1. Introduction
Aflatoxins, which mainly exist in grains, nuts, cotton seeds, as well as some related products, are toxic metabolites and strong carcinogens from a class of fungi (
Aspergillus flavus and
Aspergillus parasiticus, e.g.) [
1]. Aflatoxin M1 (ATM1), as the hydroxylated metabolite of aflatoxin B1 (ATB1), is usually present in the animal milk contaminated by ATB1. Because of their stronger toxic effects than ATB1 on public health, many governments have provided maximum acceptable limits for residual ATM1 in foodstuffs, especially in milk products [
2]. For example, according to Chinese national standards, aflatoxin content cannot exceed 0.5 μg/kg in milk and 5 μg/kg in milk powder, whereas the USA has higher regulations of 500 ng/kg [
3]. Thus, the food administration agencies in almost all countries have dedicated much effort to developing sensitive analytical methods for monitoring ultratrace levels of ATM1 (<0.05 μg/kg) in foods [
4]. Current strategies for ultrasensitive detection of ATM1 are based mainly on thin-layer chromatography (TLC), high performance liquid chromatography (HPLC) or UV light spectroscopy after extraction and clean-up procedures [
3]. These methods are sufficiently sensitive and accurate; however, they often require sophisticated, expensive and heavy instruments that may not be available in laboratories with fewer resources; these methods are especially not fit for mass screening [
4]. Therefore, it is urgent to develop affordable, portable and sensitive methodologies for the screening of ATM1 at ultralow concentrations in foodstuffs.
The immunoassay techniques which are based on the highly specific recognition ability with corresponding antigens by the antibodies have been successfully applied for the detection of aflatoxin [
5]. At present, the main immunoassay for ATM1 analysis is carried out using competitive ELISA (cELISA) kits, with confirmation by HPLC (the official method) [
6,
7]. It is based on a competitive reaction between the free ATM1 in the sample and an ATM1-horseradish peroxidase conjugate, for an immobilized monoclonal antibody for ATM1 on the bottom of 96-well plate monitored by chronoamperometry. The assay is inexpensive and has been used in practical screening analysis. However, it also has some inherent flaws which are difficult to overcome, such as: (1) lower sensitivity compared with HPLC due to the difficulty of determination the pictogram level of ATM1; (2) multiple preparative and washing steps are required to complete the assay, and common time-to-results can take as long as two hours; (3) it also requires a series of laborious sample processing procedures and clean-up to yield reproducible results [
8]. Thus, despite their success, there are still pressing needs for ultrasensitive detection strategy of ATM1, especially in the separation and enrichment of analytes from food samples containing highly abundant nonspecific protein and lipid matrixes for point-of-care testing. Recently, there has been an emerging trend of establishing new analytical methods, such as electrochemical and chemiluminescent methods for toxins [
7], which not only retains the simplicity, low cost, and portable analytical devices, but also provides new opportunities in the development of precise and sensitive diagnostic devices. Electrochemiluminescent immunoassay (ECLIA) has been proven to be a highly sensitive and selective immunoassay, which combines analytical advantages of electrochemiluminescence (ECL) (sensitive, cost effective, absence of background optical signal, and ease of control by changing electrode potential) with the specificity of immunoassay. Thus ECLIA is suitable for point-of-care determination of ultratrace levels of ATM1 in food.
The most important step to develop an ultrasensitive ECLIA sensor is to fabricate a signal tag labeled with the antibody of ATM1 (ATM1 Ab) [
8]. There have been many reports on the signal tags based on Ru(bpy)
32+ and luminol [
9]. Strongly luminescent semiconductor quantum dots (QDs) (CdS, PbS, CdTe and ZnS) have found potential applications in biological imaging and labeling, which also have unique advantages such as excellent opto-electronic properties [
10,
11]. Among the compound semiconductors, water-soluble CdTe has attracted growing interests on versatility in surface modification with various biomolecules [
12]. Although ECLIA analysis based on QDs has many advantages, the reports concerning the detection of toxins with QDs ECL are relatively scarce. The reasons are partially because that the ECL of semiconductor QDs is weaker than that of conventional luminescent reagents such as luminal or Ru(bpy)
32+. It is reported that carbon nanotube (CNT) can be used to enhance the ECL of CdS QDs film by reducing the injection barrier of electrons to the QDs [
13]. Moreover, Wang and Mountziaris [
14] have reported on immunoassays employing ZnSe QDs conjugated to proteins to form QD-based biomolecular sensors. This phenomenon enables the development of homogeneous, separation-free immunoassays for rapid quantitative detection of proteins in solution. The development of the QDs-CNT compound requires conjugation of both ingredients through polymer. Poly(diallyldimethylammonium chloride) PDDA is a strong candidate for further modification to develop nanocomposites [
14]. With readily available free amine groups, this polymer offers more uniform coating of the carbon nanotubes, thus improving the solubility of CNTs, and reducing the potential toxic effects if used on a labeling antibody. Moreover, a single CNT can encapsulate dozens of CdTe QDs (as the signal source) to produce amplification of an ECL signal. Moreover, the surface of the hybrid particles can also be labeled with attached antibodies as tags. Taking into consideration the above advantages, the CdTe QDs-CNTs composites were fabricated and employed for immobilizing ATM1 monoclonal antibody (ATM1 Ab1) on it in the article. Thus a novel signal tag (ATM1 Ab1/CdTe QDs-CNTs) for ECL biosensors was synthesized.
