Cytotoxicity and Glycan-Binding Properties of an 18 kDa Lectin Isolated from the Marine Sponge Halichondria okadai
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal and Chemicals
2.2. Purification of Lectin
2.3. Determination of Molecular Mass Using Gel Permeation Chromatography and SDS-PAGE
2.4. Detection of Saccharides in Polypeptide of the Lectin
2.5. Hemagglutination and Carbohydrate-Binding Specificity
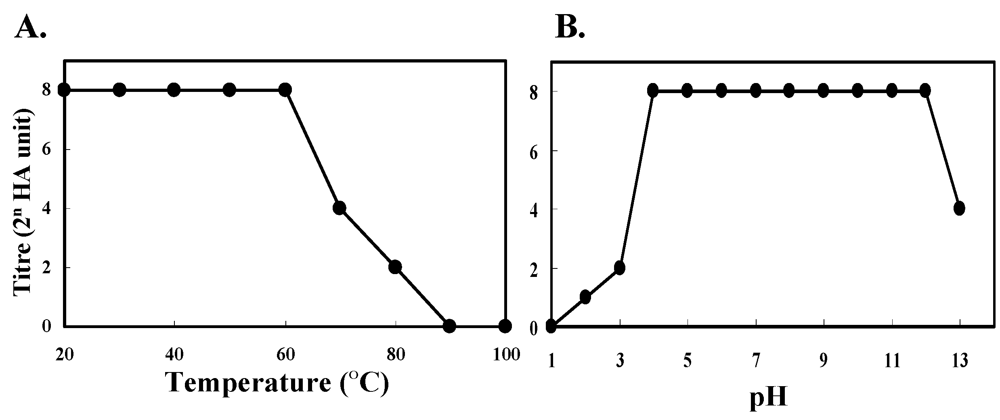
2.6. Effects of Divalent Cations, Sulfhydryl Preservation Reagent, Temperature and pH
2.7. Kinetic Analysis Using Surface Plasmon Resonance
2.8. Glycan-Binding Property by FACT
2.9. The Cytotoxicity
3. Results and Discussion
3.1. Purification of HOL-18
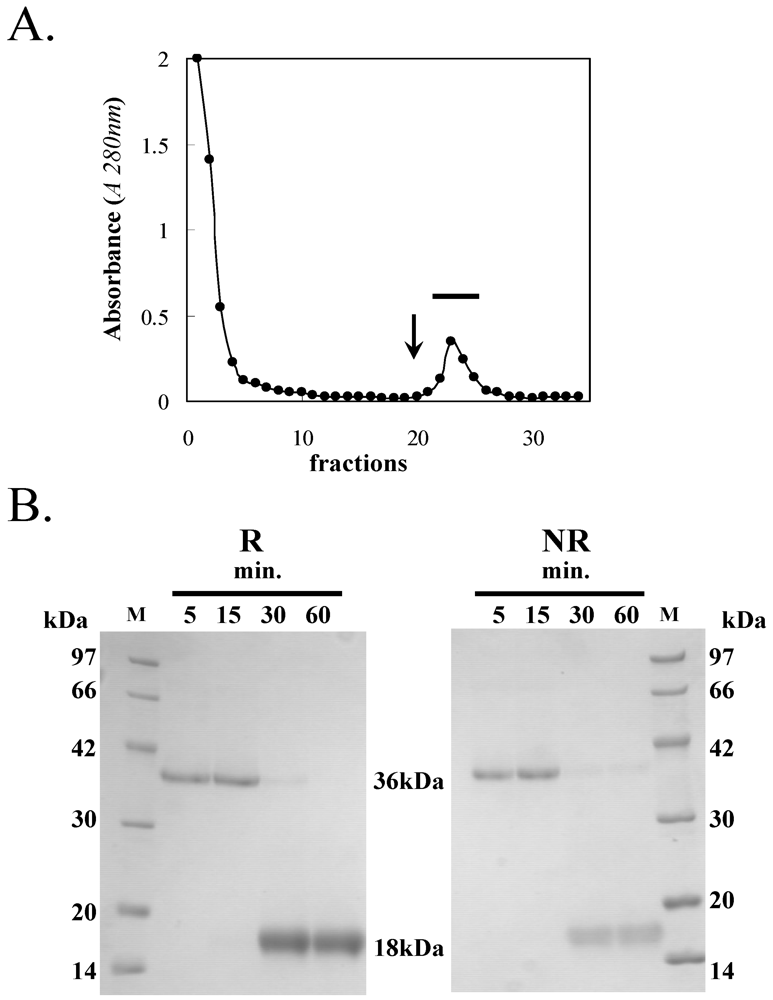
| Steps | Titer (HU) | Volume (mL) | Total activity a | Protein conc. (mg mL−1) | Specific activity b | Purification ratio (fold) | Recovery of activity (%) |
|---|---|---|---|---|---|---|---|
| Crude extract obtained by TBS | 1024 | 200 | 204,800 | 2.3 | 2.2 | 1 | 100 |
| Chitooligo-agarose | 4096 | 10 | 40,960 | 0.7 | 585 | 292 | 20 |
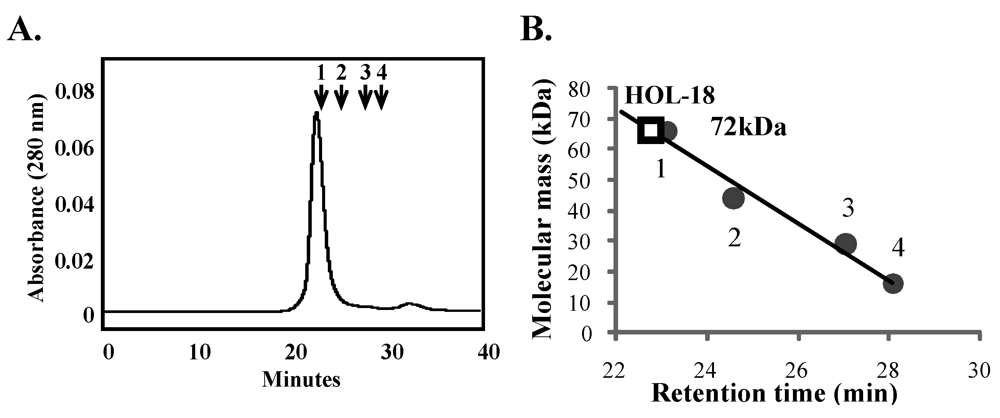
3.2. Saccharide-Binding Specificity of HOL-18
| Saccharides | Minimum inhibitory concentration (mM) |
|---|---|
| Chitobiose | 0.8 |
| Chitotriose | 0.4 |
| D-Galactose | N.I. a |
| Methyl α-N-Acetyl D-glucosamine | 3.13 |
| Methyl β-N-Acetyl D-glucosamine | 3.13 |
| Methyl α-N-Acetyl D-galactosamine | 6.25 |
| Methyl β-N-Acetyl D-galactosamine | 12.5 |
| D-Glucose | N.I. |
| D-Mannose | N.I. |
| L-Fucose | N.I. |
| Lactose | N.I. |
