous and Future Work
In Conclusion
Appendix information 4: Article discussion ‘Cyanobacteria and BMAA exposure from desert dust: A possible link to sporadic ALS among Gulf War veterans [60]’
Incomplete Description of Methods
Incomplete Description of Results
Incorrect Data Visualisation
In Conclusion
Appendix information 5: Article discussion ‘Reactivity of β-methylamino-l-alanine in complex sample matrixes complicating detection and quantification by mass spectrometry [57]’
Adduct and Complex Formation and the Detection of Mass-to-Charge Ratio (m/z) 119
Implications for Sample Analysis
Recommended Analytical Procedure
In Conclusion
Appendix information 6: Article discussion ‘Nitrogen starvation results in the production of β-N-methylamino-l-alanine [76]’
Flaws in Experimental Setup
Suboptimal Analysis
Presentation of Raw Data
Obscured Data Presentation
Incomplete Data Presentation
In Conclusion
Appendix information 7: Reporting quality of methods and results
| ref | method | Pre column derivatization | Sample origin | Growth conditions/moment of sampling | Storage conditions | Sample origin and conditions | Volumes and weights | Derivatization protocol | Processing repeatable | Hardware described | Method described | Method of quantification | Analysis repeatable | Cal curve/linearity | LOD/LOQ* defined | LOD/LOQ standard reported | LOD/LOQ sample reported | Precision | Recovery | Method performance | Chrom/spectrum standard | Chrom/spectrum sample | Chrom/spectrum spiked sample | BMAA identification |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [13] | LC-MS | Y | ~ | - | - | - | - | - | - | + | - | x | - | - | - | - | - | - | - | - | - | - | - | - |
| [13] | LC-FLD | Y | ~ | - | - | - | - | - | - | ~ | ~ | ~ | ~ | - | - | + | - | - | ~ | ~ | + | + | - | + |
| [24] | LC-FLD | Y | + | + | + | + | - | - | - | + | + | - | ~ | - | - | + | - | - | - | - | + | + | + | + |
| [24] | LC-MS/MS | Y | + | + | + | + | - | - | - | + | + | x | ~ | - | - | - | - | - | - | - | - | ~ | - | - |
| [14] | AA | N | + | - | - | ~ | + | x | + | + | + | - | + | - | - | - | - | - | - | - | - | + | - | ~ |
| [14] | LC-MS/MS | Y | + | - | - | ~ | - | - | - | + | ~ | ~ | ~ | - | - | - | - | - | - | - | - | + | - | ~ |
| [14] | LC-MS | Y | + | - | - | ~ | ~ | ~ | ~ | + | + | x | + | - | - | - | - | - | - | - | - | ~ | ~ | - |
| [14] | LC-UV | Y | + | - | - | ~ | + | + | + | ~ | + | ~ | + | + | - | + | - | + | - | ~ | - | ~ | - | - |
| [14] | LC-FLD | Y | + | - | - | ~ | + | + | + | + | + | + | + | - | - | - | - | - | - | - | ~ | ~ | ~ | ~ |
| [66] | LC-FLD | Y | + | + | + | + | ~ | - | - | + | ~ | + | ~ | ~ | + | ~ | ~ | - | - | ~ | - | - | ~ | - |
| [75] | LC-MS | N | ~ | - | - | - | + | x | + | + | + | + | + | + | + | + | - | - | + | + | + | - | + | + |
| [46] | LC-UV | Y | + | + | - | ~ | ~ | - | - | ~ | ~ | - | ~ | - | - | - | - | - | - | - | + | + | - | + |
| [46] | LC-MS | Y | + | + | - | ~ | ~ | - | - | + | + | x | + | - | - | - | - | - | - | - | + | + | - | + |
| [46] | LC-MS/MS | Y | + | + | - | ~ | - | - | - | + | + | x | + | - | - | - | - | - | - | - | - | ~ | - | - |
| [46] | LC-FLD | Y | + | + | - | ~ | ~ | - | - | + | + | ~ | + | - | - | + | - | - | - | ~ | + | + | - | + |
| [44] | GC-MS | Y | + | + | + | + | - | - | - | + | + | + | + | + | - | + | - | - | - | ~ | + | ~ | ~ | ~ |
| [47] | LC-MS/MS | N | ~ | - | - | - | ~ | x | + | + | + | + | + | + | + | - | + | + | - | + | + | + | + | + |
| [53] | 1H-NMR | X | ~ | - | - | - | ~ | x | ~ | ~ | + | + | + | + | + | + | - | + | + | + | + | - | + | + |
| [36] | LC-FLD | Y | ~ | - | - | - | + | + | + | + | + | x | + | + | + | + | + | - | + | + | + | + | + | + |
| [48] | LC-MS/MS | N | + | + | + | + | + | x | + | + | + | + | + | - | + | + | + | - | + | + | + | + | - | + |
