Surface Plasmon Resonance Biosensor Method for Palytoxin Detection Based on Na+,K+-ATPase Affinity
Abstract
:1. Introduction
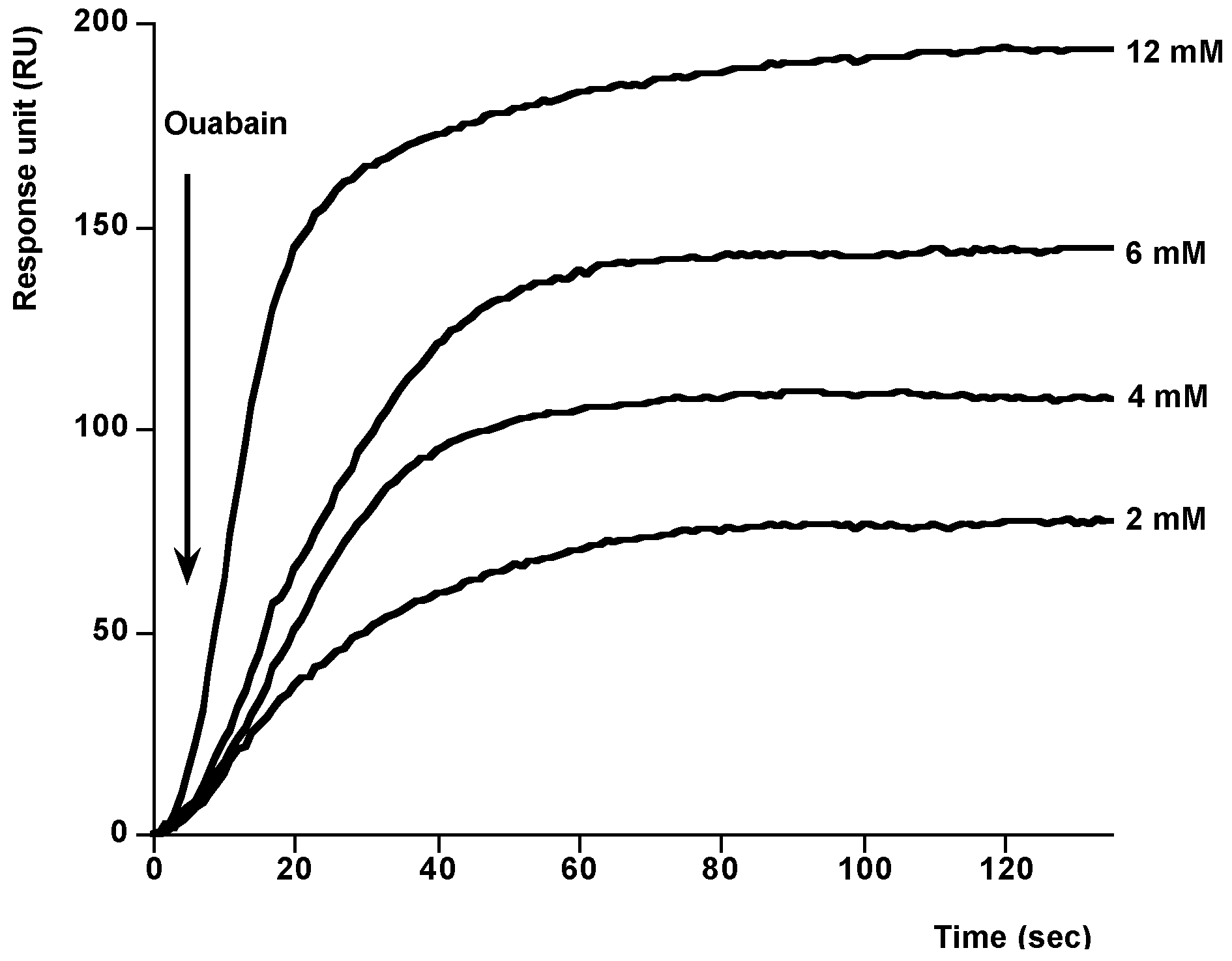
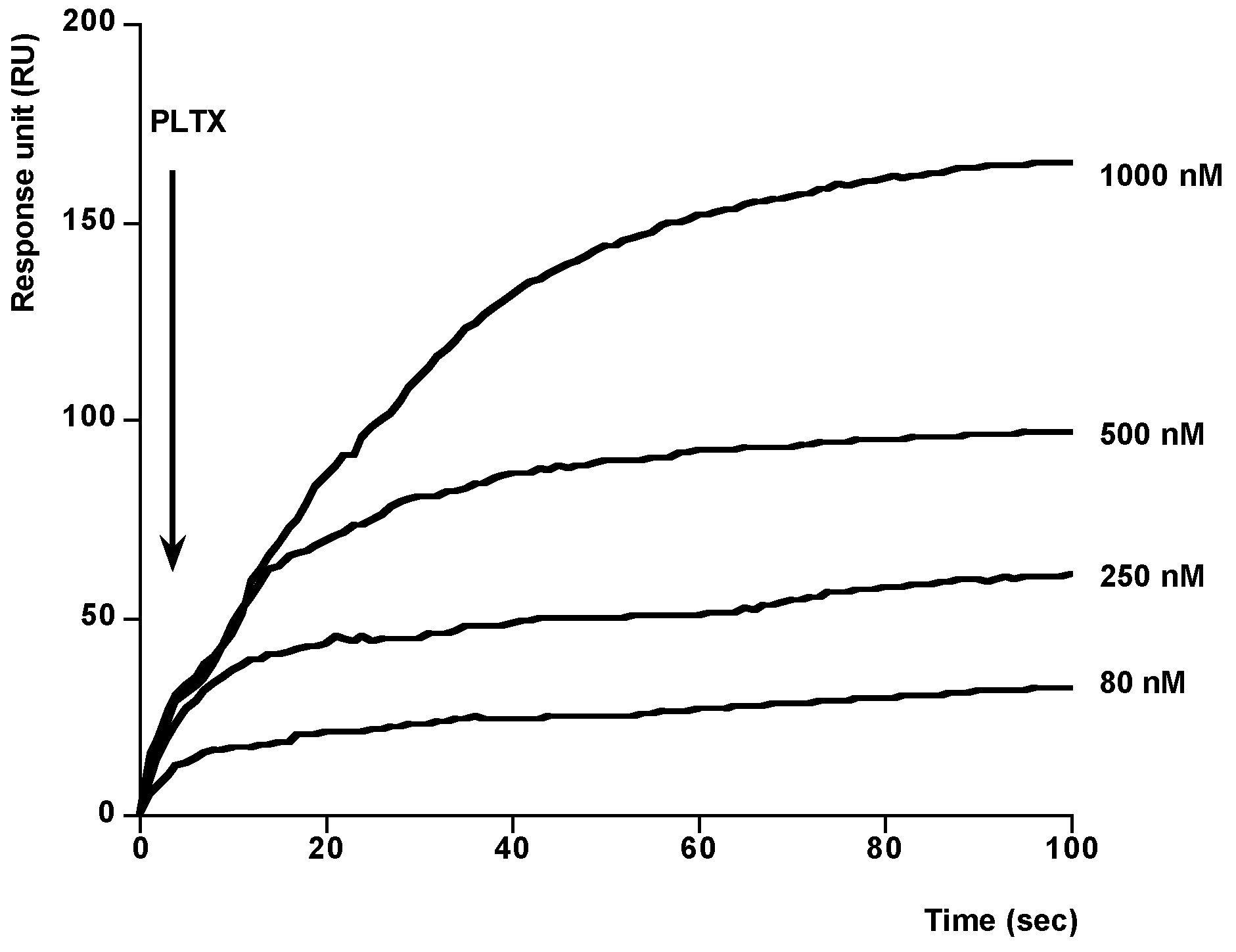
2. Results and Discussion
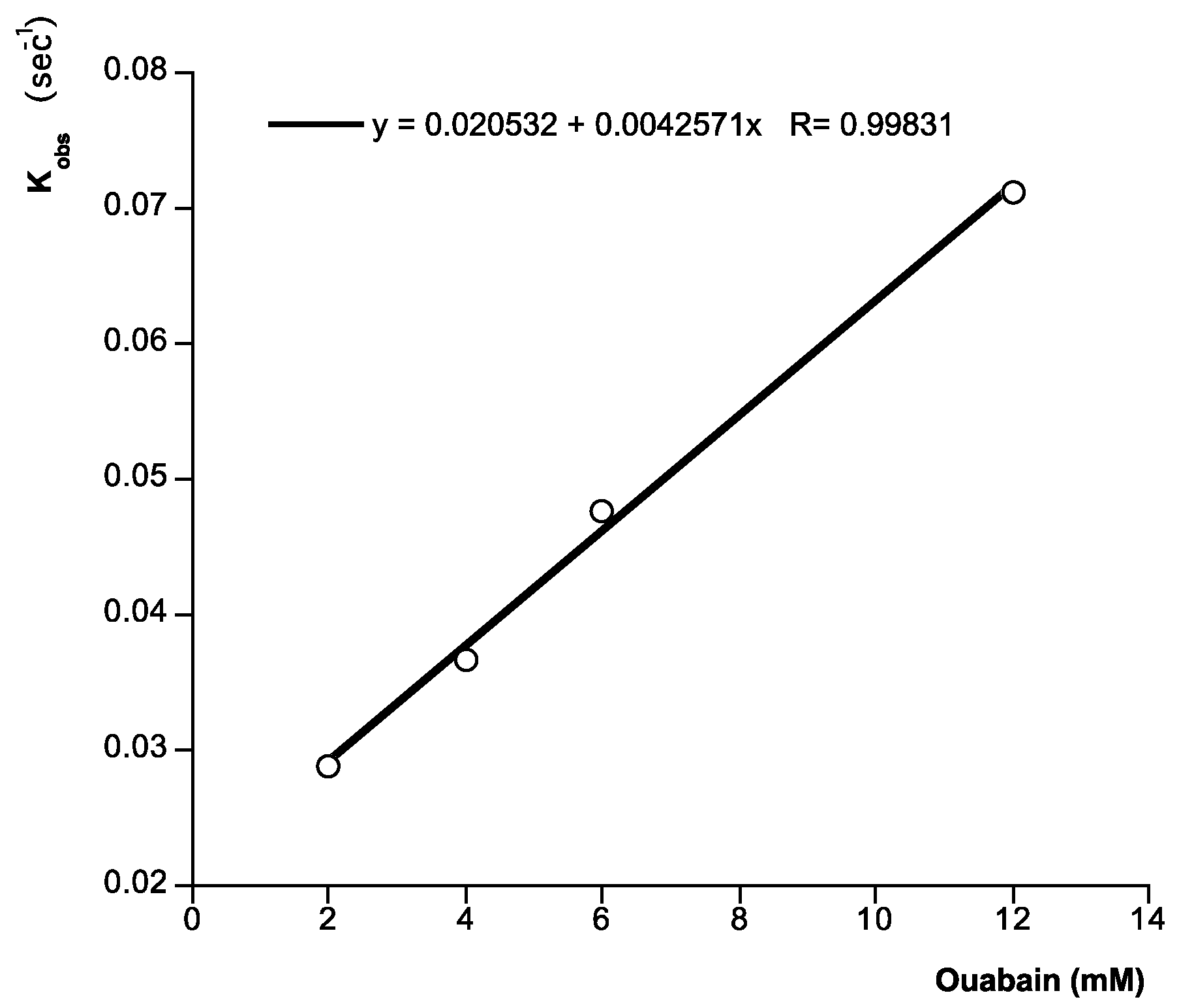
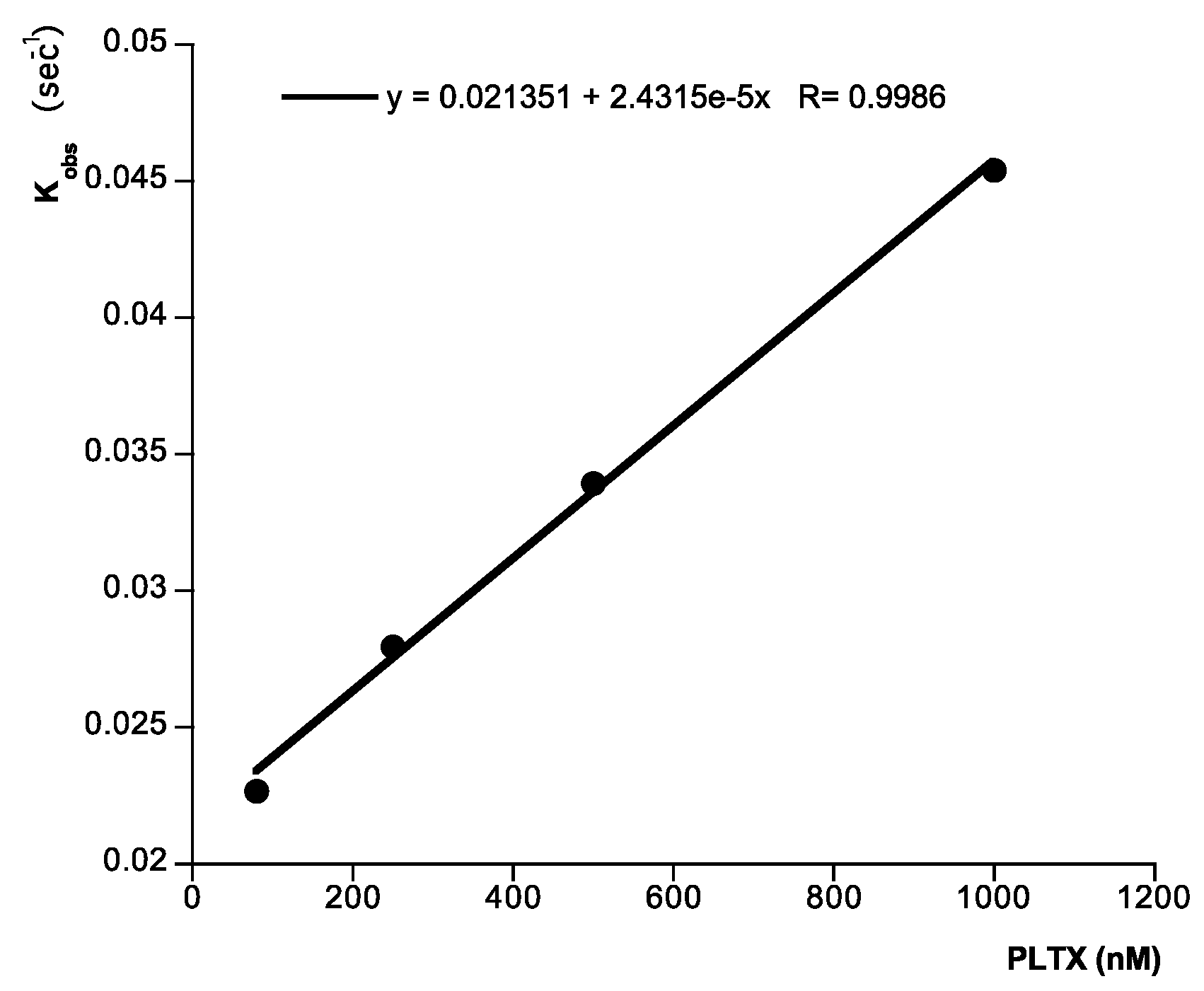
| PLTX (µM) Biosensor assay | PLTX (µM) FP assay | |
|---|---|---|
| Extract 1 | 56.2.8 ± 8.83 | 68.8 ± 9.8 |
| Extract 2 | 670 ± 31.6 | 761.1 ± 4.5 |
| P. reticulatum | - | - |
3. Materials and Methods
3.1. Instrumentation
3.2. Chemicals
3.3. Methods
3.3.1. Surface Activation and Ligand Inmobilization
3.3.2. Binding of PLTX or Ouabain to Immobilized Na+,K+-ATPase
3.4. Dinoflagellate Extracts
3.5 Statistical Analysis
4. Conclusion
Acknowledgments
References
- Ito, E.; Yasumoto, T. Toxicological studies on palytoxin and ostreocin-D administered to mice by three different routes. Toxicon 2009, 54, 244–251. [Google Scholar] [CrossRef]
- Gleibs, S.; Mebs, D. Distribution and sequestration of palytoxin in coral reef animals. Toxicon 1999, 37, 1521–1527. [Google Scholar] [CrossRef]
- Fernández, D.A.; Louzao, M.C.; Vilariño, N.; Espiña, B.; Fraga, M.; Vieytes, M.R.; Román, A.; Poli, M.; Botana, L.M. The kinetic, mechanistic and cytomorphological effects of palytoxin in human intestinal cells (Caco-2) explain its lower-than-parenteral oral toxicity. FEBS J. 2013, 280, 3906–3919. [Google Scholar] [CrossRef]
- Deeds, J.R.; Schwartz, M.D. Human risk associated with palytoxin exposure. Toxicon 2010, 56, 150–162. [Google Scholar] [CrossRef]