| Sucrose | N.I. |
| Glycoproteins | Minimum inhibitory concentration (mg/mL) |
| PSM | 0.01 |
| BSM | 0.125 |
| Fetuin | 0.125 |
3.3. Glycan Moiety of HOL-18 Polypeptide
3.4. Association and Dissociation Rates of the Lectin

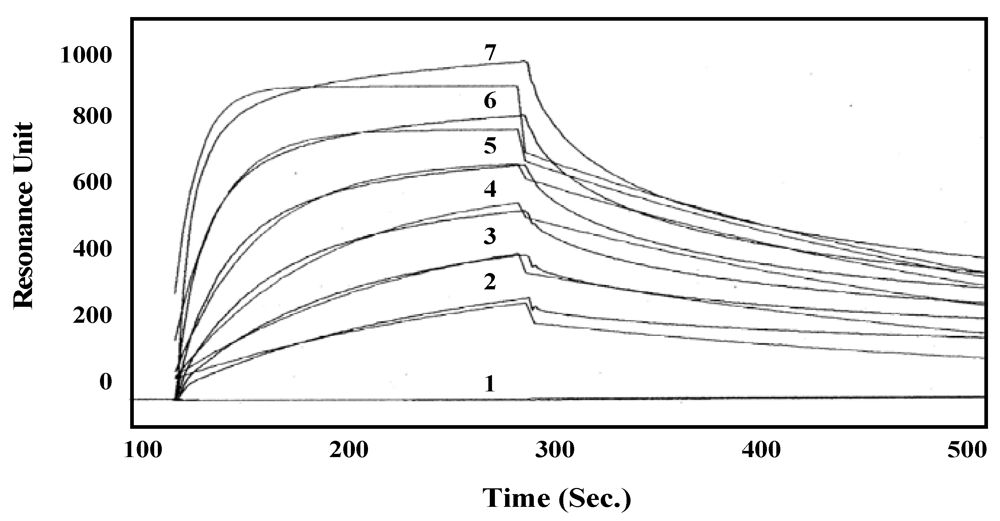
3.5. The Glycan-Binding Property
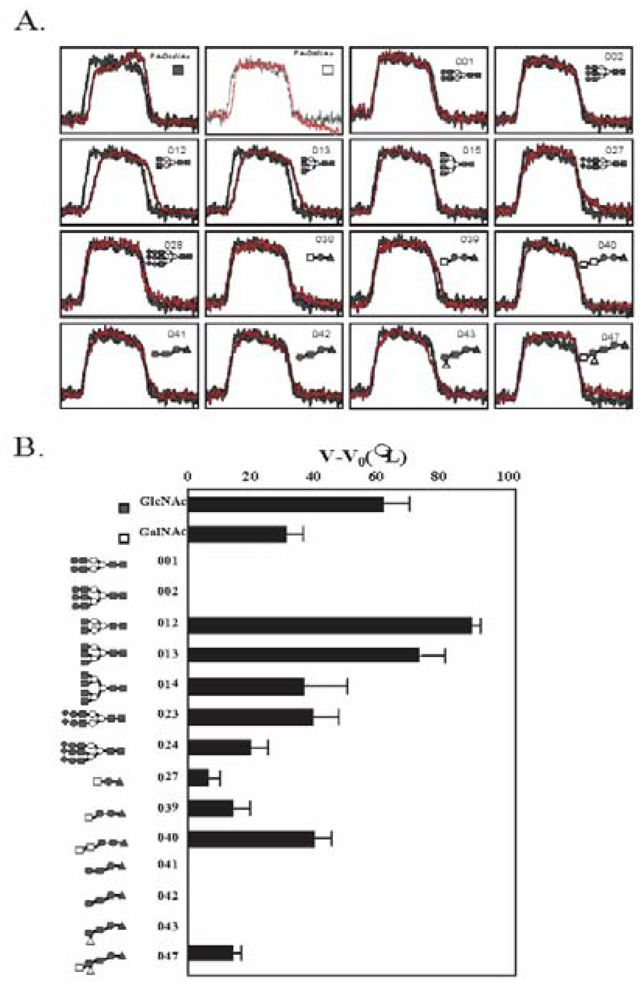
| PA No. | Glycoprotein type PA-oligosaccharide | PA No. | Glycosphingolipid type PA-oligosaccharide |
|---|---|---|---|
| [001] |  | [027] | GalNAcβ1-4Galβ1-4Glc-PA |
| [002] | 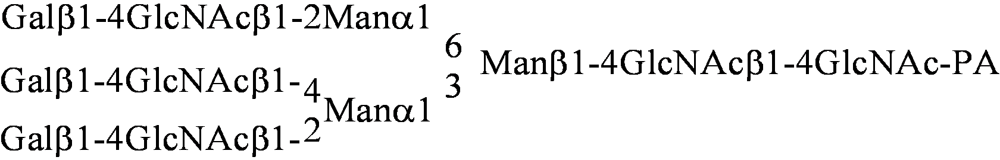 | [039] | GalNAcβ1-3Galα1-4Galβ1-4Glc-PA |
| [012] | 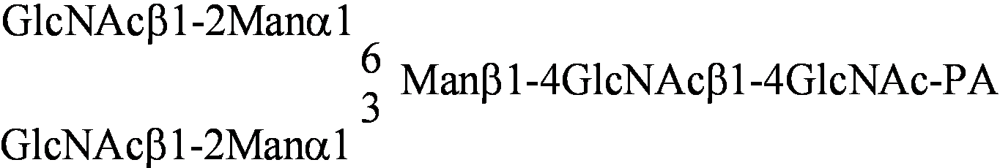 | [040] | GalNAcα1-3GalNAcβ1-3Galα1-4Galβ1-4Glc-PA |
| [013] | 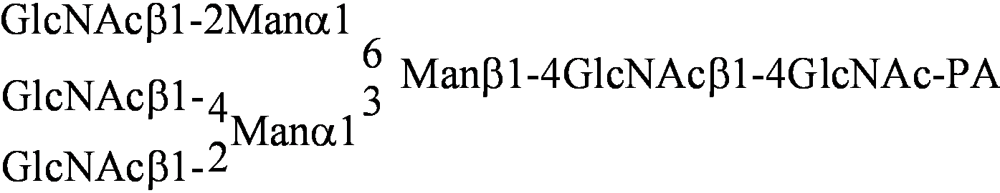 | [041] | Galβ1-4GlcNAcβ1-3Galβ1-4Glc-PA |
| [014] | 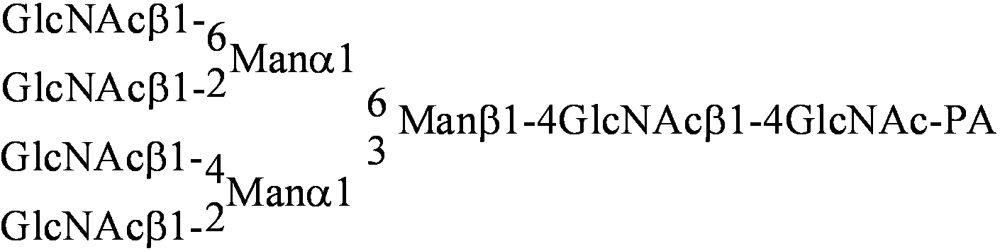 | [042] | Galβ1-3GlcNAcβ1-3Galβ1-4Glc-PA |
| [023] |  | [043] | Fucα1-2Galβ1-3GlcNAcβ1-3Galβ1-4Glc-PA |
| [024] |  | [047] | GalNAcα1-3(Fucα1-2)Galβ1-3GlcNAcβ1-3Galβ1-4Glc-PA |
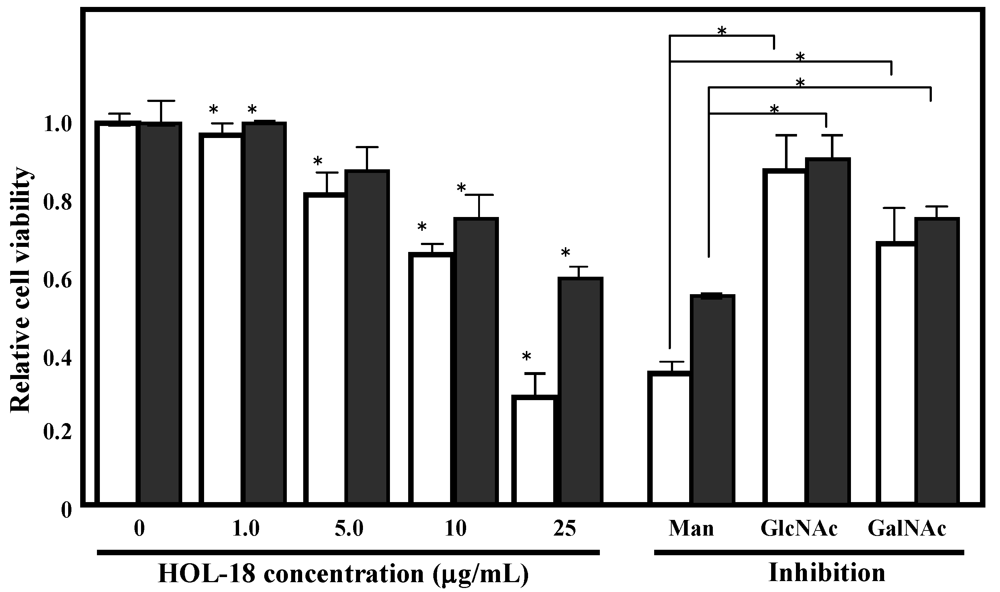
3.6. Glycan-Dependent Cytotoxicity
4. Conclusions
Acknowledgments
References
- Ogawa, T.; Watanabe, M.; Naganuma, T.; Muramoto, K. Diversified carbohydrate-binding lectins from marine resources. J. Amino Acids 2011, 2011. [Google Scholar]
- Paunto, P.C.; de Sukva, M.A.; Linardi, A.; Buzin, M.P.; Melo, S.E.S.F.C.; Mello, S.M.; Prado-Franceschi, J.; Hyslop, S. Biological activities of a lectin from Bothrops jararacussu snake venom. Toxicon 2006, 47, 21–31. [Google Scholar] [CrossRef]
