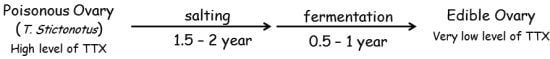
Removal of Toxin (Tetrodotoxin) from Puffer Ovary by Traditional Fermentation
Abstract
:1. Introduction

2. Results
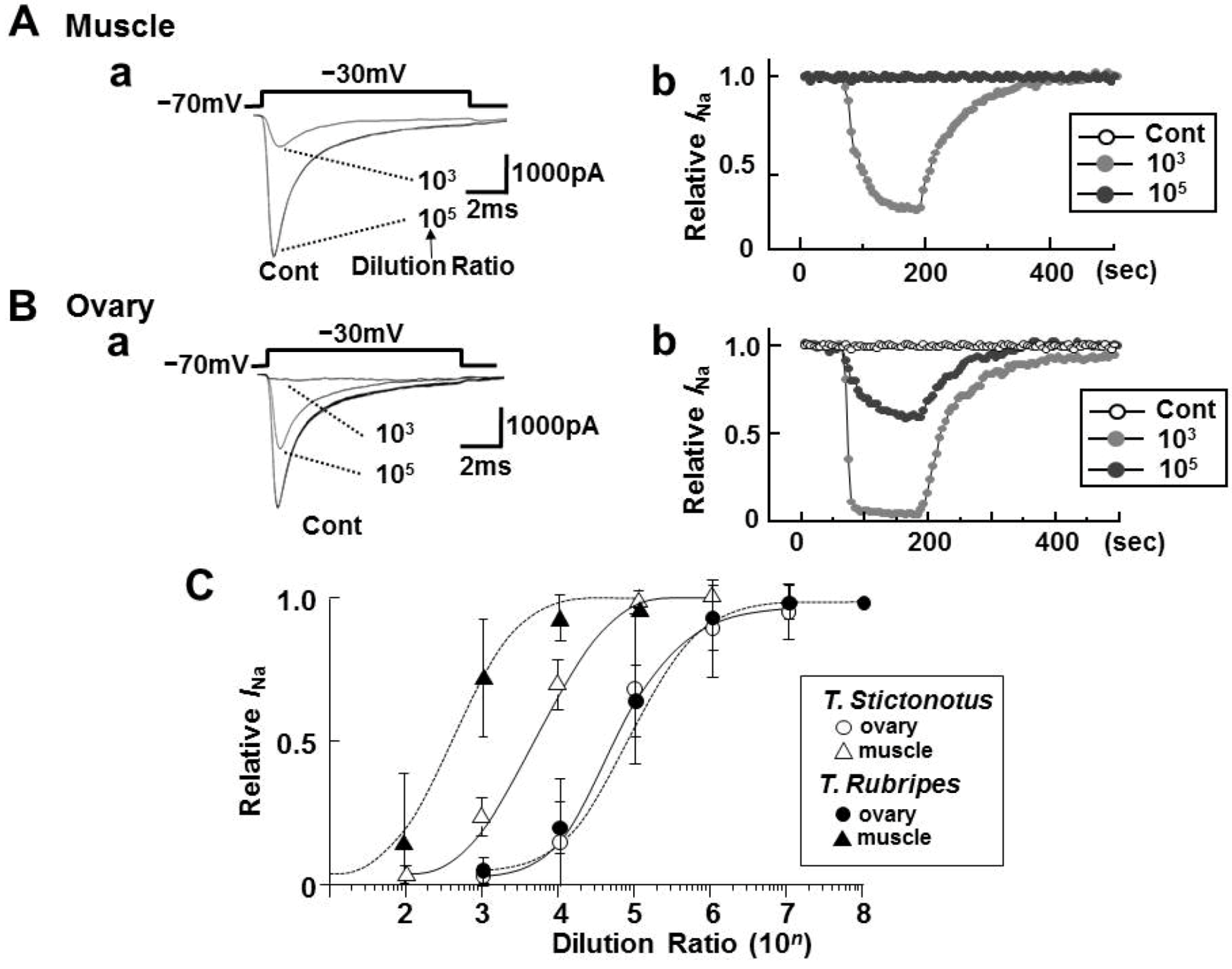
2.1. Effects of Toxin Extracts from the Muscle and Ovary of Puffers on INa
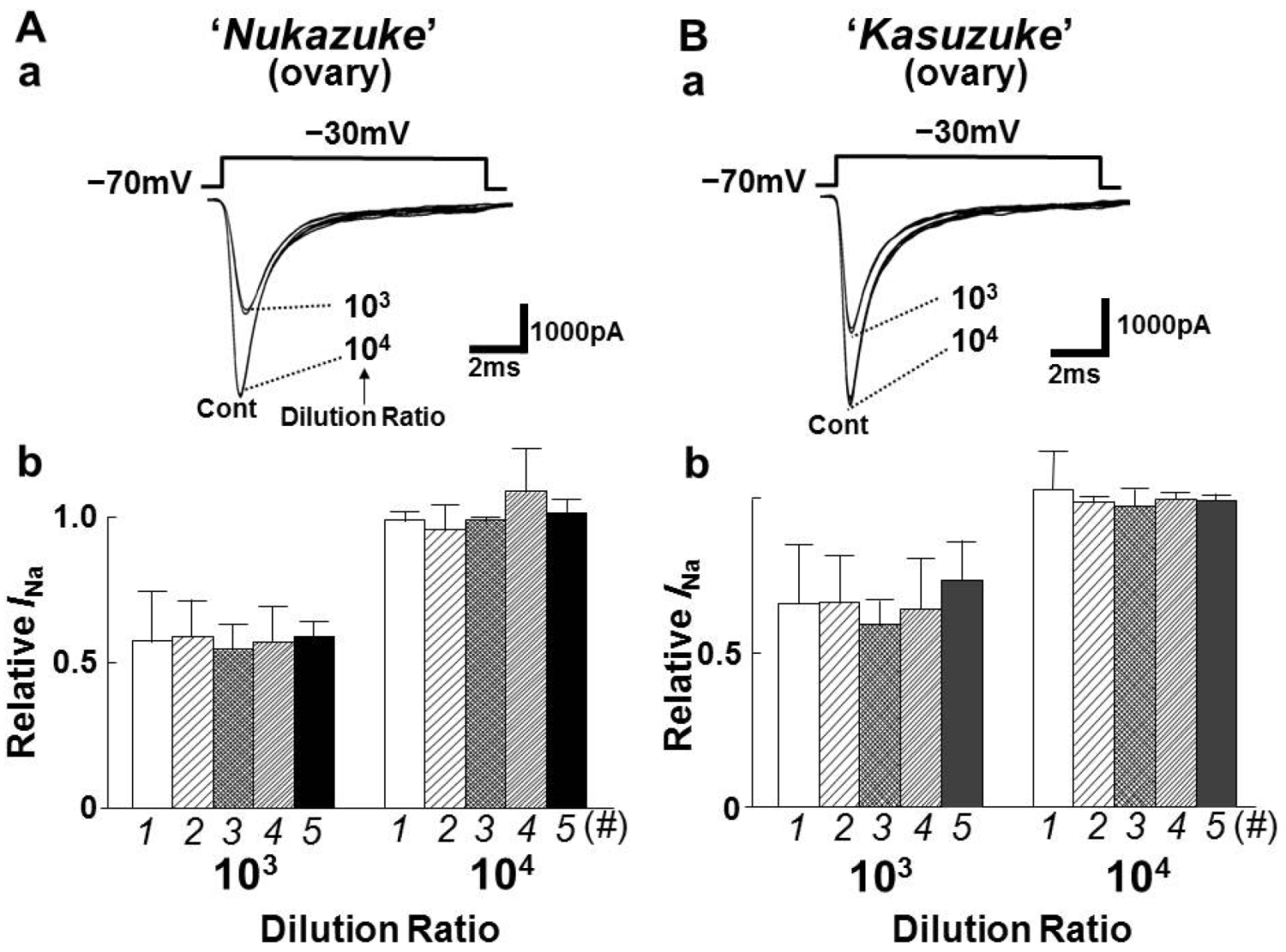
2.2. Effects of Toxin Extracts from “Nukazuke” and “Kasuzuke” Ovaries of T. Stictonotus
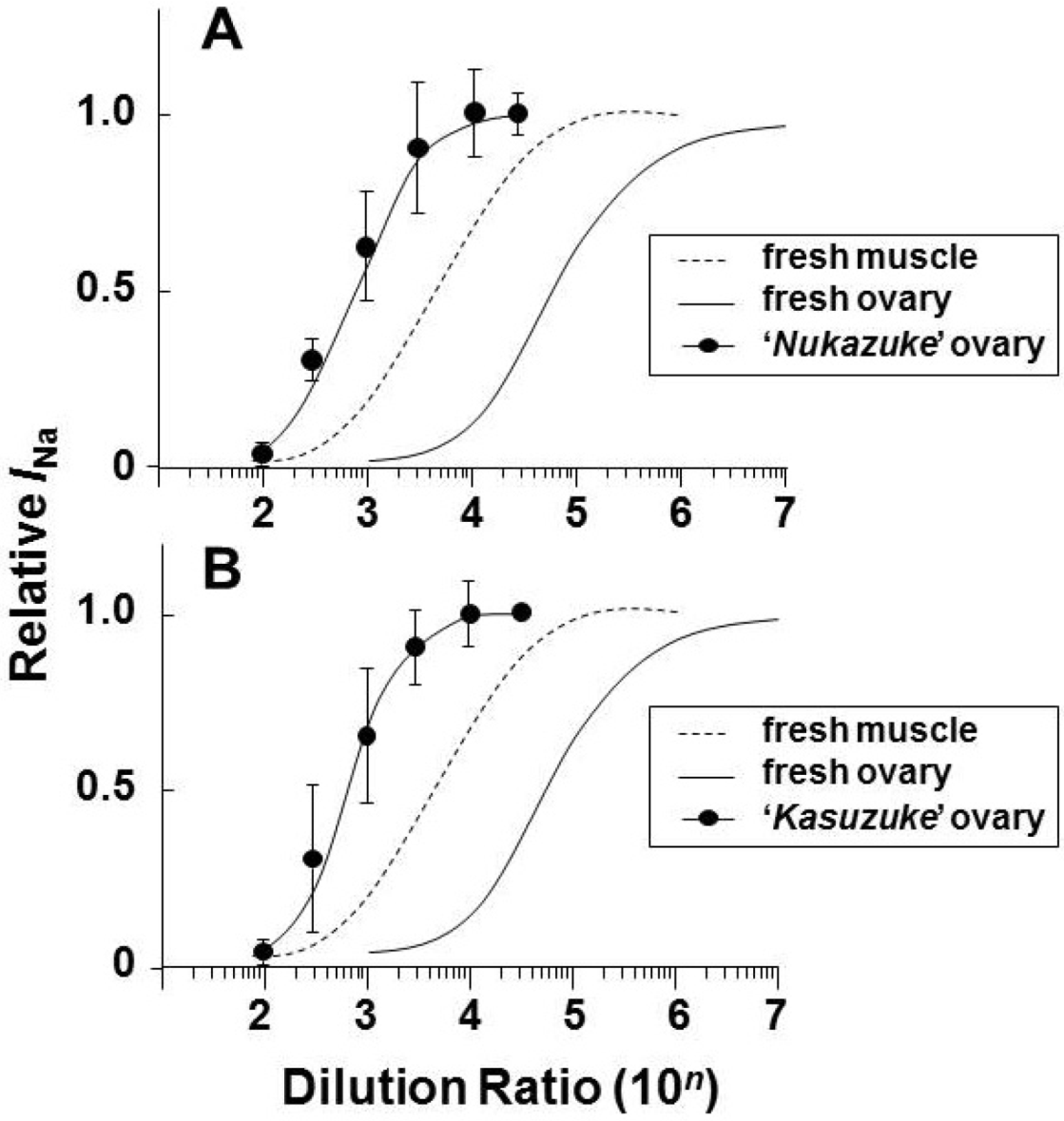
3.3. Estimated Toxin Amount in the Fresh and Fermented Ovaries of T. stictonotus
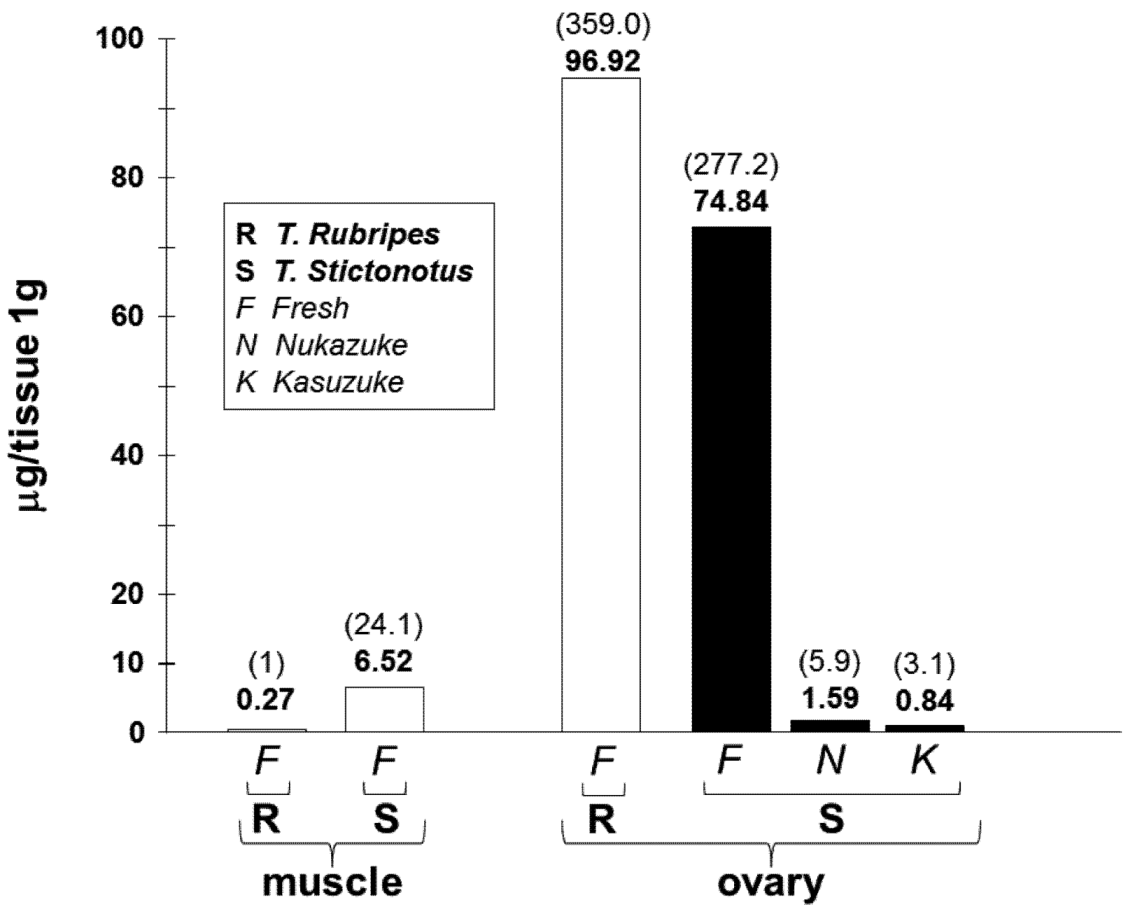
3. Discussions
4. Experimental Section
4.1. Puffers
4.2. Toxin Extract from the Fresh Muscle, and the Fresh, “Nukazuke” and “Kasuzuke” Ovaries of Puffers
4.3. Cell Preparation
4.4. Sodium Current Recording
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Ozawa, C. Toxicity of puffer roe pickled in rice-bran. J. Food Hyg. Safety Sci. 1983, 24, 258–262. [Google Scholar] [CrossRef]
