BiP Negatively Affects Ricin Transport
Abstract
:1. Introduction
2. Results
2.1. BiP Protects against Ricin Toxicity

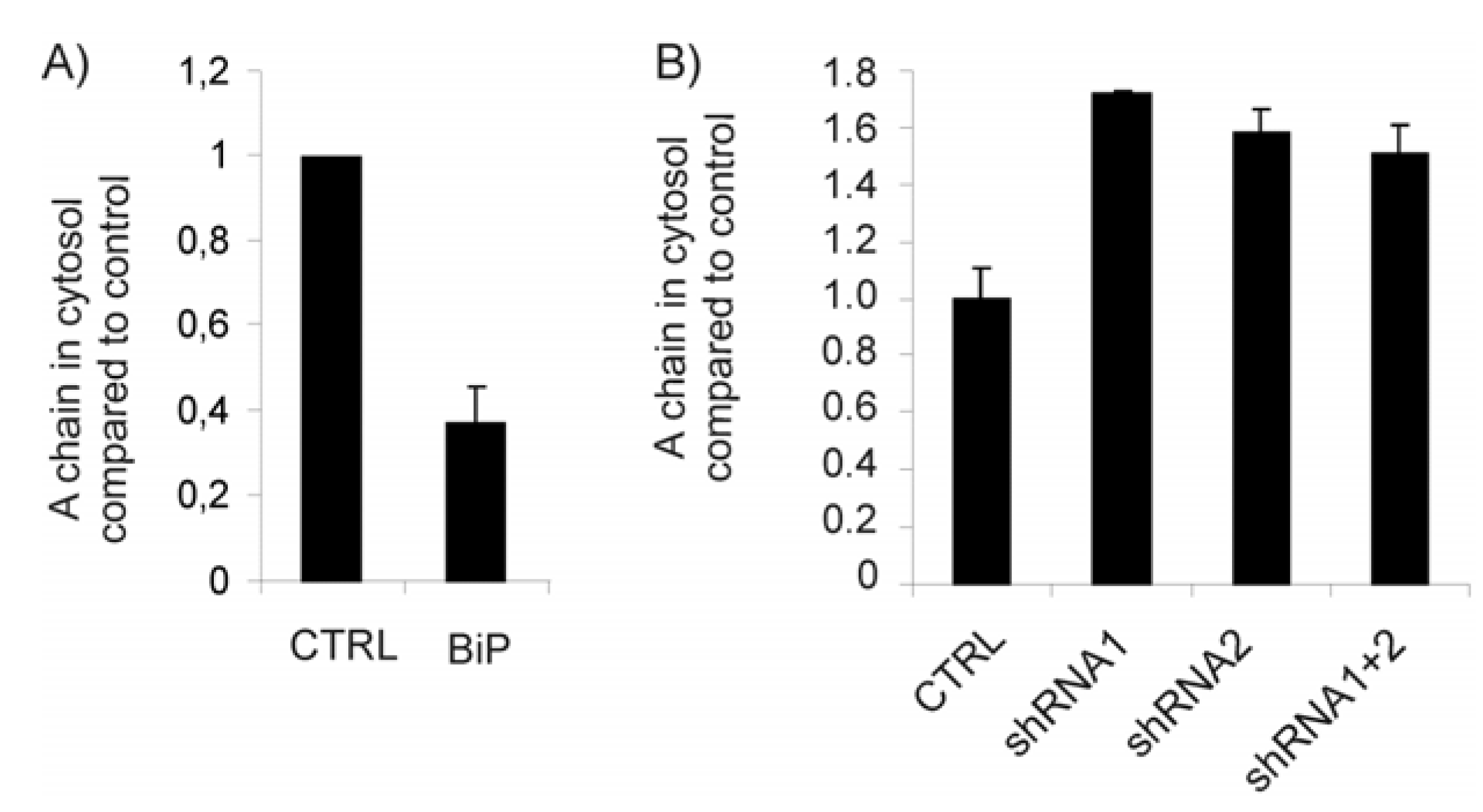
2.2. Depletion of BiP Sensitizes Cells towards Ricin Toxicity

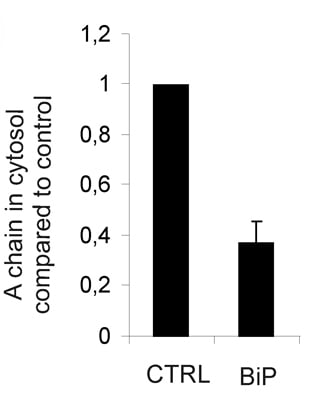
2.3. The Protein Level of BiP Affects Ricin Translocation to the Cytosol
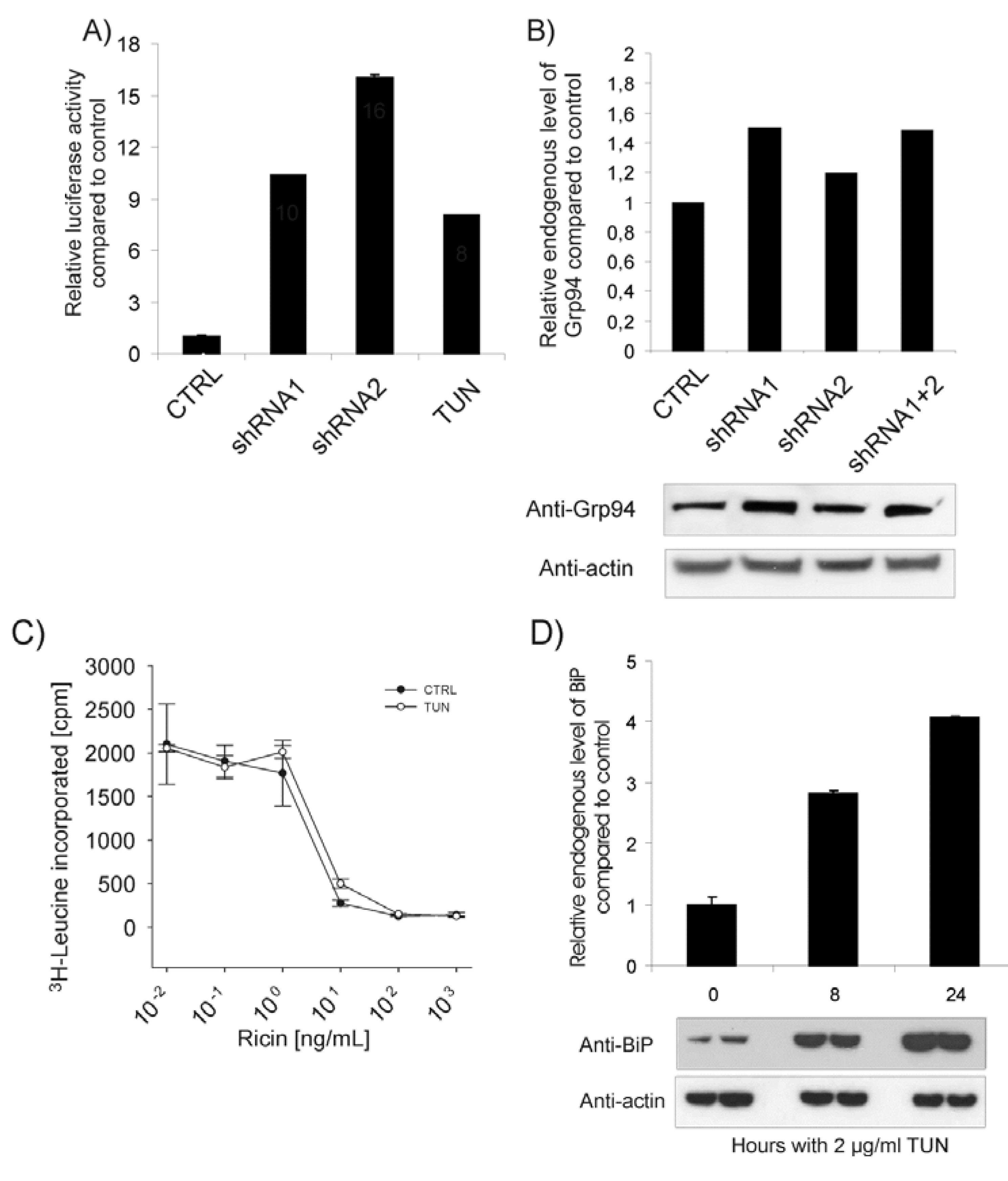
2.4. ER Stress Does Not Cause Increased Ricin Toxicity per se
3. Discussion
4. Experimental Section
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. DNA Constructs, Transfection, Protein Expression and Pull down
4.4. Ricin Toxicity and Measurement of Protein Synthesis
4.5. Sulfation of Ricin Sulf-1 and Permeabilisation of Cells
4.6. ATF6 Activation Assay
Acknowledgments
Conflict of Interest
References
- Dudek, J.; Benedix, J.; Cappel, S.; Greiner, M.; Jalal, C.; Muller, L.; Zimmermann, R. Functions and pathologies of bip and its interaction partners. Cell. Mol. Life Sci. CMLS 2009, 66, 1556–1569. [Google Scholar] [CrossRef]
- Bole, D.G.; Hendershot, L.M.; Kearney, J.F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J. Cell Biol. 1986, 102, 1558–1566. [Google Scholar] [CrossRef]
- Haas, I.G.; Wabl, M. Immunoglobulin heavy chain binding protein. Nature 1983, 306, 387–389. [Google Scholar] [CrossRef]
- Hendershot, L.M. The er function bip is a master regulator of er function. Mount Sinai J. Med. N. Y. 2004, 71, 289–297. [Google Scholar]
- Sandvig, K.; Torgersen, M.L.; Engedal, N.; Skotland, T.; Iversen, T.G. Protein toxins from plants and bacteria: Probes for intracellular transport and tools in medicine. FEBS Lett. 2010, 584, 2626–2634. [Google Scholar] [CrossRef]
- Bellisola, G.; Fracasso, G.; Ippoliti, R.; Menestrina, G.; Rosen, A.; Solda, S.; Udali, S.; Tomazzolli, R.; Tridente, G.; Colombatti, M. Reductive activation of ricin and ricin a-chain immunotoxins by protein disulfide isomerase and thioredoxin reductase. Biochem. Pharmacol. 2004, 67, 1721–1731. [Google Scholar] [CrossRef]
- Spooner, R.A.; Watson, P.D.; Marsden, C.J.; Smith, D.C.; Moore, K.A.; Cook, J.P.; Lord, J.M.; Roberts, L.M. Protein disulphide-isomerase reduces ricin to its a and b chains in the endoplasmic reticulum. Biochem. J. 2004, 383, 285–293. [Google Scholar] [CrossRef]