| [61] | LC-MS | Y | + | - | - | - | - | - | - | ~ | - | ~ | - | - | - | + | - | - | ~ | ~ | - | - | - | - |
| [62] | LC-MS/MS | N | ~ | - | - | - | - | x | - | + | ~ | - | ~ | - | - | - | - | - | - | - | ~ | ~ | ~ | ~ |
| [62] | LC-FLD | Y | ~ | - | - | - | + | + | + | + | + | + | + | - | - | + | - | - | - | - | - | - | - | - |
| [73] | LC-MS/MS | Y | + | + | + | + | + | - | ~ | + | + | x | + | - | - | - | - | - | - | - | - | ~ | - | - |
| [63] | LC-MS/MS | Y | - | - | - | - | + | - | - | + | + | x | + | - | - | - | - | - | - | - | - | + | - | ~ |
| [60] | AA | N | - | + | - | - | - | x | - | + | + | x | + | - | - | - | - | - | - | - | - | + | - | ~ |
| [60] | LC-MS/MS | Y | - | + | - | - | - | - | - | + | + | x | + | - | - | - | - | - | - | - | ~ | ~ | - | - |
| [60] | LC-FLD | Y | - | + | - | - | - | - | - | + | ~ | x | ~ | - | - | - | - | - | - | - | + | + | - | + |
| [60] | LC-MS | Y | - | + | - | - | - | - | - | + | ~ | x | ~ | - | - | + | - | - | - | ~ | + | + | - | + |
| [87] | LC-MS/MS | N | + | + | - | ~ | ~ | x | - | + | + | x | + | + | + | + | + | + | ~ | + | ~ | ~ | - | - |
| [87] | LC-MS | N | + | + | - | ~ | ~ | x | - | + | + | x | + | + | + | + | - | + | ~ | + | + | + | - | + |
| [87] | LC-MS/MS | Y | + | + | - | ~ | ~ | - | - | + | + | x | + | - | - | - | - | - | - | - | - | - | - | - |
| [27] | LC-MS/MS | Y | + | + | + | + | ~ | + | + | + | + | + | + | - | + | + | - | + | - | ~ | + | + | - | + |
| [23] | LC-MS/MS§ | Y | + | + | + | + | ~ | + | + | + | + | + | + | - | p | p | - | p | - | p | + | + | - | + |
| [28] | LC-FLD | Y | + | + | ~ | + | + | - | ~ | ~ | + | + | + | + | - | - | + | - | + | ~ | - | + | + | + |
| [28] | LC-MS/MS | Y | + | + | ~ | + | - | - | - | + | ~ | x | ~ | - | - | - | - | - | - | - | ~ | ~ | - | - |
| [49] | LC-MS/MS | N | + | + | + | + | + | x | + | + | + | + | + | - | + | - | + | - | + | ~ | + | + | - | + |
| [45] | LC-MS | Y | + | + | + | + | - | - | - | + | + | ~ | + | + | + | + | - | + | - | + | + | - | - | ~ |
| [76] | LC-MS/MS | Y | + | + | + | + | - | - | - | + | + | x | + | - | - | - | - | - | - | - | - | + | - | ~ |
| [42] | LC-FLD | Y | + | + | ~ | + | ~ | + | + | + | ~ | - | ~ | - | + | + | - | - | - | ~ | ~ | - | - | - |
| [50] | CE-UV | X | + | + | ~ | + | ~ | x | + | + | + | + | + | + | + | + | - | + | + | + | + | - | ~ | ~ |
| [67] | LC-MS | Y | - | - | + | - | ~ | - | - | + | + | ~ | + | - | - | - | - | - | + | ~ | - | ~ | - | - |
| [35] | LC-FLD^ | Y | + | + | + | + | + | + | + | + | + | x | + | - | + | - | + | - | + | + | p | p | p | p |
| [71] | LC-MS/MS | N | + | + | + | + | + | x | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| [69] | LC-FLD | Y | + | - | - | - | + | + | + | + | - | - | - | + | + | + | - | ~ | + | + | + | + | - | + |
| [30] | LC-FLD | Y | + | ~ | + | + | - | - | - | ~ | + | ~ | ~ | - | - | + | - | - | ~ | ~ | + | + | - | + |
| [30] | LC-MS/MS | Y | + | ~ | + | + | - | - | - | - | + | x | ~ | - | - | - | - | - | - | - | ~ | ~ | - | - |
| [31] | LC-MS/MS | Y | ~ | - | + | - | ~ | - | - | + | + | x | + | - | + | - | + | + | - | ~ | + | + | + | + |
| [43] | LC-MS/MS | N | + | + | + | + | + | x | + | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + |
| [43] | LC-MS/MS | Y | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + |
| [43] | LC-FLD | Y | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| [32] | LC-MS/MS | Y | + | - | - | - | ~ | + | ~ | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| [29] | LC-MS/MS | Y | ~ | + | + | ~ | - | - | - | ~ | ~ | - | ~ | - | - | + | + | - | + | ~ | ~ | ~ | + | ~ |
| [68] | LC-MS/MS | Y | + | + | - | + | ~ | - | - | + | + | + | + | + | + | + | + | + | + | + | + | + | - | + |
| [72] | LC-MS/MS | N | + | + | + | + | + | x | + | + | + | + | + | + | + | + | + | + | + | + | + | - | ~ | ~ |