- Alfonso, A.; Fernández-Araujo, A.; Alfonso, C.; Caramés, B.; Tobio, A.; Louzao, M.C.; Vieytes, M.R.; Botana, L.M. Palytoxin detection and quantification using the fluorescence polarization technique. Anal. Biochem. 2012, 424, 64–70. [Google Scholar] [CrossRef]
- Aligizaki, K.; Katikou, P.; Milandri, A.; Diogène, J. Ocurrence of palytoxin-group toxins in seafood and future strategies to complement the present state of the art. Toxicon 2011, 57, 390–399. [Google Scholar] [CrossRef]
- Botana, L.M.; Fernández-Araujo, A.; Alfonso, A.; Antelo, J.M.; Davila, T.; Alfonso, C.; Katikou, P. Warm seawater microalgae: Growth and toxic profile of Ostreopsis spp. from European costs. Oceanography 2013, 1, 1–6. [Google Scholar]
- Bellocci, M.; Ronzitti, G.; Milandri, A.; Melchiorre, N.; Grillo, C.; Poletti, R.; Yasumoto, T.; Rossini, G.P. A cytolytic assay for the measurement of palytoxin based on a cultured monolayer cell line. Anal. Biochem. 2008, 374, 48–55. [Google Scholar] [CrossRef]
- Bignami, G.S. A rapid and sensitive hemolysis neutralization assay for palytoxin. Toxicon 1993, 31, 817–820. [Google Scholar] [CrossRef]
- Bignami, G.S.; Raybould, T.J.; Sachinvala, N.D.; Grothaus, P.G.; Simpson, S.B.; Lazo, C.B.; Byrnes, J.B.; Moore, R.E.; Vann, D.C. Monoclonal antibody-based enzyme-linked immunoassays for the measurement of palytoxin in biological samples. Toxicon 1992, 30, 687–700. [Google Scholar] [CrossRef]
- Boscolo, S.; Pelin, M.; de Bortoli, M.; Fontanive, G.; Barreras, A.; Berti, F.; Sosa, S.; Chaloin, O.; Bianco, A.; Yasumoto, T.; et al. Sandwich ELISA assay for the quantitation of palytoxin and its analogs in natural samples. Environ. Sci. Technol. 2013, 47, 2034–2042. [Google Scholar] [CrossRef]