- Spooner, R.A.; Lord, J.M. How ricin and shiga toxin reach the cytosol of target cells: Retrotranslocation from the endoplasmic reticulum. Curr. Top. Microbiol. Immunol. 2012, 357, 19–40. [Google Scholar]
- Spooner, R.A.; Hart, P.J.; Cook, J.P.; Pietroni, P.; Rogon, C.; Hohfeld, J.; Roberts, L.M.; Lord, J.M. Cytosolic chaperones influence the fate of a toxin dislocated from the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 2008, 105, 17408–17413. [Google Scholar] [CrossRef]
- Li, S.; Spooner, R.A.; Allen, S.C.; Guise, C.P.; Ladds, G.; Schnoder, T.; Schmitt, M.J.; Lord, J.M.; Roberts, L.M. Folding-competent and folding-defective forms of ricin a chain have different fates after retrotranslocation from the endoplasmic reticulum. Mol. Biol. Cell 2010, 21, 2543–2554. [Google Scholar] [CrossRef]
- Wesche, J.; Rapak, A.; Olsnes, S. Dependence of ricin toxicity on translocation of the toxin a-chain from the endoplasmic reticulum to the cytosol. J. Biol. Chem. 1999, 274, 34443–34449. [Google Scholar] [CrossRef]
- Slominska-Wojewodzka, M.; Gregers, T.F.; Walchli, S.; Sandvig, K. Edem is involved in retrotranslocation of ricin from the endoplasmic reticulum to the cytosol. Mol. Biol. Cell 2006, 17, 1664–1675. [Google Scholar] [CrossRef]
- Sokolowska, I.; Walchli, S.; Wegrzyn, G.; Sandvig, K.; Slominska-Wojewodzka, M. A single point mutation in ricin a-chain increases toxin degradation and inhibits edem1-dependent er retrotranslocation. Biochem. J. 2011, 436, 371–385. [Google Scholar] [CrossRef]
- Lord, J.M.; Roberts, L.M.; Lencer, W.I. Entry of protein toxins into mammalian cells by crossing the endoplasmic reticulum membrane: Co-opting basic mechanisms of endoplasmic reticulum-associated degradation. Curr. Top. Microbiol. Immunol. 2005, 300, 149–168. [Google Scholar]
- Bassik, M.C.; Kampmann, M.; Lebbink, R.J.; Wang, S.; Hein, M.Y.; Poser, I.; Weibezahn, J.; Horlbeck, M.A.; Chen, S.; Mann, M.; et al. A systematic mammalian genetic interaction map reveals pathways underlying ricin susceptibility. Cell 2013, 152, 909–922. [Google Scholar] [CrossRef]
