A Metabolomic Approach to Clarifying the Effect of AST-120 on 5/6 Nephrectomized Rats by Capillary Electrophoresis with Mass Spectrometry (CE-MS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Treatment
2.3. Blood Pressure and Biochemical Measurement
2.4. CE-MS Measurement for Metabolome Analysis
2.5. Statistics
3. Results
3.1. Biological Profiles and CE-MS Analysis
| Sham (n = 3) | Control (n = 13) | AST-120 (n = 11) | |||
|---|---|---|---|---|---|
| 4 weeks | 0 week | 4 weeks | 0 week | 4 weeks | |
| BW (g) | 493 ± 15 | 452 ± 22 | 490 ± 19 | 462 ± 17 | 486 ± 43 |
| SBP (mmHg) | 124 ± 7 ** | 173 ± 20 | 177 ± 21 | 169 ± 23 | 169 ± 24 |
| Cr (mg/dL) | 0.60 ± 0.10 ** | 1.03 ± 0.12 | 1.06 ± 0.26 | 1.04 ± 0.18 | 0.98 ± 0.26 |
| Ccr (mL/min) | 2.09 ± 0.29 ** | 1.06 ± 0.26 | 1.29 ± 0.38 | 1.11 ± 0.28 | 1.43 ± 0.4 |
3.2. Anions
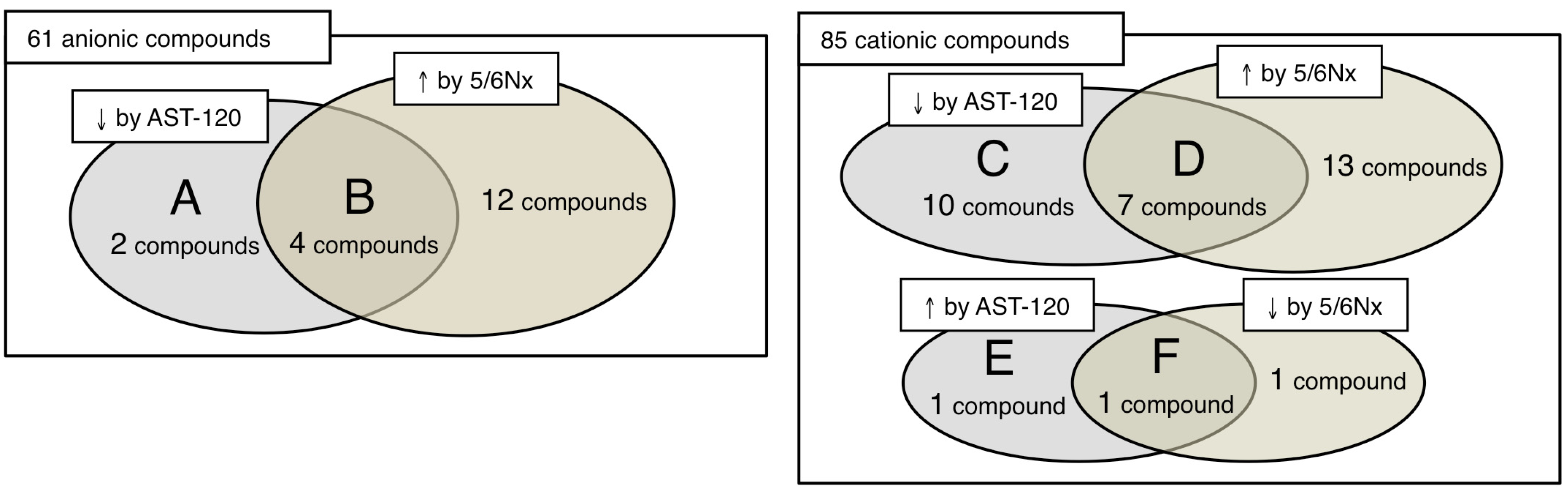
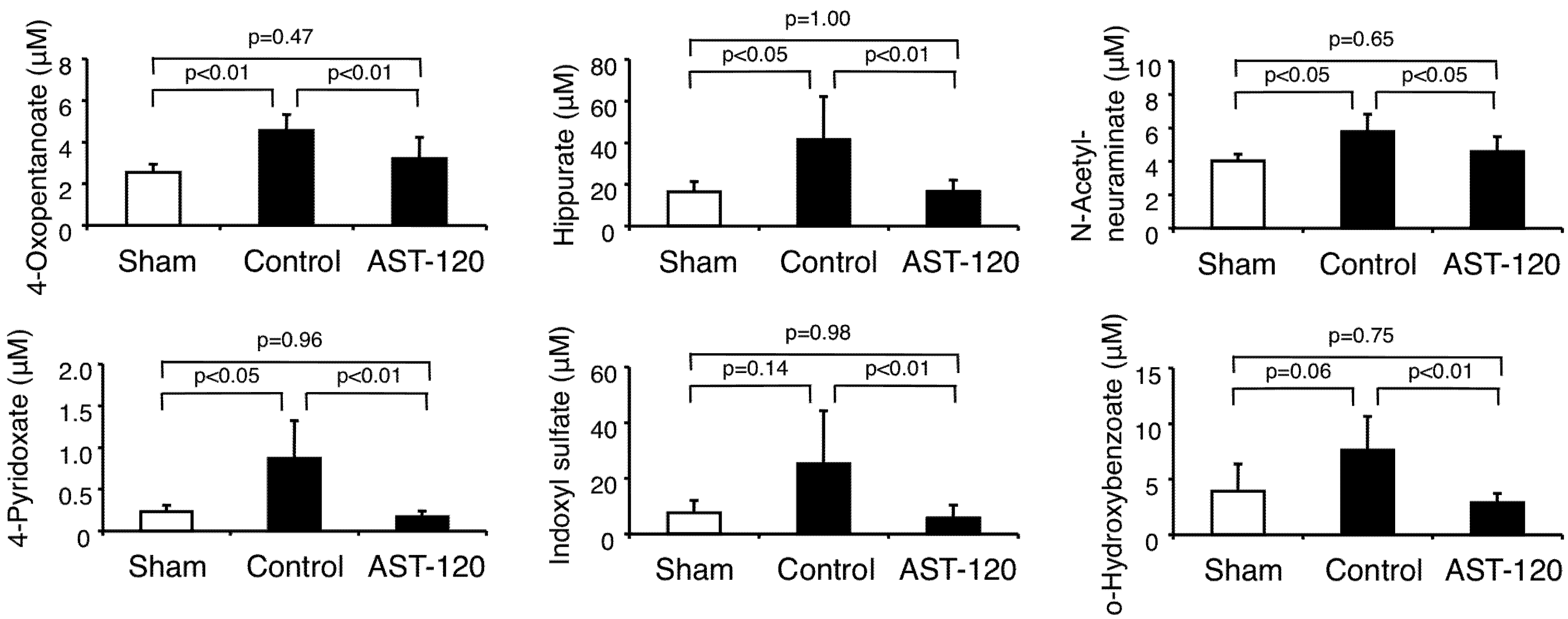
3.3. Cations
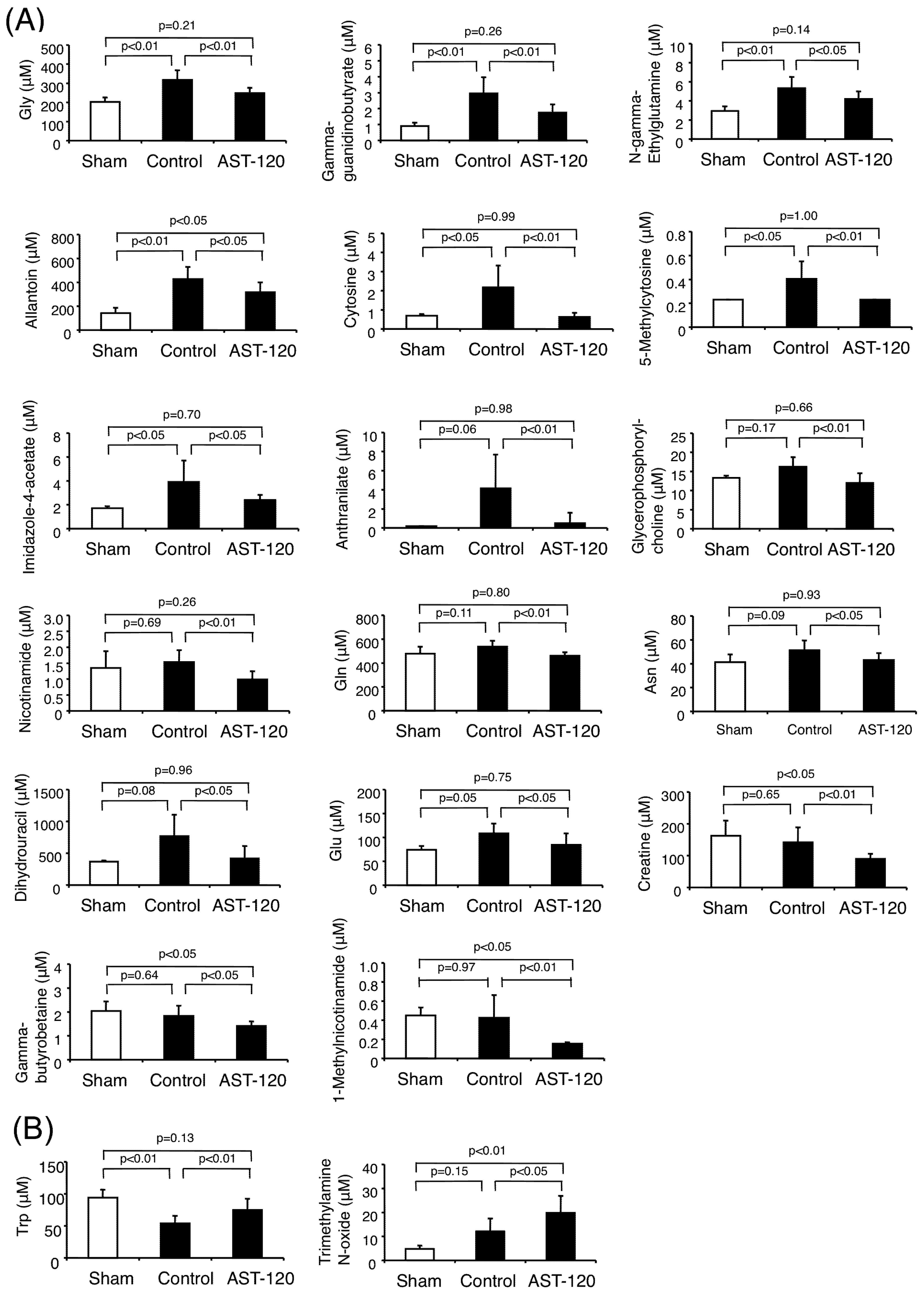
4. Discussion
| Anionic compounds | In this experiment | Reported by Kikuchi et al. [9] | Reported in human by Toyohara T. et al. [13] | Others | References |