- Sriwilaijaroen, N.; Kondo, S.; Yagi, H.; Wilairat, P.; Hiramatsu, H.; Ito, M.; Ito, Y.; Kato, K.; Suzuki, Y. Analysis of N-glycans in embrhonated chicken egg chorioallantoic and amniotic cells responsible for binding and adaptation of human and avian influenza viruses. Glycoconj. J. 2009, 26, 433–443. [Google Scholar] [CrossRef]
- Bai, R.; Nguyen, T.L.; Burnett, J.C.; Atasoylu, O.; Munro, M.H.; Pettit, G.R.; Smith, A.B.; Gussio, R.; Hamel, E. Interaction of halichondrin B and eribulin with tubulin. J. Chem. Inf. Model 2011, 51, 1393–1404. [Google Scholar]
- Schröder, H.C.; Breter, H.C.; Fattorusso, E.; Ushijima, H.; Wiens, M.; Steffen, R.; Batel, R.; Müller, W.E.G. Okadaic acid, an apoptogenic toxin form symbiotic parasitic annelids in the demosponge Suberites domuncula. Appl. Environ. Microbiol. 2006, 72, 4907–4916. [Google Scholar]
- Pfeifer, K.; Haasemann, M.; Gamulin, V.; Bretting, H.; Fahrenholz, F.; Müller, W.E.G. S-type lectins occur also in invertebrates: High conservation of the carbohydrate recognition domain in the lectin genes from the marine sponge Geodia cydonium. Glycobiology 1993, 3, 179–184. [Google Scholar] [CrossRef]
- Schröder, H.C.; Boreiko, A.; Korzhev, M.; Tahir, M.N.; Tremel, W.; Eckert, C.; Ushijima, H.; Müller, I.M.; Müller, W.E.G. Co-expression and functional interaction of silicatein with galectin; matrix-guided formation of siliceous spicules in the marine demosponge Suberites domuncula. J. Biol. Chem. 2006, 281, 12001–12009. [Google Scholar]