| [54] | ELISA | X | + | + | + | + | + | x | + | + | + | + | + | + | + | + | + | - | + | + | x | x | x | x |
| [54] | LC-MS/MS# | N | + | + | + | + | + | x | + | + | + | + | + | p | p | p | p | p | p | p | p | p | p | p |
| [33] | LC-MS/MS | Y | + | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| [34] | LC-MS/MS | Y | + | - | + | ~ | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| [74] | LC-MS/MS§ | Y | + | + | - | ~ | p | p | p | + | ~ | x | + | - | - | - | - | - | - | - | + | + | - | + |
| [51] | LC-MS/MS | Y | + | + | ~ | + | ~ | ~ | ~ | + | ~ | - | ~ | - | - | - | - | - | + | ~ | - | - | - | - |
| [92] | LC-FLD | Y | + | ~ | + | + | + | + | + | + | + | + | + | + | + | - | + | - | - | ~ | - | - | + | ~ |
| [92] | LC-MS/MS | Y | + | ~ | + | + | + | + | + | + | + | - | ~ | + | + | - | + | - | - | ~ | - | - | + | ~ |
| [25] | LC-MS/MS | Y | + | + | + | + | ~ | - | ~ | + | ~ | + | ~ | + | - | - | - | - | - | - | + | + | - | + |
| [26] | LC-MS/MS% | Y | ~ | p | p | + | p | p | p | p | p | p | p | p | p | p | p | p | p | p | + | ~ | - | ~ |
Appendix information 8: Criteria used for classification in Figure 4
| Group* | Positive results for BMAA reported | At least one highly selective method used | Sensitive method used | Identification correctly reported | Quantification correctly reported | References |
|---|---|---|---|---|---|---|
| 1 | + | + | + | + | + | [25,32,34,48,68] |
| 2 | + | + | + | + | - | [23,27,31,74] |
| 3 | - | n.a. | + | + | n.a. | [35,36,43,47,49,71,75] |
| 4 | - | + | - | + | n.a. | [53] |
| 5 | + | - | n.a. | n.a. | n.a. | [13,42,44,45,50,54,61,66,67,69] |
| 6 | n.a. | + | n.a. | - | n.a. | [14,24,26,28,29,30,33,46,51,60,62,63,72,73,76,87,92] |
References
- Chiu, A.S.; Gehringer, M.M.; Welch, J.H.; Neilan, B.A. Does α-amino-β-methylaminopropionic acid (BMAA) play a role in neurodegeneration? Int. J. Environ. Res. Public Health 2011, 8, 3728–3746. [Google Scholar] [CrossRef]
- Bradley, W.G.; Mash, D.C. Beyond Guam: The cyanobacteria/BMAA hypothesis of the cause of ALS and other neurodegenerative diseases. Amyotroph. Lat. Scler. 2009, 10, 7–20. [Google Scholar]
- Pablo, J.; Banack, S.A.; Cox, P.A.; Johnson, T.E.; Papapetropoulos, S.; Bradley, W.G.; Buck, A.; Mash, D.C. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer’s disease. Acta Neurol. Scand. 2009, 120, 216–225. [Google Scholar] [CrossRef]
- Spencer, P.S.; Nunn, P.B.; Hugon, J.; Ludolph, A.C.; Ross, S.M.; Roy, D.N.; Robertson, R.C. Guam amyotrophic lateral sclerosis-Parkinsonism-dementia linked to a plant excitant neurotoxin. Science 1987, 237, 517–522. [Google Scholar]
- Vega, A.; Bell, E.A. α-amino-β-methylaminopropionic acid, a new amino acid from seeds of Cycas circinalis. Phytochemistry 1967, 6, 759–762. [Google Scholar] [CrossRef]
- Whiting, M.G. Toxicity of cycads. Econ. Bot. 1963, 17, 271–302. [Google Scholar] [CrossRef]
- Duncan, M.W.; Kopin, I.J.; Garruto, R.M.; Lavine, L.; Markey, S.P. 2-Amino-3 (methylamino)-propionic acid in cycad-derived foods is an unlikely cause of amyotrophic lateral sclerosis/parkinsonism. Lancet 1988, 332, 631–632. [Google Scholar]
- Wilson, J.; Shaw, C.A. Commentary on: Return of the cycad hypothesis—Does the amyotrophic lateral sclerosis/parkinsonism dementia complex (ALS/PDC) of Guam have new implications for global health? Neuropathol. Appl. Neurobiol. 2006, 32, 341–343. [Google Scholar] [CrossRef]
- Montine, T.J.; Li, K.; Perl, D.P.; Galasko, D. Lack of β-methylamino-l-alanine in brain from controls, AD, or Chamorros with PDC. Neurology 2005, 65, 768–769. [Google Scholar] [CrossRef]
- Kisby, G.E.; Spencer, P.S. Is neurodegenerative disease a long-latency response to early-life genotoxin exposure? Int. J. Env. Res. Public Health 2011, 8, 3889–3921. [Google Scholar] [CrossRef]
