Correlation of ATP Citrate Lyase and Acetyl CoA Levels with Trichothecene Production in Fusarium graminearum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Precocene II on ACL Expression
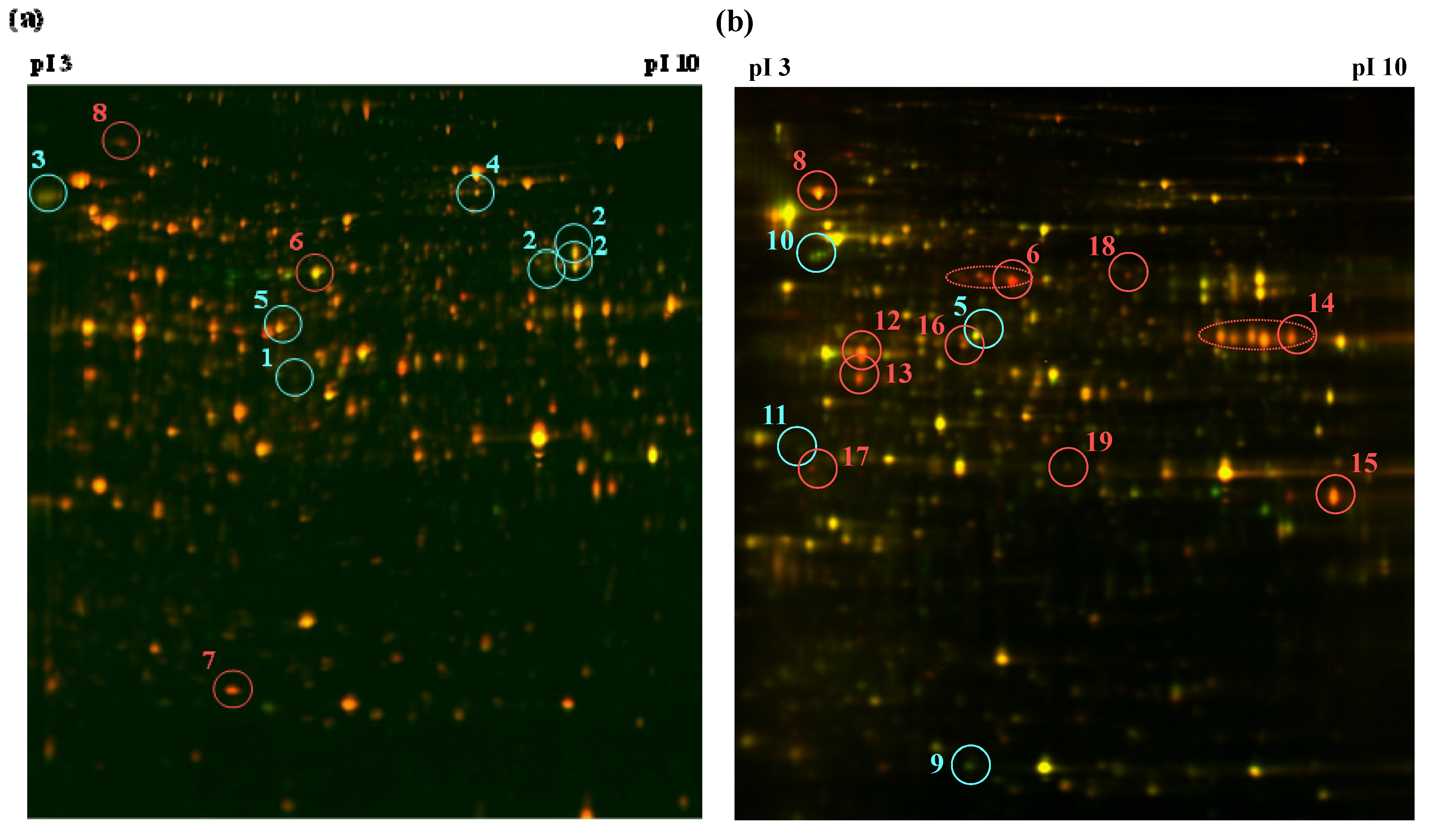
| Spot | Protein | Fold change a | pI b | kDa | Expect | Matched peptides | Sequence coverage (%) | |
|---|---|---|---|---|---|---|---|---|
| 2 days | 4 days | |||||||
| 1 | Formamidase | −1.43 | - | 5.6 | 44.2 | 3.1 × 10−10 | 9 | 29 |
| 2 | Serine carboxypeptidase | −1.53 | - | 6.5 | 59.7 | 0.0019 | 5 | 10 |
| 3 | Serine carboxypeptidase | −1.54 | - | 5.1 | 63.6 | 0.029 | 6 | 13 |
| 4 | Peroxidase catalase 2 | −1.43 | - | 5.8 | 81.3 | 3.1× 10−11 | 11 | 17 |
| 5 | ATP citrate lyase | −1.27 | −1.59 | 5.5 | 53.3 | 5.0 × 10−7 | 8 | 26 |
| 6 | Pyruvate decarboxylase | +1.60 | +4.29 | 5.6 | 63.5 | 2.0 × 10−13 | 12 | 34 |
| 7 | Glycolipid transfer protein | +1.33 | - | 5.7 | 22.4 | 6.0 × 10−5 | 5 | 30 |
| 8 | Cell division cycle protein 48 | +1.35 | +2.85 | 4.9 | 90.5 | 0.00087 | 6 | 11 |
| 9 | Superoxide dismutase | - | −2.27 | 5.6 | 27.7 | 4.0 × 10−7 | 6 | 29 |
| 10 | Kinesin heavy chain | - | −2.56 | 5.7 | 104.1 | 19 | 4 | 6 |
| 11 | Diphosphomevalonate decarboxylase | - | −1.77 | 5.3 | 41.0 | 2.2 | 3 | 7 |