- Ciminiello, P.; DellʼAversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Guerrini, F.; Pezzolesi, L.; Pistocchi, R.; et al. Stereochemical studies on ovatoxin-a. J. Am. Chem. Soc. 2012, 134, 1869–1875. [Google Scholar] [CrossRef]
- Ciminiello, P.; DellʼAversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Tartaglione, L. LC-MS of palytoxin and its analogues: State of the art and future perspectives. Toxicon 2011, 57, 376–389. [Google Scholar] [CrossRef]
- Ciminiello, P.; DellʼAversano, C.; Iacovo, E.D.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Battocchi, C.; Crinelli, R.; Carloni, E.; Magnani, M.; et al. Unique toxin profile of a Mediterranean Ostreopsis cf. ovata strain: HR LC-MS(n) characterization of ovatoxin-f, a new palytoxin congener. Chem. Res. Toxicol. 2012, 25, 1243–1252. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Iacovo, E.D.; Fattorusso, E.; Forino, M.; Tartaglione, L.; Yasumoto, T.; Battocchi, C.; Giacobbe, M.; Amorim, A.; et al. Investigation of toxin profile of mediterranean and atlantic strains of Ostreopsis cf. siamensis (Dinophyceae) by liquid chromatography-high resolution mass spectrometry. Harmful Algae 2013, 23, 19–27. [Google Scholar] [CrossRef]
- Lenoir, S.; Ten-Hage, L.; Tuquet, J.; Quod, J.P.; Bernard, C.; Hennion, M.C. First evidence of palytoxin analogies from an Ostreopsis mascarensis (Dinophyceae) benthic bloom in southwestern Indian Ocean. J. Phycol. 2004, 40, 1042–1051. [Google Scholar] [CrossRef]
- Riobo, P.; Franco, J.M. Palytoxins: Biological and chemical determination. Toxicon 2011, 57, 368–375. [Google Scholar] [CrossRef]