Another key issue for the ECLIA sensor is to choose a convenient method for immobilizing the signal probe on the electrode and maintain the biocompatibility of the antibody to greatest extent. However, it is very difficult to achieve the aim, because whatever the chemical grafting or physical adsorption method employed, it can inevitably generate deactivation to antibody and reduce its identification ability for antigen. And once the antigen is conjugated with the antibody, it is difficult for the electrode to be reused. Moreover, the antibodies are very expensive. Thus, if a kind of material with recognition feature for ATM1 can be utilized to immobilize on the electrode instead of the antibody, a label-free ECL electrode would be obtained, which will greatly reduce the cost and simplify the fabrication process. Graphene oxides (GO), as one kind of flat monolayer of carbon atoms tightly packed into a two-dimensional honeycomb lattice, has come to the forefront of research in electrochemical sensors recently, because of its unique electronic properties and high electrical conductivity [
15,
16]. Furthermore, GO can also been served as an extraordinarily wonderful adsorbent material for enriching aromatic organic compounds in a complex matrix [
17]. Because ATM1 is a compound with a large aromatic ring, GO will thus be used to absorb ATM1 and separate the ATM1 from the tested samples in this paper. Furthermore, the ATM1 Ab1/CdTe-CNT signal tag can be employed to conjugate with the ATM1 absorbed on GO to form a “sandwich” immunocomplex (GO/ATM1/ATM1 Ab1/CdTe-CNT), which can generate ECL signal on the electrode. Certainly, other aromatic ring-containing substances can also be adsorbed on the surface of GO. However, the signal tag labeled with ATM1 Ab1, cannot react with other absorbed matter other than ATM1. Thus the ECL signal can only reflect the amount of ATM1 enriched on GO. The “sandwich” detection scheme (
Scheme 1) is significantly different from the conventional competitive enzyme immunoassay (cELISA) for toxin. Firstly, when the concentration of ATM1 is increased, the proposed method will generate an enhanced detection signal, while the signals in cELISA decrease, because less enzyme-labeled antigen as competitive substances would bind to the bottom of the microplate, which can catalyze the substrate to produce the signal. Usually, most of the interference substances (such as protein and lipid) in food samples have high resistance, which can decrease the electrochemical signal. Therefore, the new immunoassay results can better reflect the real content of the analyte. Secondly, the scheme is similar to double-antibody sandwich reaction with two recognition sites (one from GO, and the other from the antibody on the signal tag), which will have higher recognition ability for ATM1 than competitive ELISA with only one recognition site. To the best of our knowledge, no report about establishing ECL immunoassay on the above “sandwich” scheme has been published.
Scheme 1.
The “sandwich” immunoassay for detection of ATM1.
Scheme 1.
The “sandwich” immunoassay for detection of ATM1.
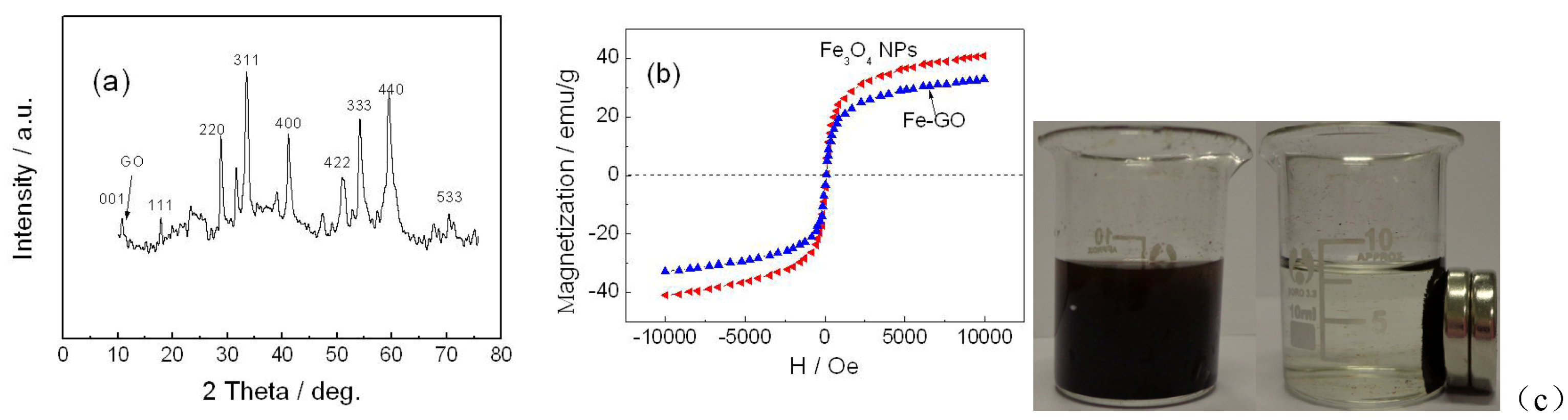
In recent years, magnetic separation technology is a useful and convenient tool that has recently been considered in sample treatment and separation from complex background in foodstuff [
18]. The magnetic component participation allows for a controllable rebinding process that may offer a rapid and simple alternative to conventional centrifugation and filtration [
19]. For instance, graphene loaded with magnetic materials could realize the retrieval and separation of graphene from dispersion rapidly and effectively. Moreover, at the same time maintain the intrinsic properties of graphene at extreme [
19]. Nanometer-sized magnetic particles of iron (Fe
3O
4 NPs), are potential candidates in magnetic separation and biomedical applications [
20]. Thus, the hybrid material combining magnetic Fe
3O
4 NPs and graphene absorbent has the potential to provide a simple, flexible, and highly selective pretreated method that may be much more practical than the complicated centrifugation procedure employed in extraction of ATM1 in food samples. Herein, the magnetic GO (Fe-GO) was prepared as absorbent for ATM1. It also has other advantages: firstly, after ATM1 was extracted by Fe-GO, the complex can be easily modified on the surface of electrode by a magnet. Secondly, GO with high conductivity can also accelerate the electron transfer speed, which would amplify the ECL signal [
21,
22]. Thirdly, the magnetic probes can be easily immobilized on the surface of the screen printed carbon electrode (SPCE) after adding a magnet on the bottom of the plane electrode [
23].
In this work, we report a novel ultrasensitive “sandwich” immunoassay scheme for ATM1, the immunocomplex was conjugated among magnetic Fe-GO absorbent, the free ATM1 in the sample and the immobilized monoclonal antibody from CdTe-CNT signal tag based on a SPCE electrode. The magnetic hybrid particles (Fe-GO), by the aid of high affinity graphene with aromatic pollutants, thereby assure the high selective adsorption of ATM1. Moreover, it can be separated easily after extracting ATM1 in food samples by external magnetic field, and avoid the interference of complex matrix. The novel signal probe (ATM1 Ab1/CdTe QDs-CNTs) can greatly enhance the ECL signal of the immunosensor. The assay was employed to detect ATM1 in real milk samples and a detection limit of 0.3 pg/mL was achieved. The immunosensor exhibited many functions in simple instrumentation, high sensitivity, disposable, magnetic separation and enrichment, which may find promising applications for toxin analysis in food.