| 12 | Enolase | - | +2.32 | 5.0 | 47.5 | 1.0 × 10−10 | 10 | 35 |
| 13 | Carboxypeptidase S1 | - | +2.18 | 5.5 | 52.3 | 6.3 × 10−7 | 8 | 28 |
| 14 | Acid phosphatase | - | +1.85 | 6.6 | 47.8 | 0.15 | 4 | 10 |
| 15 | Alcohol dehydrogenase 1 | - | +2.39 | 7.6 | 42.0 | 0.0061 | 5 | 19 |
| 16 | Aldehyde dehydrogenase | - | +1.60 | 5.4 | 53.9 | 1.0 × 10−12 | 11 | 32 |
| 17 | 40S ribosomal protein 50 | - | +2.34 | 4.8 | 31.8 | 3.1 × 10−10 | 8 | 30 |
| 18 | Pyruvate kinase | - | +2.78 | 5.8 | 60.0 | 4.8 × 10−5 | 7 | 13 |
| 19 | UDP glucose epimerase | - | +1.69 | 5.8 | 42.0 | 0.0051 | 5 | 20 |
2.2. Effects of Carbon Sources on Acetyl CoA Amount and ACL2 mRNA Level
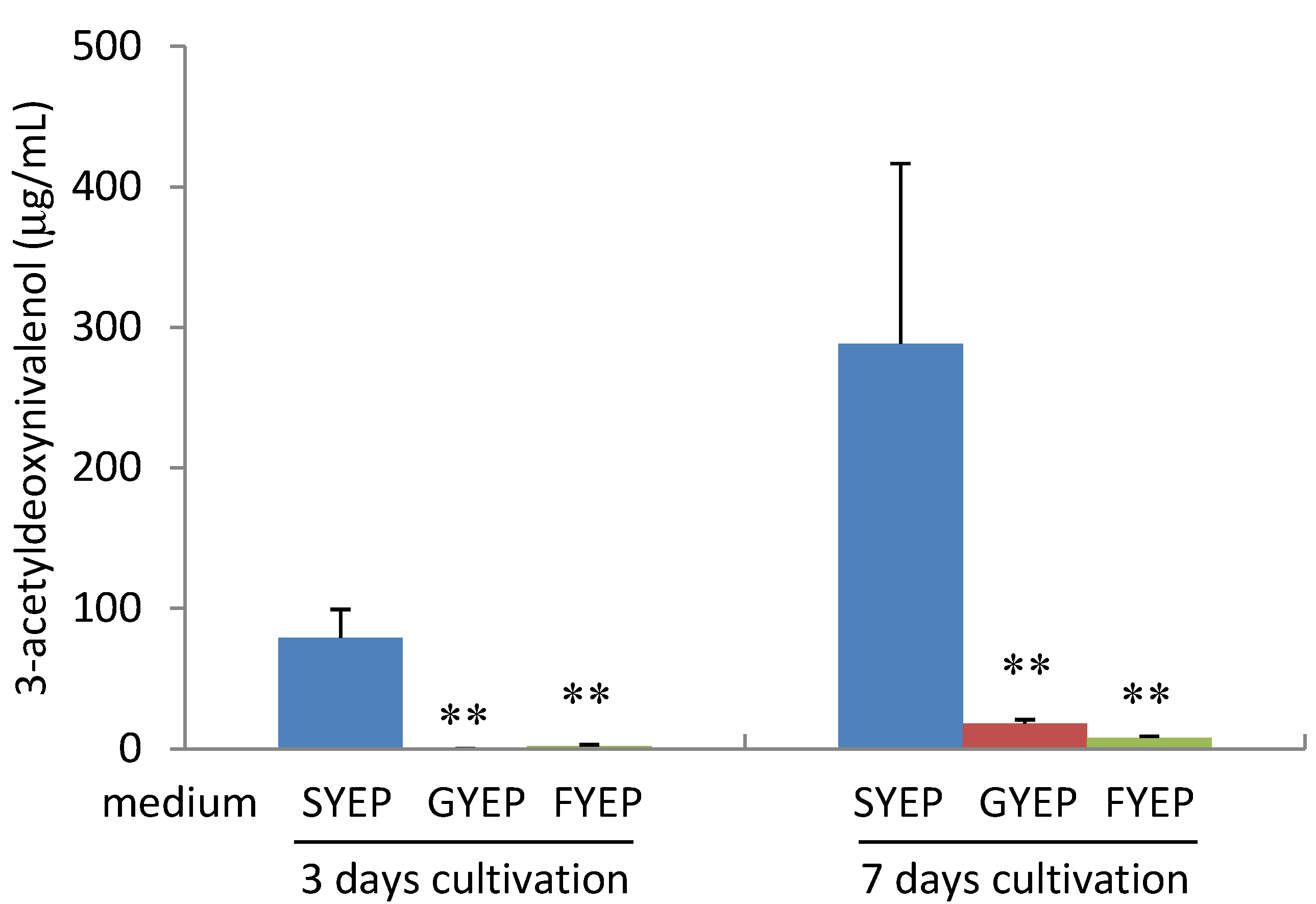
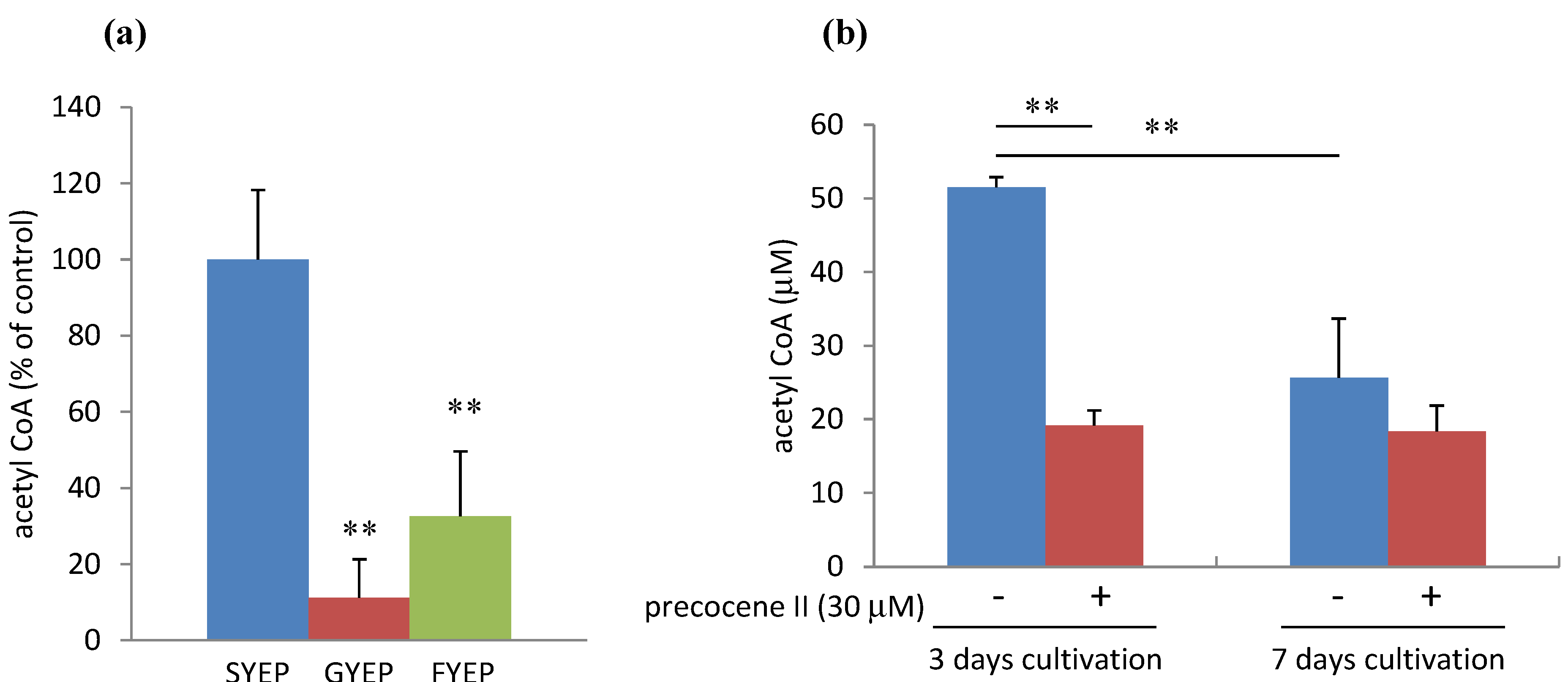

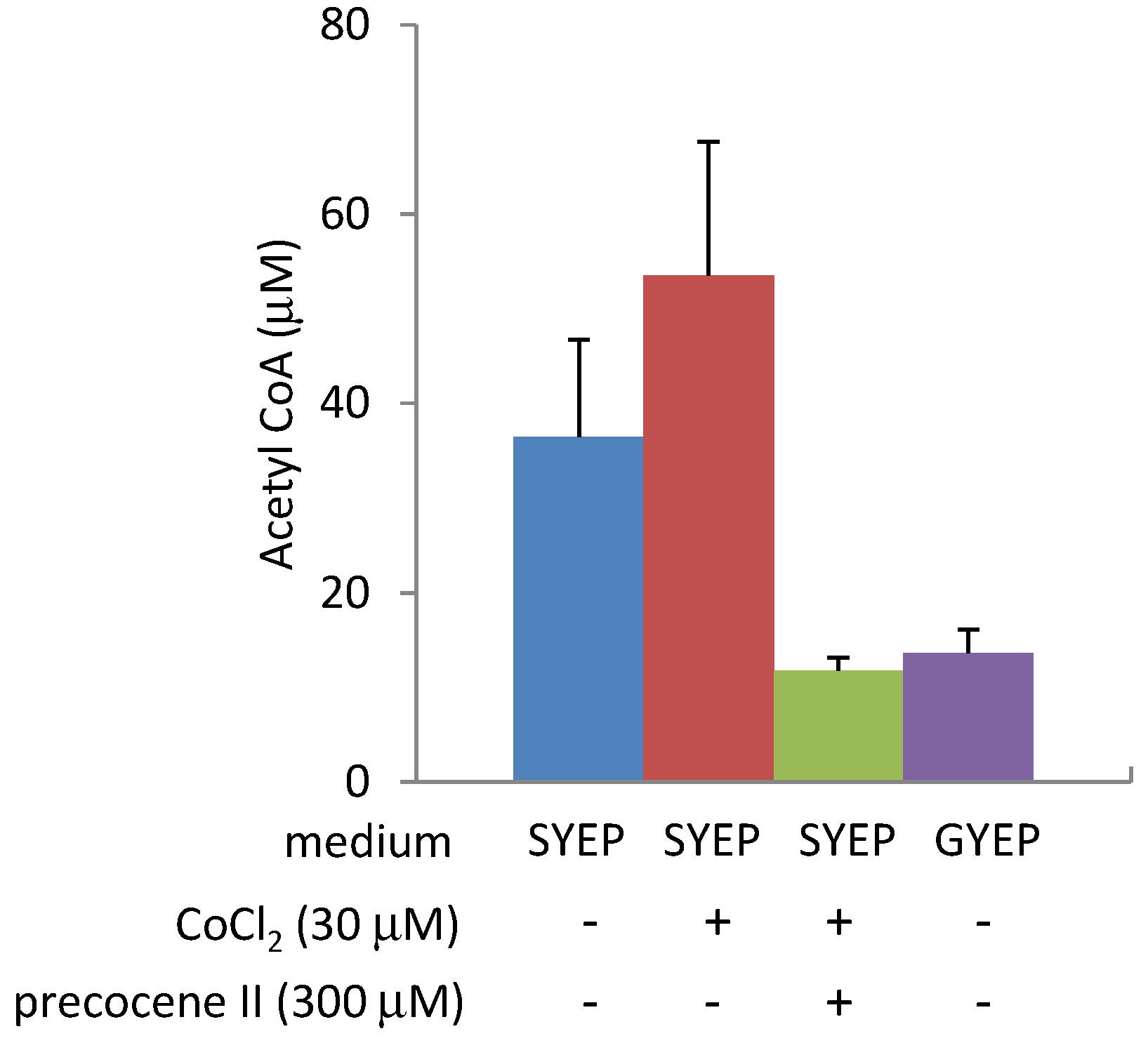
2.3. Effects of Precocene II on Acetyl CoA Amount and ACL2 mRNA Level
2.4. Effects of Cobalt Chloride on Acetyl CoA Amount and ACL2 mRNA Level
3. Experimental Section
3.1. F. graminearum Culture Conditions and Analysis of 3-Acetyldeoxynivalenol
3.2. 2D-DIGE Analysis
3.3. In-Gel Digestion and Protein Identification by Mass Spectrometry
3.4. Analysis of Acetyl CoA
3.5. RT-PCR Analysis