- Moreau, D.; Kumar, P.; Wang, S.C.; Chaumet, A.; Chew, S.Y.; Chevalley, H.; Bard, F. Genome-wide rnai screens identify genes required for ricin and pe intoxications. Dev. Cell 2011, 21, 231–244. [Google Scholar] [CrossRef]
- Falguieres, T.; Johannes, L. Shiga toxin b-subunit binds to the chaperone bip and the nucleolar protein b23. Biol. Cell Auspices Eur. Cell Biol. Organ. 2006, 98, 125–134. [Google Scholar]
- Yu, M.; Haslam, D.B. Shiga toxin is transported from the endoplasmic reticulum following interaction with the luminal chaperone hedj/erdj3. Infect. Immun. 2005, 73, 2524–2532. [Google Scholar] [CrossRef]
- Winkeler, A.; Godderz, D.; Herzog, V.; Schmitz, A. Bip-dependent export of cholera toxin from endoplasmic reticulum-derived microsomes. FEBS Lett. 2003, 554, 439–442. [Google Scholar] [CrossRef]
- Tsai, B.; Rapoport, T.A. Unfolded cholera toxin is transferred to the er membrane and released from protein disulfide isomerase upon oxidation by ero1. J. Cell Biol. 2002, 159, 207–216. [Google Scholar] [CrossRef]
- Tsai, B.; Rodighiero, C.; Lencer, W.I.; Rapoport, T.A. Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell 2001, 104, 937–948. [Google Scholar] [CrossRef]
- Tamayo, A.G.; Slater, L.; Taylor-Parker, J.; Bharti, A.; Harrison, R.; Hung, D.T.; Murphy, J.R. Grp78(bip) facilitates the cytosolic delivery of anthrax lethal factor (lf) in vivo and functions as an unfoldase in vitro. Mol. Microbiol. 2011, 81, 1390–1401. [Google Scholar] [CrossRef]
- Shen, J.; Snapp, E.L.; Lippincott-Schwartz, J.; Prywes, R. Stable binding of atf6 to bip in the endoplasmic reticulum stress response. Mol. Cell. Biol. 2005, 25, 921–932. [Google Scholar] [CrossRef]
- Rapak, A.; Falnes, P.O.; Olsnes, S. Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol. Proc. Natl. Acad. Sci. USA 1997, 94, 3783–3788. [Google Scholar] [CrossRef]
- Wei, J.; Gaut, J.R.; Hendershot, L.M. In vitro dissociation of bip-peptide complexes requires a conformational change in bip after atp binding but does not require atp hydrolysis. J. Biol. Chem. 1995, 270, 26677–26682. [Google Scholar] [CrossRef]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of bip and er stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef]