3. Experimental Section
3.1. Materials and Reagents
Graphene oxide (purity 99.9995%) and single carbon nanotube were obtained from Aladdin Chemistry Co. Ltd. (Shanghai, China). Aflatotoxin M1 (ATM1) was purchased from sigma Co. Ltd. (St. Louis, MO, USA). CdTe was synthesized according literature [
12]. Milk samples were obtained from local supermarkets. The monoclonal antibodies against ATM1 (MAb) were purchased from Maine Biotechnology Services (Meridian Life Science, Inc., Memphis, TN, USA). Casein, bovine albumin (BSA), Tween20, potassium permanganate (KMnO
4, 98 wt%), sulfuric acid (H
2SO
4, 98 wt%), phosphorus pentoxide (P
2O
5, 99 wt%), hydrogen peroxide (H
2O
2, 30 wt%), hydrochloric acid (HCl, 37 wt%) and potassium peroxydisulfate (K
2S
2O
8, 99 wt%) were obtained from Shanghai Chemical Reagent Co. Ltd. (Shanghai, China). All reagents were of analytical grade and were prepared using deionized water.
3.2. Apparatus
A model 550 microplate reader (Bio-Rad Laboratories, Shanghai, China) was used to read the absorbance on ELISA plates at 655 nm. ECL signals were measured with a MPI-A multifunctional electrochemical and chemiluminescent analytical system (Remax Electronic Instrument Limited Co., Xi’an, China, 350 nm–650 nm) by a conventional three-electrode configuration at room temperature. SPCE (3 mm diameter) was used as a working electrode; an SCE and a Pt wire were used as a reference and auxiliary electrodes, respectively. The spectral width of the photomultiplier tube (PMT) was 200–800 nm and the voltage of the PMT was 500–800 V in the detection process. Transmission electron microscopy (TEM, Hitachi, Tokyo, Japan). Fluorescence spectrometer (Hitachi, Tokyo, Japan). X-ray diffraction (XRD, Bruker D8 Focus, Breman, Germany). Ultrasonic cleaner (Kudos, Shanghai, China). Supercentrifuge (Anting, Shanghai, China).
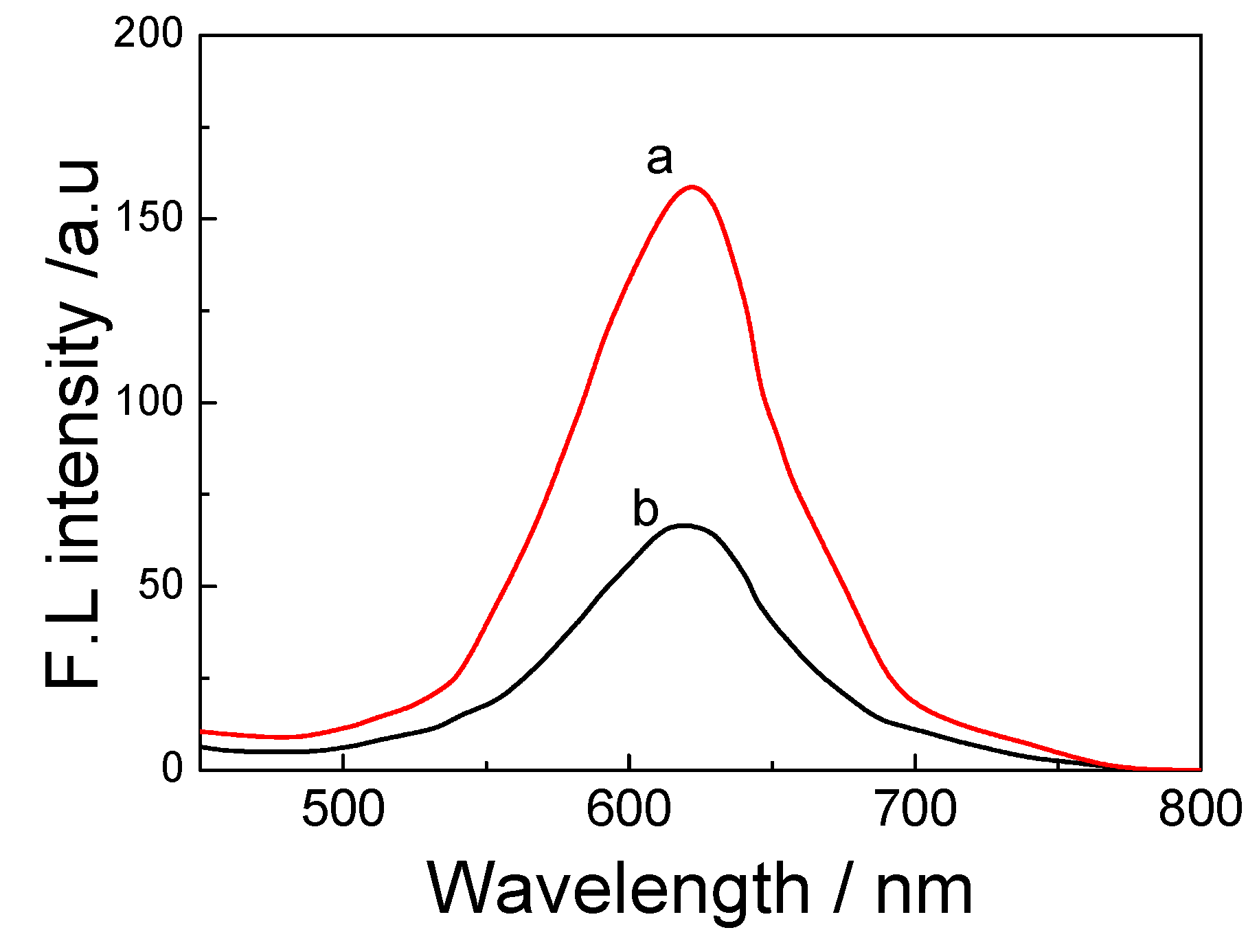