3.6. Data Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health 2005, 8, 39–69. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Luo, Q.; Yuan, S.; Zhou, M. Characterization and fitness of carbendazim-resistant strains of Fusarium graminearum (wheat scab). Pest. Manag. Sci. 2007, 63, 1201–1207. [Google Scholar] [CrossRef]
- Kimura, M.; Tokai, T.; Takahashi-Ando, N.; Ohsato, S.; Fujimura, M. Molecular and genetic studies of Fusarium trichothecene biosynthesis: Pathways, genes, and evolution. Biosci. Biotechnol. Biochem. 2007, 71, 2105–2123. [Google Scholar] [CrossRef]
- Hohn, T.M.; Krishna, R.; Proctor, R.H. Characterization of a transcriptional activator controlling trichothecene toxin biosynthesis. Fungal Genet. Biol. 1999, 26, 224–235. [Google Scholar] [CrossRef]
- Seong, K.-Y.; Pasquali, M.; Zhou, X.; Song, J.; Hilburn, K.; McCormick, S.; Dong, Y.; Xu, J.-R.; Kistler, H.C. Global gene regulation by Fusarium transcription factor Tri6 and Tri10 reveals adaptation for toxin biosynthesis. Mol. Microbiol. 2009, 72, 354–367. [Google Scholar] [CrossRef]
- Peplow, A.W.; Tag, A.G.; Garifullina, G.F.; Beremand, M.N. Identification of new genes positively regulated by Tri10 and a regulatory network for trichothecene mycotoxin production. Appl. Environ. Microbiol. 2003, 69, 2731–2736. [Google Scholar]