- Rossi, R.; Castellano, V.; Scalco, E.; Serpe, L.; Zingone, A.; Soprano, V. New palytoxin-like molecules in Mediterranean Ostreopsis cf. ovata (dinoflagellates) and in Palythoa tuberculosa detected by liquid chromatography-electrospray ionization time-of-flight mass spectrometry. Toxicon 2010, 56, 1381–1387. [Google Scholar] [CrossRef]
- Artigas, P.; Gadsby, D.C. Large diameter of palytoxin.induced Na/K pump channels and modulation of palytoxin interaction by Na/K pump ligands. J. Gen. Physiol. 2004, 123, 357–376. [Google Scholar] [CrossRef]
- Habermann, E. Palytoxin acts through Na+,K+-ATPase. Toxicon 1989, 27, 1171–1187. [Google Scholar] [CrossRef]
- Rakowski, R.F.; Artigas, P.; Palma, F.; Holmgren, M.; de Weer, P.; Gadsby, D.C. Sodium flux ratio in Na/K pump-channels opened by palytoxin. J. Gen. Physiol. 2007, 130, 41–54. [Google Scholar] [CrossRef]
- Vale-Gonzalez, C.; Gómez-Limia, B.; Vieytes, M.R.; Botana, L.M. Effects of the marine phycotoxin palytoxin on neuronal pH in primary cultures of cerebellar granule cells. J. Neurosci. Res. 2007, 85, 90–98. [Google Scholar] [CrossRef]
- Vale-Gonzalez, C.; Pazos, M.J.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Study of the neuronal effects of ouabain and palytoxin and their binding to Na,K-ATPases using an optical biosensor. Toxicon 2007, 15, 541–552. [Google Scholar]
- Tubaro, A.; del Favero, G.; Beltramo, D.; Ardizzone, M.; Forino, M.; de Bortoli, M.; Pelin, M.; Poli, M.; Bignami, G.; Ciminiello, P.; et al. Acute oral toxicity in mice of a new palytoxin analog: 42-Hydroxy-palytoxin. Toxicon 2011, 57, 755–763. [Google Scholar] [CrossRef]