- Chen, X.; Shen, J.; Prywes, R. The luminal domain of atf6 senses endoplasmic reticulum (er) stress and causes translocation of atf6 from the er to the golgi. J. Biol. Chem. 2002, 277, 13045–13052. [Google Scholar] [CrossRef]
- Shen, J.; Chen, X.; Hendershot, L.; Prywes, R. Er stress regulation of atf6 localization by dissociation of bip/grp78 binding and unmasking of golgi localization signals. Dev. Cell 2002, 3, 99–111. [Google Scholar] [CrossRef]
- Kapulkin, W.J.; Hiester, B.G.; Link, C.D. Compensatory regulation among er chaperones in c. Elegans. FEBS Lett. 2005, 579, 3063–3068. [Google Scholar] [CrossRef]
- Suzuki, T.; Lu, J.; Zahed, M.; Kita, K.; Suzuki, N. Reduction of grp78 expression with sirna activates unfolded protein response leading to apoptosis in hela cells. Arch. Biochem. Biophys. 2007, 468, 1–14. [Google Scholar] [CrossRef]
- Shen, J.; Prywes, R. Er stress signaling by regulated proteolysis of atf6. Methods 2005, 35, 382–389. [Google Scholar] [CrossRef]
- Ye, J.; Rawson, R.B.; Komuro, R.; Chen, X.; Dave, U.P.; Prywes, R.; Brown, M.S.; Goldstein, J.L. Er stress induces cleavage of membrane-bound atf6 by the same proteases that process srebps. Mol. Cell 2000, 6, 1355–1364. [Google Scholar] [CrossRef]
- Christianson, J.C.; Olzmann, J.A.; Shaler, T.A.; Sowa, M.E.; Bennett, E.J.; Richter, C.M.; Tyler, R.E.; Greenblatt, E.J.; Harper, J.W.; Kopito, R.R. Defining human erad networks through an integrative mapping strategy. Nat. Cell Biol. 2012, 14, 93–105. [Google Scholar]
- Paton, A.W.; Beddoe, T.; Thorpe, C.M.; Whisstock, J.C.; Wilce, M.C.; Rossjohn, J.; Talbot, U.M.; Paton, J.C. Ab5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone bip. Nature 2006, 443, 548–552. [Google Scholar] [CrossRef]
- Hegde, N.R.; Chevalier, M.S.; Wisner, T.W.; Denton, M.C.; Shire, K.; Frappier, L.; Johnson, D.C. The role of bip in endoplasmic reticulum-associated degradation of major histocompatibility complex class i heavy chain induced by cytomegalovirus proteins. J. Biol. Chem. 2006, 281, 20910–20919. [Google Scholar]