- Banack, S.A.; Cox, P.A. Distribution of the neurotoxic nonprotein amino acid BMAA in Cycas micronesica. Bot. J. Linn. Soc. 2003, 143, 165–168. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce β-N-methylamino-l-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef]
- Banack, S.A.; Johnson, H.E.; Cheng, R.; Cox, P.A. Production of the neurotoxin BMAA by a marine cyanobacterium. Mar. Drugs 2007, 5, 180–196. [Google Scholar] [CrossRef]
- Karamyan, V.T.; Speth, R.C. Animal models of BMAA neurotoxicity: a critical review. Life Sci. 2008, 82, 233–246. [Google Scholar] [CrossRef]
- Banack, S.A.; Caller, T.A.; Stommel, E.W. The cyanobacteria derived toxin beta-N-methylamino-l-alanine and amyotrophic lateral sclerosis. Toxins 2010, 2, 2837–2850. [Google Scholar] [CrossRef]
- Okle, O.; Stemmer, K.; Deschl, U.; Dietrich, D.R. L-BMAA induced ER stress and enhanced caspase 12 cleavage in human neuroblastoma SH-SY5Y cells at low nonexcitotoxic concentrations. Toxicol. Sci. 2013, 131, 217–224. [Google Scholar] [CrossRef]
- Murch, S.J.; Cox, P.A.; Banack, S.A.; Steele, J.C.; Sacks, O.W. Occurrence of β-methylamino-l-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol. Scand. 2004, 110, 267–269. [Google Scholar] [CrossRef]
- Murch, S.J.; Cox, P.A.; Banack, S.A. A mechanism for slow release of biomagnified cyanobacterial neurotoxins and neurodegenerative disease in Guam. Proc. Natl. Acad. Sci. USA 2004, 101, 12228–12231. [Google Scholar] [CrossRef]
- Snyder, L.R.; Cruz-Aguado, R.; Sadilek, M.; Galasko, D.; Shaw, C.A.; Montine, T.J. Lack of cerebral BMAA in human cerebral cortex. Neurology 2009, 72, 1360–1361. [Google Scholar]
- Snyder, L.R.; Hoggard, J.C.; Montine, T.J.; Synovec, R.E. Development and application of a comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry method for the analysis of L-β-methylamino-alanine in human tissue. J. Chromatogr. A 2010, 1217, 4639–4647. [Google Scholar] [CrossRef]
- Duncan, M.W. Good mass spectrometry and its place in good science. J. Mass Spectrom. 2012, 47, 795–809. [Google Scholar] [CrossRef]
- Jonasson, S.; Eriksson, J.; Berntzon, L.; Spáčil, Z.; Ilag, L.L.; Ronnevi, L.O.; Rasmussen, U.; Bergman, B. Transfer of a cyanobacterial neurotoxin within a temperate aquatic ecosystem suggests pathways for human exposure. Proc. Natl. Acad. Sci. USA 2010, 107, 9252–9257. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Banack, S.A.; Lindsay, J.; Morrison, L.F.; Cox, P.A.; Codd, G.A. Co-occurrence of β-N-methylamino-l-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British waterbodies, 1990–2004. Environ. Microbiol. 2008, 10, 702–708. [Google Scholar] [CrossRef]
- Jiang, L.; Eriksson, J.; Lage, S.; Jonasson, S.; Shams, S.; Mehine, M.; Ilag, L.L.; Rasmussen, U. Diatoms: A novel source for the neurotoxin BMAA in aquatic environments. PLoS One 2014, 9, e84578. [Google Scholar]
- Jiang, L.; Ilag, L.L. Detection of endogenous BMAA in dinoflagellate (Heterocapsa triquetra) hints at evolutionary conservation and environmental concern. Pubraw Sci. 2014, 2, 1–8. [Google Scholar]
- Spáčil, Z.; Eriksson, J.; Jonasson, S.; Rasmussen, U.; Ilag, L.L.; Bergman, B. Analytical protocol for identification of BMAA and DAB in biological samples. Analyst 2010, 135, 127–132. [Google Scholar] [CrossRef]
- Brand, L.E.; Pablo, J.; Compton, A.; Hammerschlag, N.; Mash, D.C. Cyanobacterial blooms and the occurrence of the neurotoxin, beta-N-methylamino-l-alanine (BMAA), in South Florida aquatic food webs. Harmful Algae 2010, 9, 620–635. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Banack, S.A.; Kotut, K.; Krienitz, L.; Codd, G.A. Amino acid neurotoxins in feathers of the Lesser Flamingo. Phoeniconaias Minor. Chemosphere 2013, 90, 835–839. [Google Scholar]