- Dresh, R.R.; Zanetti, G.D.; Lerner, C.B.; Trindade, V.M.; Henriques, A.T.; Vozári-Hampe, M.M. ACL-1, a lectin from the marine sponge Axinella corrugate: Isolation, characterization and chemotactic activity. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 148, 23–30. [Google Scholar]
- Schröder, H.C.; Ushijima, H.; Krasko, A.; Gamulin, V.; Thakur, N.L.; Diehl-Seifert, B.; Müller, I.M.; Müller, W.E.G. Emergence and disappearance of an immune molecule, an antimicrobial lectin, in basal metazoa; a tachylectin-related protein in the sponge Suberites domuncula. J. Biol. Chem. 2003, 278, 32810–32817. [Google Scholar]
- Funayama, N.; Nakatsukasa, M.; Kuraku, S.; Takechi, K.; Dohi, M.; Iwabe, N.; Miyata, T.; Agata, K. Isolation of Ef silicatein and Ef lectin as molecular markers for sclerocytes and cells involved in innate immunity in the freshwater sponge Ephydatia fluviatilis. Zool. Sci. 2005, 22, 1113–1122. [Google Scholar] [CrossRef]
- Kawsar, S.M.A.; Fujii, Y.; Matsumoto, R.; Ichikawa, T.; Tateno, H.; Hirabayashi, J.; Yasumitsu, H.; Dogasaki, C.; Hosono, M.; Nitta, K.; Hamako, J.; Matsui, T.; Ozeki, Y. Isolation, purification, characterization and glycan-binding profile of a D-galactoside specific lectin from the marine sponge, Halichondria okad. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 150, 349–357. [Google Scholar] [CrossRef]
- Kawagishi, H.; Yamawaki, M.; Isobe, S.; Usui, T.; Kimura, A.; Chiba, S. Two lectins from the marine sponge Halichondria okadai. An N-acetyl-sugar-specific lectin (HOL-I) and an N-acetyllactosamine-specific lectin (HOL-II). J. Biol. Chem. 1994, 269, 1375–1379. [Google Scholar]