| 4-Oxopentanoate | ↑ by 5/6Nx, ↓ by AST-120 | ↑ in CKD patients | |||
| Hippurate | ↑ by 5/6Nx, ↓ by AST-120 | ↑ by 5/6Nx, ↓ by AST-120 | ↑ in CKD patients | ↑ in uremia | [19] |
| N-Acetylneuraminate | ↑ by 5/6Nx, ↓ by AST-120 | Not Detected | |||
| 4-Pyridoxate | ↑ by 5/6Nx, ↓ by AST-120 | Not Detected | |||
| Indoxyl sulfate | N.S. by 5/6Nx, ↓ by AST-120 | ↑ by 5/6Nx, ↓ by AST-120 | ↑ in CKD patients | ↑ in uremia | [8,19] |
| o-Hydroxybenzoate | N.S. by 5/6Nx, ↓ by AST-120 | Not Detected | |||
| Phenyl sulfate | NA | ↑ by 5/6Nx, ↓ by AST-120 | NA | ||
| 4-Ethylphenyl sulfate | NA | ↑ by 5/6Nx, ↓ by AST-120 | NA | ||
| p-Cresyl sulfate | NA | ↑ by 5/6Nx, ↓ by AST-120 | NA | ||
| Cationic compounds | In this experiment | Repoted by Kikuchi et al. [9] | Reported in human by Toyohara T. et al. [13] | Others | References |
| Gly | ↑ by 5/6Nx, ↓ by AST-120 | N.S. | |||
| g-Guanidinobutyrate | ↑ by 5/6Nx, ↓ by AST-120 | N.S. | ↑ in uremia | [19] | |
| N-g-Ethylglutamine | ↑ by 5/6Nx, ↓ by AST-120 | Not Detected | |||
| Allantoin | ↑ by 5/6Nx, ↓ by AST-120 | ↑ in CKD patients | ↑ in CKD patients | [20] | |