- Mondo, K.; Hammerschlag, N.; Basile, M.; Pablo, J.; Banack, S.A.; Mash, D.C. Cyanobacterial neurotoxin β-N-methylamino-l-alanine (BMAA) in Shark Fins. Mar. Drugs 2012, 10, 509–520. [Google Scholar] [CrossRef]
- Jiang, L.; Aigret, B.; De Borggraeve, W.M.; Spacil, Z.; Ilag, L.L. Selective LC-MS/MS method for the identification of BMAA from its isomers in biological samples. Anal. Bioanal. Chem. 2012, 403, 1719–1730. [Google Scholar] [CrossRef]
- Christensen, S.J.; Hemscheidt, T.K.; Trapido-Rosenthal, H.; Laws, E.A.; Bidigare, R.R. Detection and quantification of β-methylamino-l-alanine in aquatic invertebrates. Limnol. Oceanogr. Methods 2012, 10, 891–898. [Google Scholar] [CrossRef]
- Field, N.C.; Metcalf, J.S.; Caller, T.A.; Banack, S.A.; Cox, P.A.; Stommel, E.W. Linking β-methylamino-l-alanine exposure to sporadic amyotrophic lateral sclerosis in Annapolis, MD. Toxicon 2013, 70, 179–183. [Google Scholar] [CrossRef]
- Lampinen Salomonsson, M.; Hansson, A.; Bondesson, U. Development and in-house validation of a method for quantification of BMAA in mussels using dansyl chloride derivatization and ultra performance liquid chromatography tandem mass spectrometry. Anal. Methods 2013, 5, 4865–4874. [Google Scholar] [CrossRef]
- Niedzwiadek, B.; Scott, P.M.; Lau, B.P.Y. Monitoring of shrimp and farmed fish sold in Canada for cyanobacterial toxins. J. Food Prot. 2012, 75, 160–163. [Google Scholar] [CrossRef]
- Scott, P.M.; Niedzwiadek, B.; Rawnben, D.F.K.; Lau, P.Y. Liquid chromatographic determination of the cyanobacterial toxin β-N-methylamino-l-alanine in algae food supplements, freshwater fish, and bottled water. J. Food Prot. 2009, 72, 1769–1773. [Google Scholar]
- Van De Waal, D.B.; Verspagen, J.M.H.; Lürling, M.; Van Donk, E.; Visser, P.M.; Huisman, J. The ecological stoichiometry of toxins produced by harmful cyanobacteria: An experimental test of the carbon-nutrient balance hypothesis. Ecol. Lett. 2009, 12, 1326–1335. [Google Scholar] [CrossRef]
- Sivonen, K.; Jones, G.J. Cyanobacterial Toxins. In Toxic Cyanobacteria in Water: A Guide to their Public Health Consequences, Monitoring and Management; Chorus, I., Bartram, J., Eds.; E&FN Spon: London, UK, 1999; pp. 41–111. [Google Scholar]
- Lürling, M.; Faassen, E.J. Dog poisonings associated with a Microcystis aeruginosa bloom in The Netherlands. Toxins 2013, 5, 556–567. [Google Scholar] [CrossRef]
- Faassen, E.J.; Lürling, M. Occurrence of the microcystins MC-LW and MC-LF in Dutch surface waters and their contribution to total microcystin toxicity. Mar. Drugs 2013, 11, 2643–2654. [Google Scholar] [CrossRef]
- Messineo, V.; Bogialli, S.; Melchiorre, S.; Sechi, N.; Lugliè, A.; Casiddu, P.; Mariani, M.A.; Padedda, B.M.; Corcia, A.D.; Mazza, R.; et al. Cyanobacterial toxins in Italian freshwaters. Limnologica 2009, 39, 95–106. [Google Scholar] [CrossRef]
- Cervantes Cianca, R.C.; Baptista, M.S.; Lopes, V.R.; Vasconcelos, V.M. The non-protein amino acid β-N-methylamino-l-alanine in Portuguese cyanobacterial isolates. Amino Acids 2012, 42, 2473–2479. [Google Scholar] [CrossRef]
- Faassen, E.J.; Gillissen, F.; Lürling, M. A comparative study on three analytical methods for the determination of the neurotoxin BMAA in cyanobacteria. PLoS One 2012, 7, e36667. [Google Scholar]
- Esterhuizen, M.; Downing, T.G. β-N-Methylamino-l-alanine (BMAA) in novel South African cyanobacterial isolates. Ecotoxicol. Environ. Saf. 2008, 71, 309–313. [Google Scholar] [CrossRef]
- Esterhuizen-Londt, M.; Downing, S.; Downing, T.G. Improved sensitivity using liquid chromatography mass spectrometry (LC-MS) for detection of propyl chloroformate derivatised β-N-methylamino-l-alanine (BMAA) in cyanobacteria. Water SA 2011, 37, 133–138. [Google Scholar]