- Hirabayashi, J.; Arata, Y.; Kasai, K-I. Frontal affinity chromatography as a tool for elucidation of sugar recognition properties of lectins. Methods Enzymol. 2003, 362, 353–368. [Google Scholar]
- Hirabayashi, J.; Hashidate, T.; Arata, Y.; Nishi, N.; Nakamura, T.; Hirashima, M.; Urashima, T.; Oka, T.; Futai, M.; Müller, W.E.G.; Yagi, F.; Kasai, K. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim.Biophys. Acta 2002, 1572, 232–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar]
- Wiechelman, K.J.; Braun, R.D.; Fitzpatrick, J.D. Investigation of the bicinchoninic acid protein assay: Identification of the groups responsible for color formation. Anal. Biochem. 1988, 175, 231–237. [Google Scholar]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal.Biochem. 1985, 150, 76–85. [Google Scholar]
- Kyhse-Andersen, J. Electroblotting of multiple gels a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J. Biochem. Biophys. Methods 1984, 10, 203–209. [Google Scholar]
- Dutta, C.; Henry, H.L. Detection of hemoprotein peroxidase activity on polyvinylidene difluoride membrane. Anal. Biochem. 1990, 184, 96–99. [Google Scholar] [CrossRef]
- Gourdine, J.P.; Cioci, G.; Miguet, L.; Unverzagt, C.; Silva, D.V.; Varrot, A.; Gautier, C.; Smith-Ravin, E.J.; Imberty, A. High affinity interaction between a bivalve C-type lectin and a biantennary complex-type N-glycan revealed by crystallography and microcalorimetry. J. Biol. Chem. 2008, 283, 30112–30120. [Google Scholar]