- Lee, S.; Son, H.; Lee, J.; Min, K.; Choi, G.J.; Kim, J.-C.; Lee, Y.-W. Functional analysis of two acetyl coenzyme A synthases in the ascomycetes Gibberella zeae. Eukaryot. Cell 2011, 10, 1043–1052. [Google Scholar] [CrossRef]
- Wynn, J.P.; Hamid, A.A.; Midgley, M.; Ratledge, C. Widespread occurrence of ATP: citrate lyase and carnitine acetyltransferase in filamentous fungi. World J. Microbiol. Biotechnol. 1998, 14, 145–147. [Google Scholar]
- Son, H.; Lee, J.; Park, A.R.; Lee, Y.-W. ATP citrate lyase is required for nomal sexual ans asexual development in Gibberella zeae. Fungal Genet. Biol. 2011, 48, 408–417. [Google Scholar] [CrossRef]
- Son, H.; Min, K.; Lee, J.; Choi, G.J.; Kim, J.-C.; Lee, Y.-W. Mitochondrial carnitine-dependant acetyl coenzyme A transport is required for normal sexual and asexual development of the ascomycete Gibberella zeae. Eukaryot. Cell 2012, 11, 1143–1153. [Google Scholar] [CrossRef]
- Jiao, F.; Kawakami, A.; Nakajima, T. Effects of different carbon sources on trichothecene production and Tri gene expression by Fusarium graminearum in liquid culture. FEMS Microbiol. Lett. 2008, 285, 212–219. [Google Scholar] [CrossRef]