- Amzil, Z.; Sibat, M.; Chomerat, N.; Grossel, H.; Marco-Miralles, F.; Lemee, R.; Nezan, E.; Sechet, V. Ovatoxin-a and palytoxin accumulation in seafood in relation to Ostreopsis cf. ovata blooms on the French Mediterranean coast. Mar. Drugs 2012, 10, 477–496. [Google Scholar] [CrossRef]
- Alcala, A.C.; Alcala, L.C.; Garth, J.S.; Yasumura, D.; Yasumoto, T. Human fatality due to ingestion of the crab Demania reynaudii that contained a palytoxin-like toxin. Toxicon 1988, 26, 105–107. [Google Scholar] [CrossRef]
- Yasumoto, T. Fish poisoning due to toxins of microalgal origins in the Pacific. Toxicon 1998, 36, 1515–1518. [Google Scholar] [CrossRef]
- Fonfría, E.S.; Vilariño, N.; Campbell, K.; Elliot, C.; Haughey, S.A.; Ben-Gigirey, B.; Vieites, M.J.; Kawatsu, K.; Botana, L.M. Paralytic shellfish poisoning detection by surface plasmon resonance-based biosensors in shellfish matrixes. Anal. Chem. 2007, 79, 6303–6311. [Google Scholar] [CrossRef]
- Fonfría, E.S.; Vilariño, N.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Feasibility of using a surface plasmon resonance-based biosensor to detect and quantify yessotoxin. Anal. Chim. Acta 2008, 617, 167–170. [Google Scholar] [CrossRef]
- Pazos, M.J.; Alfonso, A.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Kinetic analysis of the interaction between yessotoxin and analogues and immobilized phosphodiesterases using a resonant mirror optical biosensor. Chem. Res. Toxicol. 2005, 18, 1155–1160. [Google Scholar] [CrossRef]