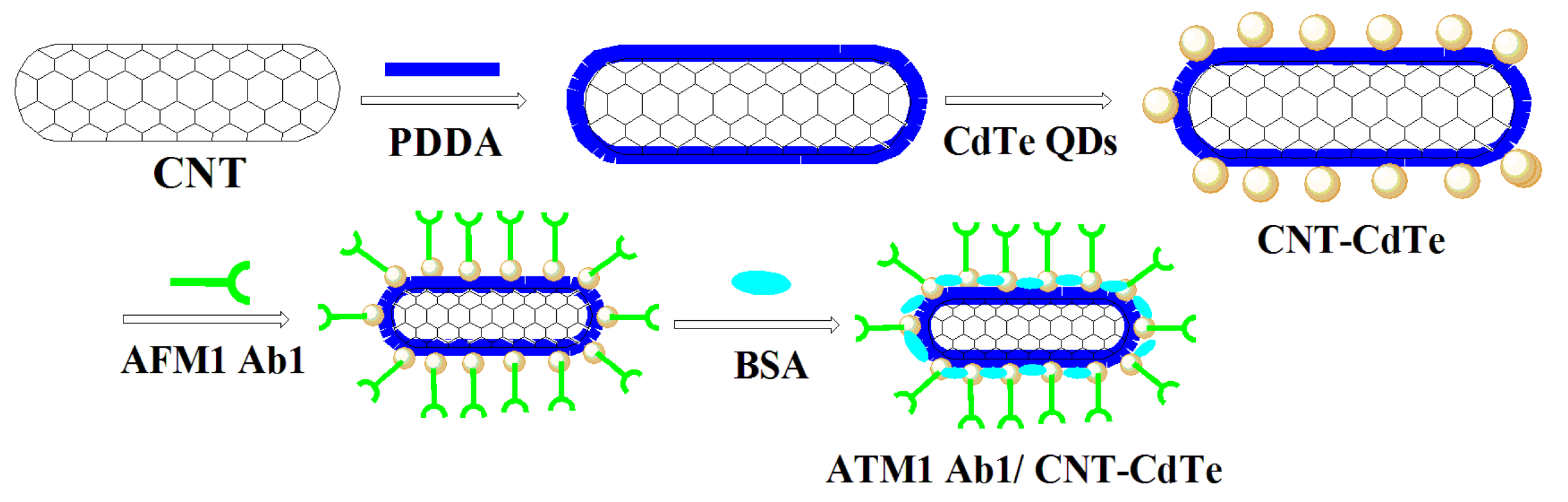
3.3. Synthesis of CdTe-CNT QDs Conjugates
Three mg of single-walled carbon nanotubes (CNT) were mixed with 1.5 mg EDC and 0.9 mg NHS, dissolved in 3 mL of DMF, and stirred at room temperature for 2 h. Then the material was ultrasonicated for 3 min at room temperature, before 3 mg of PDDA was dissolved in 1 mL of 1 mol/L NaOH. CNT and PDDA solutions were mixed together and stirred at room temperature for 1 h. The mixture was then taken and centrifuged at room temperature for 15 min at 5000 rpm. Distilled water was added to precipitate, and the centrifuge cycle repeated. The final precipitate was oven-dried for 10 min at 68 °C, and the dried weight calculated. PBS was then added to obtain 1 mg/mL concentration.
CdTe QDs with free surface –COOH groups was mixed with methanol at 1:1 volume ratio, and centrifuged 5000 rpm at room temperature for 10 min. Supernatant was then discarded, and 1 mg/mL concentration of CdTe QDs solution was prepared. CdTe QDs and CNT-PDDA solutions were combined to make up final solution of 1:1 concentration ratio. Thus the CdTe-CNT conjugate was obtained.
3.4. Antibody-Labeling CdTe-CNT QDs Conjugate (ATM1 Ab1/CdTe-CNT)
Ten µL of ATM1 monoantibody (ATM1 Ab1) concentrates was mixed with 1 mL of CdTe-CNT complex; 5 mg of NHS and 5 mg EDC was added, and shaken for 20 min at room temperature. One hundred µL of diluted antibody solution and 5% BSA, which was employed for coating the active sites of CdTe QDs, were placed in a shaker at speed 30 for 10 min at room temperature. Mixtures were then centrifuged using 100 kDa filter system at room temperature for 10 min at 5000 rpm, before the supernatant was discarded. The ATM Ab1/CdTe-CNT signal tag can be acquired (
Figure 12).
Figure 12.
The synthesis steps for ATM Ab1/CdTe-CNT signal tag.
Figure 12.
The synthesis steps for ATM Ab1/CdTe-CNT signal tag.