- Johnson, H.E.; King, S.R.; Banack, S.A.; Webster, C.; Callanaupa, W.J.; Cox, P.A. Cyanobacteria (Nostoc commune) used as a dietary item in the Peruvian highlands produce the neurotoxic amino acid BMAA. J. Ethnopharmacol. 2008, 118, 159–165. [Google Scholar] [CrossRef]
- Rosén, J.; Hellenäs, K.E. Determination of the neurotoxin BMAA (β-N-methylamino-l-alanine) in cycad seed and cyanobacteria by LC-MS/MS (Liquid Chromatography Tandem Mass Spectrometry). Analyst 2008, 133, 1785–1789. [Google Scholar] [CrossRef]
- Faassen, E.J.; Gillissen, F.; Zweers, H.A.J.; Lürling, M. Determination of the neurotoxins BMAA (β-N-methylamino-l-alanine) and DAB (α-,γ-diaminobutyric acid) by LC-MSMS in Dutch urban waters with cyanobacterial blooms. Amyotroph. Lat. Scler. 2009, 10, 79–84. [Google Scholar]
- Krüger, T.; Mönch, B.; Oppenhäuser, S.; Luckas, B. LC-MS/MS determination of the isomeric neurotoxins BMAA (β-N-methylamino-l-alanine) and DAB (2,4-diaminobutyric acid) in cyanobacteria and seeds of Cycas revoluta and Lathyrus latifolius. Toxicon 2010, 55, 547–557. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cianca, R.C.C.; Lopes, V.R.; Almeida, C.M.R.; Vasconcelos, V.M. Determination of the non protein amino acid β-N-methylamino-l-alanine in estuarine cyanobacteria by capillary electrophoresis. Toxicon 2011, 58, 410–414. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, Q.; Chen, X.; Wang, X.; Liao, X.; Jiang, L.; Wu, J.; Yang, L. Occurrence and transfer of a cyanobacterial neurotoxin β-methylamino-l-alanine within the aquatic food webs of Gonghu Bay (Lake Taihu, China) to evaluate the potential human health risk. Sci. Total Environ. 2014, 468–469, 457–463. [Google Scholar]
- Cohen, S.A. Analytical techniques for the detection of α-amino-β-methylaminopropionic acid. Analyst 2012, 137, 1991–2005. [Google Scholar] [CrossRef]
- Moura, S.; Ultramari, M.A.; de Paula, D.M.L.; Yonamine, M.; Pinto, E. 1H NMR determination of β-N-methylamino-l-alanine (L-BMAA) in environmental and biological samples. Toxicon 2009, 53, 578–583. [Google Scholar] [CrossRef]
- Faassen, E.J.; Beekman, W.; Lürling, M. Evaluation of a commercial enzyme linked immunosorbent assay (ELISA) for the determination of the neurotoxin BMAA in surface waters. PLoS One 2013, 8, e65260. [Google Scholar] [CrossRef]
- Banack, S.A.; Downing, T.G.; Spácil, Z.; Purdie, E.L.; Metcalf, J.S.; Downing, S.; Esterhuizen, M.; Codd, G.A.; Cox, P.A. Distinguishing the cyanobacterial neurotoxin β-N-methylamino-l-alanine (BMAA) from its structural isomer 2,4-diaminobutyric acid (2,4-DAB). Toxicon 2010, 56, 868–879. [Google Scholar] [CrossRef]
- Banack, S.A.; Metcalf, J.S.; Spáčil, Z.; Downing, T.G.; Downing, S.; Long, A.; Nunn, P.B.; Cox, P.A. Distinguishing the cyanobacterial neurotoxin β-N-methylamino-l-alanine (BMAA) from other diamino acids. Toxicon 2011, 57, 730–738. [Google Scholar] [CrossRef]
- Glover, W.B.; Liberto, C.M.; McNeil, W.S.; Banack, S.A.; Shipley, P.R.; Murch, S.J. Reactivity of β-methylamino-l-alanine in complex sample matrixes complicating detection and quantification by mass spectrometry. Anal. Chem. 2012, 84, 7946–7953. [Google Scholar]
- Kebarle, P.; Tang, L. From ions in solution to ions in the gas phase: The mechanism of electrospray mass spectrometry. Anal. Chem. 1993, 65, 972–986. [Google Scholar]
- SANCO. Method Validation and Quality Control Procedures for Pesticide Residues Analysis in Food and Feed. European Commission: Strasbourg, France, 2011. [Google Scholar]
- Cox, P.A.; Richer, R.; Metcalf, J.S.; Banack, S.A.; Codd, G.A.; Bradley, W.G. Cyanobacteria and BMAA exposure from desert dust: A possible link to sporadic ALS among Gulf War veterans. Amyotroph. Lat. Scler. 2009, 10, 109–117. [Google Scholar] [CrossRef]