- Pazos, M.J.; Alfonso, A.; Vieytes, M.R.; Yasumoto, T.; Botana, L.M. Study of the interaction between different phosphodiesterases and yessotoxin using a resonant mirror biosensor. Chem. Res. Toxicol. 2006, 19, 794–800. [Google Scholar] [CrossRef]
- Campbell, K.; Barnes, P.; Haughey, S.A.; Higgins, C.; Kawatsu, K.; Vasconcelos, V.; Elliott, C.T. Development and single laboratory validation of an optical biosensor assay for tetrodotoxin detection as a tool to combat emerging risks in European seafood. Anal. Bioanal. Chem. 2013, 405, 7753–7763. [Google Scholar] [CrossRef]
- McGrath, T.F.; Campbell, K.; Fodey, T.L.; OʼKennedy, R.; Elliott, C.T. An evaluation of the capability of a biolayer interferometry biosensor to detect low-molecular-weight food contaminants. Anal. Bioanal. Chem. 2013, 405, 2535–2544. [Google Scholar] [CrossRef]
- Johnsson, B.; Löfás, S.; Lindquist, G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaaction analysis in surface plasmon resonance sensors. Anal. Biochem. 1991, 198, 268–277. [Google Scholar] [CrossRef]
- Carlsson, J.; Drevin, H.; Axén, R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio) propionate, a new heterobifunctional reagent. Biochem. J. 1978, 173, 723–737. [Google Scholar]
- Pazos, M.J.; Alfonso, A.; Vieytes, M.R.; Yasumoto, T.; Vieites, J.M.; Botana, L.M. Resonant mirror biosensor detection method based on yessotoxin-phosphodiesterase interactions. Anal. Biochem. 2004, 335, 112–118. [Google Scholar] [CrossRef]
- Taniyama, S.; Arakawa, O.; Terada, M.; Nishio, S.; Takatani, T.; Mahmud, Y.; Noguchi, T. Ostreopsis sp., a possible origin of palytoxin (PTX) in parrotfish Scarus ovifrons. Toxicon 2003, 42, 29–33. [Google Scholar] [CrossRef]