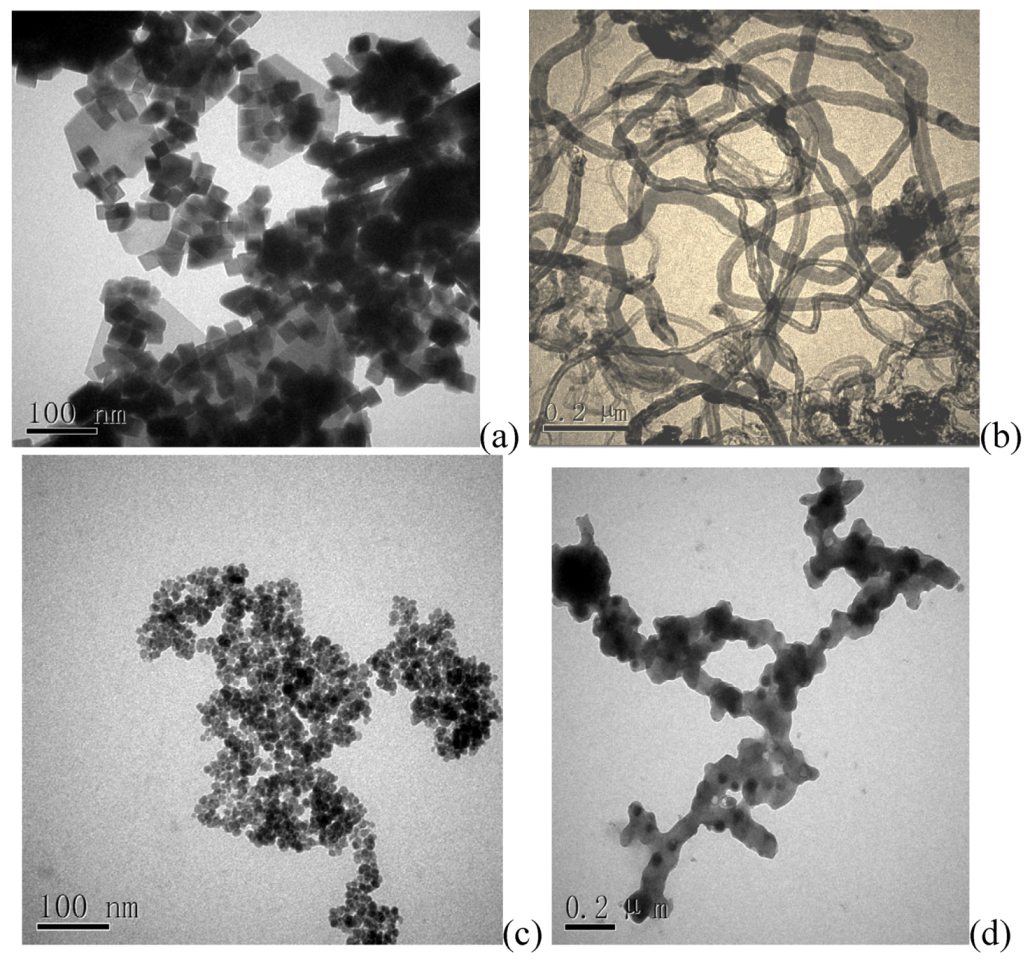
3.5. Synthesis of Fe-GO
The magnetic graphene oxide-Fe
3O
4 nanoparticles hybrid (Fe-GO) was synthesized by the
in situ chemical co-precipitation of Fe
2+ and Fe
3+ in an alkaline solution in the presence of GO according to the literature [
25].
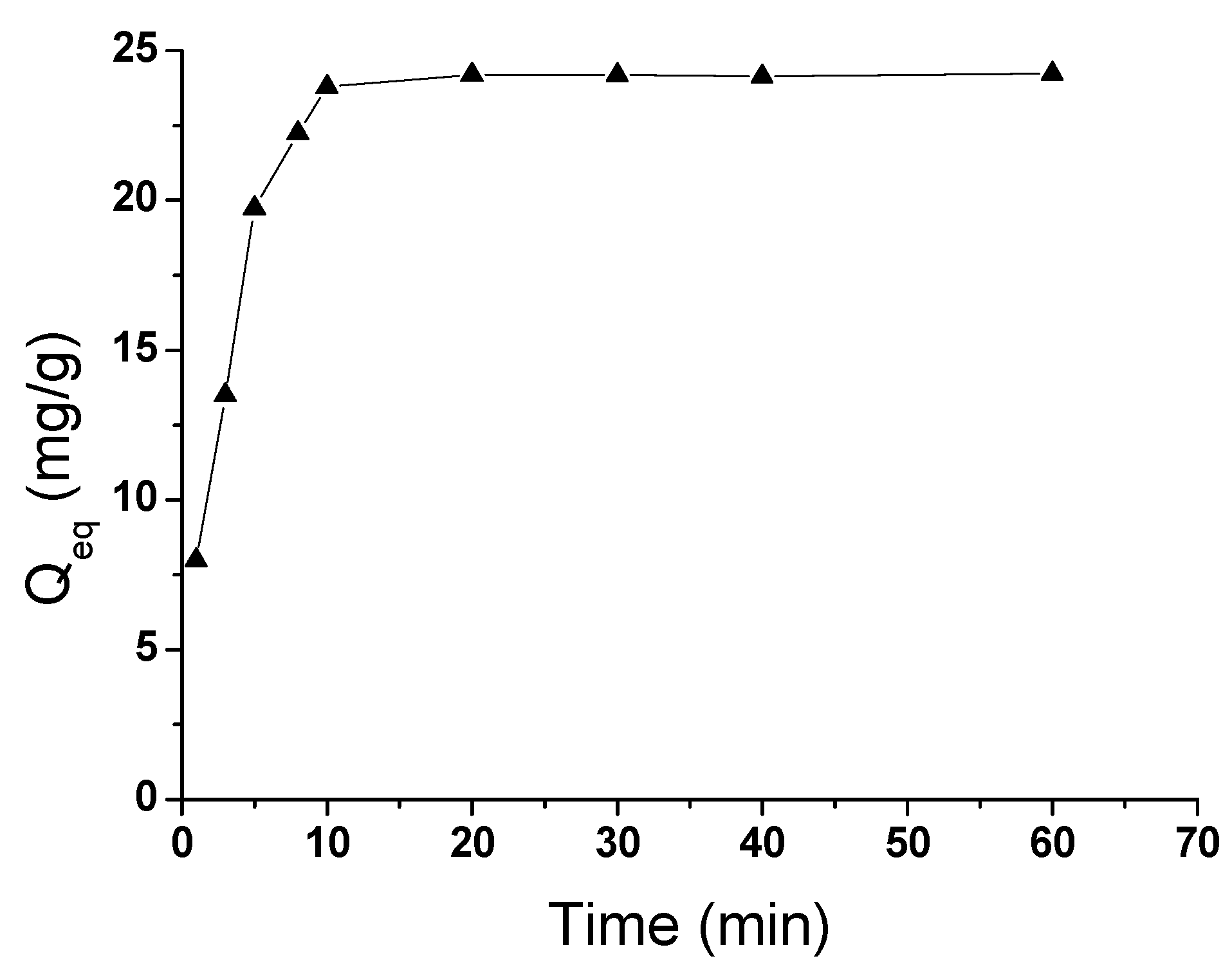
3.6. The Sandwich ECLA Procedure for Detection of ATM1 in Milk Samples
Milk samples were purchased from a grocery store. A series of milk samples spiked with different concentrations of ATM1 from 0.01 to 1.0 μg/mL were prepared. Each sample was diluted with PBS to 10 mL. The preparation of the sample consisted of centrifugation for defatting for 15 min at 6000 rpm. After centrifugation, the phases were completely separated into layers of fat, cream and skimmed milk from top to bottom, respectively. The defatted sample was tested directly.