- Ozawa, C. Sodium bicarbonate-accelerated detoxification of puffer ovaries during pickled product processing. Bull. Jpn. Soc. Sci. Fisheries 1986, 52, 2177–2181. [Google Scholar] [CrossRef]
- Sasaki, K.; Takayama, Y.; Tahara, T.; Anraku, K.; Ito, Y.; Akaike, N. Quantitative analysis of toxin extracts from various tissues of wild and cultured puffer fish by an electrophysiological method. Toxicon 2008, 51, 606–614. [Google Scholar] [CrossRef]
- Iwata, S.; Matsuura, T.; Yamamoto, S.; Tahara, T.; Shin, M.C.; Ito, Y.; Akaike, N. Inhibition of Na currents by the toxin extracts from puffer fishes captured in the sea coast of Japan. Toxicon 2010, 56, 999–1006. [Google Scholar] [CrossRef]
- Narahashi, T.; Moore, J.W.; Scott, W.R. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. J. Gen. Physiol. 1964, 47, 965–974. [Google Scholar]
- Kaneda, M.; Oyama, Y.; Ikemoto, Y.; Akaike, N. Blockade of the voltage-dependent sodium current in isolated rat hippocampal neurons by tetrodotoxin and lidocaine. Brain Res. 1989, 484, 348–351. [Google Scholar] [CrossRef]
- Kobayashi, T.; Okuzumi, M.; Fujii, T. Microflora of fermented puffer fish ovaries in rice-bran “Fugunoko Nukazuke”. Fisheries Sci. 1995, 61, 291–295. [Google Scholar]
- Kobayashi, T.; Kimura, B.; Fujii, T. Possibility of toxin declination by microbiological metabolism during manufacture of puffer fish ovaries fermented with rice-bran. Bull. Jpn. Soc. Sci. Fisheries 2003, 69, 782–786. [Google Scholar] [CrossRef]
- Noguchi, T.; Arakawa, O. Tetrodotoxin-distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs 2008, 6, 220–242. [Google Scholar] [CrossRef]
- Ikeda, K.; Emoto, U.; Tatsuno, R.; Wang, J.J.; Ngy, L.; Tanimiya, S.; Takatani, T.; Arakawa, O. Maturation-associated Chages in toxicity of the pufferfish Takifugu poecilonotus. Toxicon 2010, 55, 289–297. [Google Scholar]