- Shinohara, Y.; Kim, F.; Shimizu, M.; Goto, M.; Tosu, M.; Hasegawa, Y. Kinetic measurement of the interaction between an oligosaccharide and lectins by a biosensor based on surface plasmon resonance. Eur. J. Biochem. 1994, 223, 189–194. [Google Scholar]
- Kawano, T.; Sugawara, S.; Hosono, M.; Tatsuta, T.; Ogawa, Y.; Fujimura, T.; Taka, H.; Murayama, K.; Nitta, K. Globotriaosylceramide-expressing Burkitt’s lymphoma cells are committed to early apoptotic status by rhamnose-binding lectin from catfish eggs. Biol. Pharm. Bull. 2009, 32, 345–353. [Google Scholar]
- Piller, V.; Piller, F.; Fukuda, M. Biosysthesis of truncated O-glycans in the T cell line Jurkat. Localization of O-glycan initiation. J. Biol. Chem. 1990, 265, 9264–9271. [Google Scholar]
- Yamada, K.; Hyodo, S.; Matsuno, Y.K.; Kinoshita, M.; Maruyama, S.Z.; Osaka, Y.S.; Casal, E.; Lee, Y.C.; Kakehi, K. Rapid and sensitive analysis of mucin-type glycans using an in line flow glycan-releasing apparatus. Anal.Biochem. 2007, 371, 52–61. [Google Scholar]
- Baenziger, J.U.; Fiete, D. Structure of the complex oligosaccharides of fetuin. J. Biol. Chem. 1979, 254, 789–795. [Google Scholar]
- Ehrlich, H.; Maldonado, M.; Spindler, K.D.; Eckert, C.; Hanke, T.; Born, R.; Glebel, C.; Simon, P.; Heinemann, S. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part 1 Verongidea (demospongia Porifera). J. Exp. Zool. B Mol. Dev. Evol. 2007, 308, 347–356. [Google Scholar]