- Roney, B.R.; Renhui, L.; Banack, S.A.; Murch, S.; Honegger, R.; Cox, P.A. Consumption of fa cai Nostoc soup: A Potential for BMAA exposure from Nostoc cyanobacteria in China? Amyotroph. Lat. Scler. 2009, 10, 44–49. [Google Scholar] [CrossRef]
- Bidigare, R.R.; Christensen, S.J.; Wilde, S.B.; Banack, S.A. Cyanobacteria and BMAA: Possible linkage with avian vacuolar myelinopathy (AVM) in the south-eastern United States. Amyotroph. Lat. Scler. 2009, 10, 71–73. [Google Scholar] [CrossRef]
- Craighead, D.; Metcalf, J.S.; Banack, S.A.; Amgalan, L.; Reynolds, H.V.; Batmunkh, M. Presence of the neurotoxic amino acids β-N-methylamino-l-alanine (BMAA) and 2,4-diamino-butyric acid (DAB) in shallow springs from the Gobi Desert. Amyotroph. Lat. Scler. 2009, 10, 96–100. [Google Scholar] [CrossRef]
- Canizares-Villanueva, R.O.; Dominguez, A.R.; Cruz, M.S.; Rios-Leal, E. Chemical composition of cyanobacteria grown in diluted, aerated swine wastewater. Bioresour. Technol. 1995, 51, 111–116. [Google Scholar] [CrossRef]
- Muro-Pastor, M.I.; Reyes, J.C.; Florencio, F.J. Cyanobacteria perceive nitrogen status by sensing intracellular 2-oxoglutarate levels. J. Biol. Chem. 2001, 276, 38320–38328. [Google Scholar]
- Eriksson, J.; Jonasson, S.; Papaefthimiou, D.; Rasmussen, U.; Bergman, B. Improving derivatization efficiency of BMAA utilizing AccQ-Tag® in a complex cyanobacterial matrix. Amino Acids 2009, 36, 43–48. [Google Scholar] [CrossRef]
- Esterhuizen-Londt, M.; Downing, T.G. Solid phase extraction of β-N-methylamino-l-alanine (BMAA) from South African water supplies. Water SA 2011, 37, 523–528. [Google Scholar]
- Jiang, L.; Johnston, E.; Åberg, K.M.; Nilsson, U.; Ilag, L.L. Strategy for quantifying trace levels of BMAA in cyanobacteria by LC/MS/MS. Anal. Bioanal. Chem. 2013. [Google Scholar] [CrossRef]
- Cervantes Cianca, R.C.; Baptista, M.S.; Da Silva, L.P.; Lopes, V.R.; Vasconcelos, V.M. Reversed-phase HPLC/FD method for the quantitative analysis of the neurotoxin BMAA (β-N-methylamino-l-alanine) in cyanobacteria. Toxicon 2012, 59, 379–384. [Google Scholar] [CrossRef]
- ICH. Text on Validation of Analytical Procedures Q2A; US Food and Drug Administration: Agoda, MD, USA, 1994.
- Li, A.; Fan, H.; Ma, F.; McCarron, P.; Thomas, K.; Tang, X.; Quilliam, M.A. Elucidation of matrix effects and performance of solid-phase extraction for LC-MS/MS analysis of β-N-methylamino-l-alanine (BMAA) and 2,4-diaminobutyric acid (DAB) neurotoxins in cyanobacteria. Analyst 2012, 137, 1210–1219. [Google Scholar] [CrossRef]
- Combes, A.; El Abdellaoui, S.; Sarazin, C.; Vial, J.; Mejean, A.; Ploux, O.; Pichon, V. Validation of the analytical procedure for the determination of the neurotoxin β-N-methylamino-l-alanine in complex environmental samples. Anal. Chim. Acta 2013, 771, 42–49. [Google Scholar] [CrossRef]
- Caller, T.A.; Doolin, J.W.; Haney, J.F.; Murby, A.J.; West, K.G.; Farrar, H.E.; Ball, A.; Harris, B.T.; Stommel, E.W. A cluster of amyotrophic lateral sclerosis in New Hampshire: A possible role for toxic cyanobacteria blooms. Amyotroph. Lat. Scler. 2009, 10, 101–108. [Google Scholar] [CrossRef]
- Berntzon, L.; Erasmie, S.; Celepli, N.; Eriksson, J.; Rasmussen, U.; Bergman, B. BMAA inhibits nitrogen fixation in the cyanobacterium Nostoc sp. PCC 7120. Mar. Drugs 2013, 11, 3091–3108. [Google Scholar] [CrossRef]
- Kubo, T.; Kato, N.; Hosoya, K.; Kaya, K. Effective determination method for a cyanobacterial neurotoxin, β-N-methylamino-l-alanine. Toxicon 2008, 51, 1264–1268. [Google Scholar] [CrossRef]
- Downing, S.; Banack, S.A.; Metcalf, J.S.; Cox, P.A.; Downing, T.G. Nitrogen starvation of cyanobacteria results in the production of β-N-methylamino-l-alanine. Toxicon 2011, 58, 187–194. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques; Elsevier Academic Press: Burlington, VT, USA, 2005. [Google Scholar]