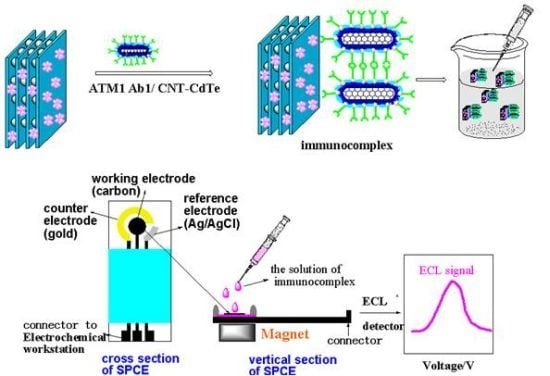
Figure 13.
The detection of ATM1 in samples by the sandwich immunoassay.
Figure 13.
The detection of ATM1 in samples by the sandwich immunoassay.
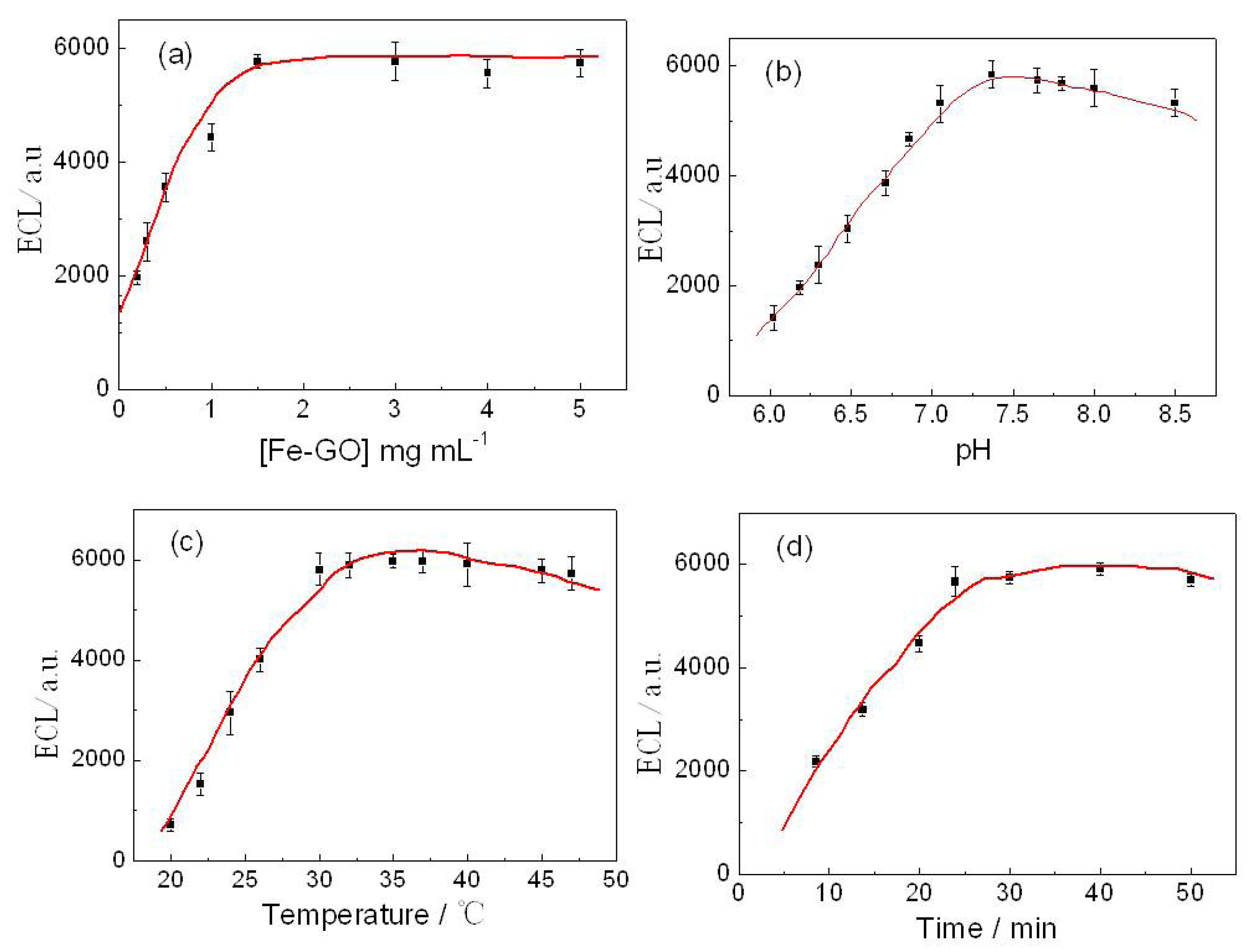
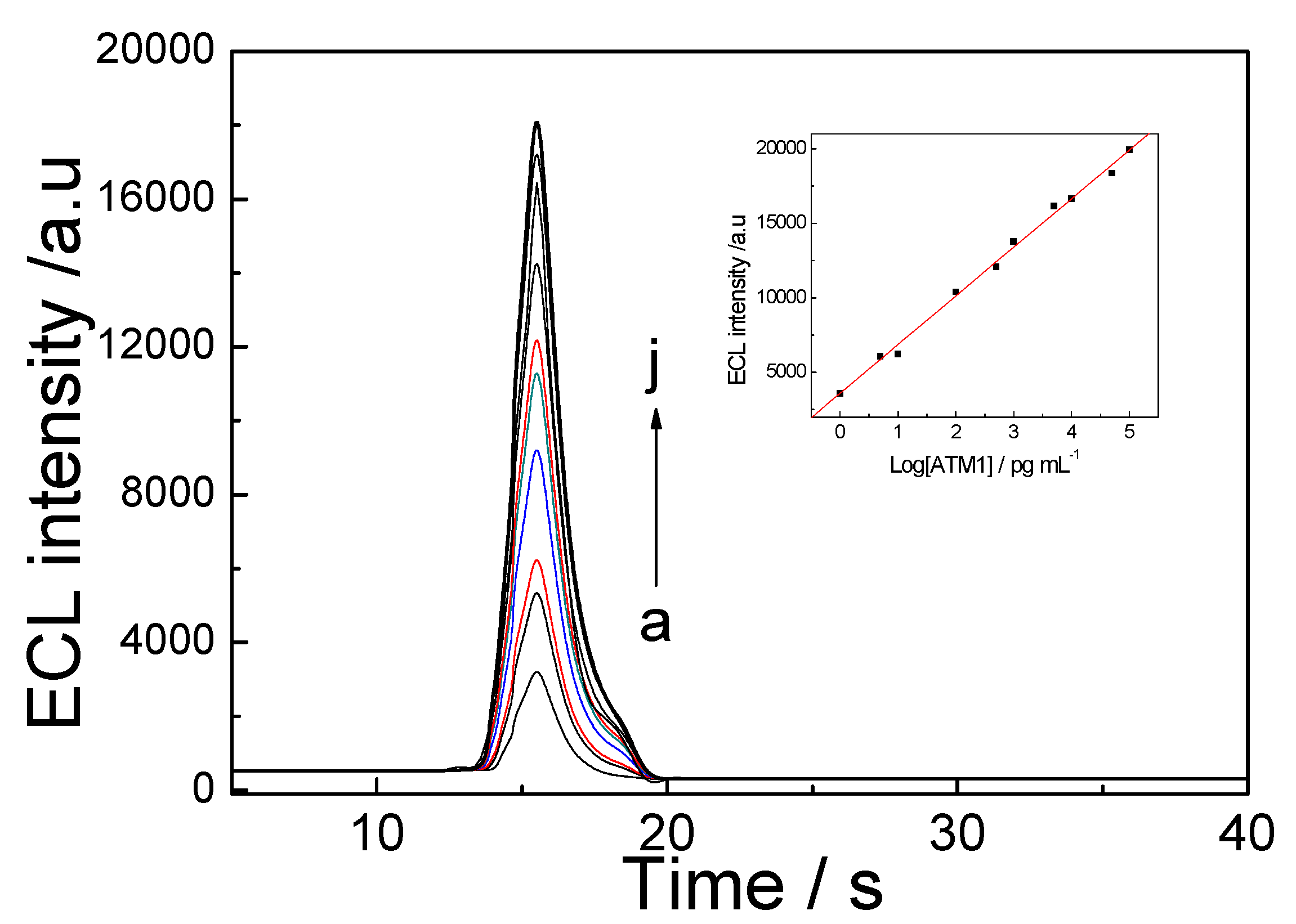
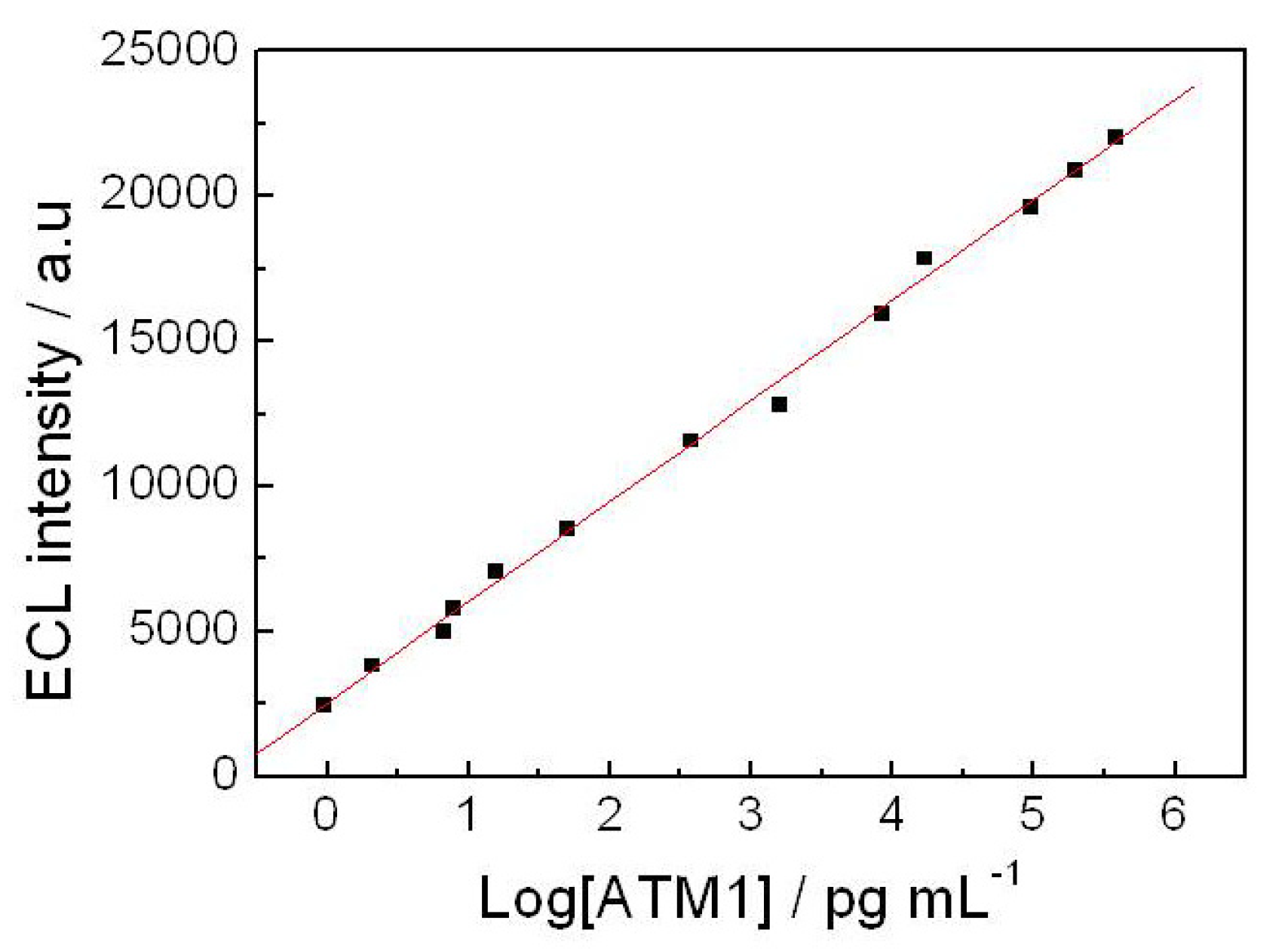
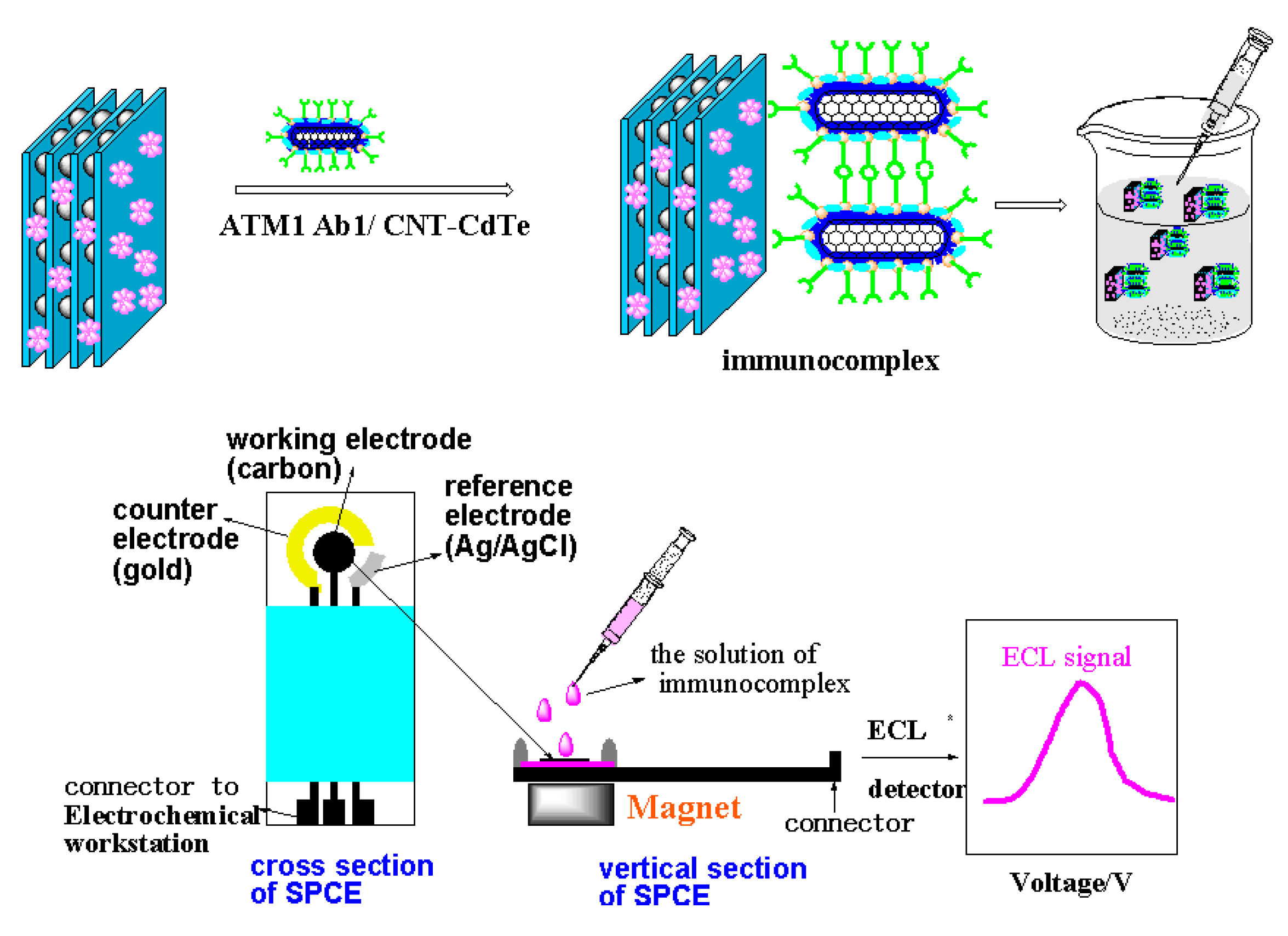
Different amounts of milk samples were then introduced into the tubes containing 300 µL of the 1.5 mg/mL Fe-GO, The mixture was incubated for 10 min at room temperature. When the reaction was over and the Fe-GO/ATM1 conjugation was formed, 200 µL of the ATM1 Ab1/CdTe-CNT was added to the above tube and incubated for another 25 min at 37 °C and then the incubation sandwich-type complex was formed. The mixture was magnetically purified for 5 min, and the precipitate was washed twice in a high-gradient magnetic field using pH 7.4 PBS buffer solution. Finally, the solution of sandwich immunocomplex was added drop-wise to the electrode’s surface, whose back was placed with a magnet in advance. The electrodes were scanned from 0 to −1.6 V with a scan rate of 100 mV/s, and ECL signals were measured (
Figure 13). After each detection, the electrode was instantaneously washed with PBS solution 5 times to remove the immunocomplex after removing the magnet. Standard curves were obtained using 1–10
5 pg/mL ATM1 for electrochemical immunoassay.
3.7. Material Characterization
The particle size and structure of the sorbents were characterized by a transmission electron microscope (TEM, JEOL 2100). Magnetic property was analyzed using a vibrating sample magnetometer (VSM, LDJ9600). The XRD characterization was performed using X-ray diffraction (Bruker D8 Focus, Breman, Germany) with Cu Kα radiation at room temperature.