- Fujita, Y.; Ohsima, N.; Hasegawa, A.; Schweizer, F.; Takeda, T.; Kiuchi, F.; Hada, N. Synthesis, inhibitory effects on nitric oxide and structure activity relationships of a glycosphingolipid from the marine sponge Aplysinella rhax and its analogues. Molecules 2011, 16, 637–651. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Matsumoto, R.; Fujii, Y.; Kawsar, S.M.A.; Kanaly, R.A.; Yasumitsu, H.; Koide, Y.; Hasan, I.; Iwahara, C.; Ogawa, Y.; Im, C.H.; et al. Cytotoxicity and Glycan-Binding Properties of an 18 kDa Lectin Isolated from the Marine Sponge Halichondria okadai. Toxins 2012, 4, 323-338. https://doi.org/10.3390/toxins4050323
Matsumoto R, Fujii Y, Kawsar SMA, Kanaly RA, Yasumitsu H, Koide Y, Hasan I, Iwahara C, Ogawa Y, Im CH, et al. Cytotoxicity and Glycan-Binding Properties of an 18 kDa Lectin Isolated from the Marine Sponge Halichondria okadai. Toxins. 2012; 4(5):323-338. https://doi.org/10.3390/toxins4050323
Chicago/Turabian StyleMatsumoto, Ryo, Yuki Fujii, Sarkar M. A. Kawsar, Robert A. Kanaly, Hidetaro Yasumitsu, Yasuhiro Koide, Imtiaj Hasan, Chihiro Iwahara, Yukiko Ogawa, Chang Hun Im, and et al. 2012. "Cytotoxicity and Glycan-Binding Properties of an 18 kDa Lectin Isolated from the Marine Sponge Halichondria okadai" Toxins 4, no. 5: 323-338. https://doi.org/10.3390/toxins4050323
APA StyleMatsumoto, R., Fujii, Y., Kawsar, S. M. A., Kanaly, R. A., Yasumitsu, H., Koide, Y., Hasan, I., Iwahara, C., Ogawa, Y., Im, C. H., Sugawara, S., Hosono, M., Nitta, K., Hamako, J., Matsui, T., & Ozeki, Y. (2012). Cytotoxicity and Glycan-Binding Properties of an 18 kDa Lectin Isolated from the Marine Sponge Halichondria okadai. Toxins, 4(5), 323-338. https://doi.org/10.3390/toxins4050323