- Yaguchi, A.; Yoshinari, T.; Tsuyuki, R.; Takahashi, H.; Nakajima, T.; Sugita-Konishi, Y.; Nagasawa, H.; Sakuda, S. Isolation and identification of precocenes and piperitone from essential oils as specific inhibitors of trichothecene production by Fusarium graminearum. J. Agric. Food Chem. 2009, 57, 846–851. [Google Scholar] [CrossRef]
- Tsuyuki, R.; Yoshinari, T.; Sakamoto, N.; Nagasawa, H.; Sakuda, S. Enhancement of trichothecene production in Fusarium graminearum by cobalt chloride. J. Agric. Food Chem. 2011, 59, 1760–1766. [Google Scholar] [CrossRef]
- Marough, R.; David, S.; Hawkins, E. The development of the DIGE system. 2D fluorescence difference gel analysis technology. Anal. Bioanal. Chem. 2005, 382, 669–678. [Google Scholar] [CrossRef]
- Yoshinari, T.; Noda, Y.; Yoda, K.; Sezaki, H.; Nagasawa, H.; Sakuda, S. Inhibitory activity of blasticidin A, a strong aflatoxin production inhibitor, on protein synthesis of yeast: Selective inhibition of aflatoxin production by protein synthesis inhibitors. J. Antibiot. 2010, 63, 309–314. [Google Scholar] [CrossRef]
- Ruijter, G.J.G.; Visser, J. Determination of intermediary metabolites in Aspergillus niger. J. Microbiol. Methods 1996, 25, 295–302. [Google Scholar] [CrossRef]
- Cuomo, C.A.; Guldener, U.; Xu, J.R.; Trail, F.; Turgeon, B.G.; di Pietro, A.; Walton, J.D.; Ma, L.J.; Baker, S.E.; Rep, M.; et al. The Fusarium graminearum genome reveals a link between localized polymprphism and pathogen specialization. Science 2007, 317, 1400–1402. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sakamoto, N.; Tsuyuki, R.; Yoshinari, T.; Usuma, J.; Furukawa, T.; Nagasawa, H.; Sakuda, S. Correlation of ATP Citrate Lyase and Acetyl CoA Levels with Trichothecene Production in Fusarium graminearum. Toxins 2013, 5, 2258-2269. https://doi.org/10.3390/toxins5112258
Sakamoto N, Tsuyuki R, Yoshinari T, Usuma J, Furukawa T, Nagasawa H, Sakuda S. Correlation of ATP Citrate Lyase and Acetyl CoA Levels with Trichothecene Production in Fusarium graminearum. Toxins. 2013; 5(11):2258-2269. https://doi.org/10.3390/toxins5112258
Chicago/Turabian StyleSakamoto, Naoko, Rie Tsuyuki, Tomoya Yoshinari, Jermnak Usuma, Tomohiro Furukawa, Hiromichi Nagasawa, and Shohei Sakuda. 2013. "Correlation of ATP Citrate Lyase and Acetyl CoA Levels with Trichothecene Production in Fusarium graminearum" Toxins 5, no. 11: 2258-2269. https://doi.org/10.3390/toxins5112258
APA StyleSakamoto, N., Tsuyuki, R., Yoshinari, T., Usuma, J., Furukawa, T., Nagasawa, H., & Sakuda, S. (2013). Correlation of ATP Citrate Lyase and Acetyl CoA Levels with Trichothecene Production in Fusarium graminearum. Toxins, 5(11), 2258-2269. https://doi.org/10.3390/toxins5112258