- Ciminiello, P.; DellʼAversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Guerrini, F.; Pistocchi, R. Complex palytoxin-like profile of Ostreopsis ovata. Identification of four new ovatoxins by high-resolution liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2735–2744. [Google Scholar] [CrossRef]
- Uemura, D. Bioactive polyethers. In Bioorganic Marine Chemestry; Scheuer, P.J., Ed.; SpringerVerlag: Berlin/Heidelberg, Germany, 1991; p. 4. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alfonso, A.; Pazos, M.-J.; Fernández-Araujo, A.; Tobio, A.; Alfonso, C.; Vieytes, M.R.; Botana, L.M. Surface Plasmon Resonance Biosensor Method for Palytoxin Detection Based on Na+,K+-ATPase Affinity. Toxins 2014, 6, 96-107. https://doi.org/10.3390/toxins6010096
Alfonso A, Pazos M-J, Fernández-Araujo A, Tobio A, Alfonso C, Vieytes MR, Botana LM. Surface Plasmon Resonance Biosensor Method for Palytoxin Detection Based on Na+,K+-ATPase Affinity. Toxins. 2014; 6(1):96-107. https://doi.org/10.3390/toxins6010096
Chicago/Turabian StyleAlfonso, Amparo, María-José Pazos, Andrea Fernández-Araujo, Araceli Tobio, Carmen Alfonso, Mercedes R. Vieytes, and Luis M. Botana. 2014. "Surface Plasmon Resonance Biosensor Method for Palytoxin Detection Based on Na+,K+-ATPase Affinity" Toxins 6, no. 1: 96-107. https://doi.org/10.3390/toxins6010096
APA StyleAlfonso, A., Pazos, M.-J., Fernández-Araujo, A., Tobio, A., Alfonso, C., Vieytes, M. R., & Botana, L. M. (2014). Surface Plasmon Resonance Biosensor Method for Palytoxin Detection Based on Na+,K+-ATPase Affinity. Toxins, 6(1), 96-107. https://doi.org/10.3390/toxins6010096