- Rainey, G.J.; Young, J.A. Antitoxins: Novel strategies to target agents of bioterrorism. Nat. Rev. Microbiol. 2004, 2, 721–726. [Google Scholar] [CrossRef]
- Smallshaw, J.E.; Richardson, J.A.; Pincus, S.; Schindler, J.; Vitetta, E.S. Preclinical toxicity and efficacy testing of rivax, a recombinant protein vaccine against ricin. Vaccine 2005, 23, 4775–4784. [Google Scholar] [CrossRef]
- Smallshaw, J.E.; Richardson, J.A.; Vitetta, E.S. Rivax, a recombinant ricin subunit vaccine, protects mice against ricin delivered by gavage or aerosol. Vaccine 2007, 25, 7459–7469. [Google Scholar] [CrossRef]
- Kreitman, R.J. Immunotoxins for targeted cancer therapy. AAPS J. 2006, 8, E532–E551. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, J.; Arenzana, N.; Tirasophon, W.; Kaufman, R.J.; Prywes, R. Activation of atf6 and an atf6 DNA binding site by the endoplasmic reticulum stress response. J. Biol. Chem. 2000, 275, 27013–27020. [Google Scholar]
- Grimmer, S.; Ying, M.; Walchli, S.; van Deurs, B.; Sandvig, K. Golgi vesiculation induced by cholesterol occurs by a dynamin- and cpla2-dependent mechanism. Traffic 2005, 6, 144–156. [Google Scholar] [CrossRef]
- Klasen, M.; Spillmann, F.J.; Lorens, J.B.; Wabl, M. Retroviral vectors to monitor somatic hypermutation. J. Immunol. Methods 2005, 300, 47–62. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gregers, T.F.; Skånland, S.S.; Wälchli, S.; Bakke, O.; Sandvig, K. BiP Negatively Affects Ricin Transport. Toxins 2013, 5, 969-982. https://doi.org/10.3390/toxins5050969
Gregers TF, Skånland SS, Wälchli S, Bakke O, Sandvig K. BiP Negatively Affects Ricin Transport. Toxins. 2013; 5(5):969-982. https://doi.org/10.3390/toxins5050969
Chicago/Turabian StyleGregers, Tone F., Sigrid S. Skånland, Sébastien Wälchli, Oddmund Bakke, and Kirsten Sandvig. 2013. "BiP Negatively Affects Ricin Transport" Toxins 5, no. 5: 969-982. https://doi.org/10.3390/toxins5050969
APA StyleGregers, T. F., Skånland, S. S., Wälchli, S., Bakke, O., & Sandvig, K. (2013). BiP Negatively Affects Ricin Transport. Toxins, 5(5), 969-982. https://doi.org/10.3390/toxins5050969