- T Hori, S.; Tanaka, T.; Murakami, R. Puffer Toxin. In Methods of Analysis in Health Science; Kanehara Co., Ltd.: Tokyo, Japan, 2005; pp. 278–285. [Google Scholar]
- Akaike, N.; Moorhouse, A.J. Techniques applications of the nerve-bouton preparation in neuropharmacology. Trends Pharmacol. Sci. 2003, 24, 44–47. [Google Scholar]
- Shin, M.C.; Wakita, M.; Xie, D.J.; Yamaga, T.; Iwata, S.; Torii, Y.; Harakawa, T.; Ginnaga, A.; Kozaki, S.; Akaike, N. Inhibition of membrane Na+ channels by A type botulinum toxin at femtomolar concentrations in central and peripheral neurons. J. Pharmacol. Sci. 2012, 118, 33–42. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Anraku, K.; Nonaka, K.; Yamaga, T.; Yamamoto, T.; Shin, M.-C.; Wakita, M.; Hamamoto, A.; Akaike, N. Removal of Toxin (Tetrodotoxin) from Puffer Ovary by Traditional Fermentation. Toxins 2013, 5, 193-202. https://doi.org/10.3390/toxins5010193
Anraku K, Nonaka K, Yamaga T, Yamamoto T, Shin M-C, Wakita M, Hamamoto A, Akaike N. Removal of Toxin (Tetrodotoxin) from Puffer Ovary by Traditional Fermentation. Toxins. 2013; 5(1):193-202. https://doi.org/10.3390/toxins5010193
Chicago/Turabian StyleAnraku, Kensaku, Kiku Nonaka, Toshitaka Yamaga, Takatoshi Yamamoto, Min-Chul Shin, Masahito Wakita, Ayaka Hamamoto, and Norio Akaike. 2013. "Removal of Toxin (Tetrodotoxin) from Puffer Ovary by Traditional Fermentation" Toxins 5, no. 1: 193-202. https://doi.org/10.3390/toxins5010193
APA StyleAnraku, K., Nonaka, K., Yamaga, T., Yamamoto, T., Shin, M.-C., Wakita, M., Hamamoto, A., & Akaike, N. (2013). Removal of Toxin (Tetrodotoxin) from Puffer Ovary by Traditional Fermentation. Toxins, 5(1), 193-202. https://doi.org/10.3390/toxins5010193