- Rippka, R. Isolation and purification of cyanobacteria. Methods Enzymol. 1988, 167, 3–27. [Google Scholar] [CrossRef]
- Marler, T.E.; Snyder, L.R.; Shaw, C.A. Cycas micronesica (Cycadales) plants devoid of endophytic cyanobacteria increase in β-methylamino-l-alanine. Toxicon 2010, 56, 563–568. [Google Scholar] [CrossRef]
- Banack, S.A.; Cox, P.A. Biomagnification of cycad neurotoxins in flying foxes: Implications for ALS-PDC in Guam. Neurology 2003, 61, 387–389. [Google Scholar] [CrossRef]
- Purdie, E.L.; Metcalf, J.S.; Kashmiri, S.; Codd, G.A. Toxicity of the cyanobacterial neurotoxin β-N-methylamino-l-alanine to three aquatic animal species. Amyotrophic Lat. Scler. 2009, 10, 67–70. [Google Scholar]
- Purdie, E.L.; Samsudin, S.; Eddy, F.B.; Codd, G.A. Effects of the cyanobacterial neurotoxin β-N-methylamino-l-alanine on the early-life stage development of zebrafish (Danio rerio). Aquat. Toxicol. 2009, 95, 279–284. [Google Scholar] [CrossRef]
- Snyder, L.R.; Cruz-Aguado, R.; Sadilek, M.; Galasko, D.; Shaw, C.A.; Montine, T.J. Parkinson-dementia complex and development of a new stable isotope dilution assay for BMAA detection in tissue. Toxicol. Appl. Pharmacol. 2009, 240, 180–188. [Google Scholar] [CrossRef]
- Nozal, M.J.; Bernal, J.L.; Toribio, M.L.; Diego, J.C.; Ruiz, A. Rapid and sensitive method for determining free amino acids in honey by gas chromatography with flame ionization or mass spectrometric detection. J. Chromatogr. A 2004, 1047, 137–146. [Google Scholar] [CrossRef]
- Cohen, S.A.; Michaud, D.P. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-droxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal. Biochem. 1993, 211, 279–287. [Google Scholar] [CrossRef]
- Cheng, R.; Banack, S.A. Previous studies underestimate BMAA concentrations in cycad flour. Amyotrophic Lat. Scler. 2009, 10, 41–43. [Google Scholar] [CrossRef]
- Li, A.; Tian, Z.; Li, J.; Yu, R.; Banack, S.A.; Wang, Z. Detection of the neurotoxin BMAA within cyanobacteria isolated from freshwater in China. Toxicon 2010, 55, 947–953. [Google Scholar] [CrossRef]
- Nunn, P.B.; O'Brien, P.; Pettit, L.D.; Pyburn, S.I. Complexes of zinc, copper, and nickel with the nonprotein amino acid L-α-amino-β-methylaminopropionic acid: A naturally occurring neurotoxin. J. Inorg. Biochem. 1989, 37, 175–183. [Google Scholar] [CrossRef]
- Bruland, K.W.; Donat, J.R.; Hutchins, D.A. Interactive influences of bioactive trace metals on biological production in Oceanic waters. Limnol. Oceanogr. 1991, 36, 1555–1577. [Google Scholar] [CrossRef]
- Dortch, Q. The interaction between ammonium and nitrate uptake in phytoplankton. Mar. Ecol. Prog. Ser. 1990, 61, 183–201. [Google Scholar] [CrossRef]
- Orr, P.T.; Jones, G.J. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol. Oceanogr. 1998, 43, 1604–1614. [Google Scholar] [CrossRef]
- Al-Sammak, M.A.; Hoagland, K.D.; Snow, D.D.; Cassada, D. Methods for simultaneous detection of the cyanotoxins BMAA, DABA, and anatoxin- A in environmental samples. Toxicon 2013, 76, 316–325. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Faassen, E.J. Presence of the Neurotoxin BMAA in Aquatic Ecosystems: What Do We Really Know? Toxins 2014, 6, 1109-1138. https://doi.org/10.3390/toxins6031109
Faassen EJ. Presence of the Neurotoxin BMAA in Aquatic Ecosystems: What Do We Really Know? Toxins. 2014; 6(3):1109-1138. https://doi.org/10.3390/toxins6031109
Chicago/Turabian StyleFaassen, Elisabeth J. 2014. "Presence of the Neurotoxin BMAA in Aquatic Ecosystems: What Do We Really Know?" Toxins 6, no. 3: 1109-1138. https://doi.org/10.3390/toxins6031109
APA StyleFaassen, E. J. (2014). Presence of the Neurotoxin BMAA in Aquatic Ecosystems: What Do We Really Know? Toxins, 6(3), 1109-1138. https://doi.org/10.3390/toxins6031109