| Cytosine | ↑ by 5/6Nx, ↓ by AST-120 | ↑ in CKD patients | |||
| 5-Methylcytosine | ↑ by 5/6Nx, ↓ by AST-120 | Not Detected | |||
| Imidazole-4-acetate | ↑ by 5/6Nx, ↓ by AST-120 | Not Detected | |||
| Anthranilate | N.S. by 5/6Nx, ↓ by AST-120 | Not Detected | |||
| Glycerophosphorylcholine | N.S. by 5/6Nx, ↓ by AST-120 | N.S. | |||
| Nicotinamide | N.S. by 5/6Nx, ↓ by AST-120 | Not Detected | |||
| Gln | N.S. by 5/6Nx, ↓ by AST-120 | ↓ in CKD patients | |||
| Asn | N.S. by 5/6Nx, ↓ by AST-120 | ↑ in CKD patients | |||
| Dihydrouracil | N.S. by 5/6Nx, ↓ by AST-120 | Not Detected | |||
| Glu | N.S. by 5/6Nx, ↓ by AST-120 | N.S. | |||
| Creatine | N.S. by 5/6Nx, ↓ by AST-120 | N.S. | ↑ in uremia | [19] | |
| g-Butyrobetaine | N.S. by 5/6Nx, ↓ by AST-120 | ↑ in CKD patients | |||
| 1-Methylnicotinamide | N.S. by 5/6Nx, ↓ by AST-120 | N.S. | |||
| Trp | ↓ by 5/6Nx, ↑ by AST-120 | ↓ in CKD patients | ↓ in uremic rats | [24] | |
| Trimethylamine N-oxide | N.S. by 5/6Nx, ↑ by AST-120 | ↑ in CKD patients | ↑ in CKD patients | [25] |
5. Conclusion
Conflict of Interest
Supplementary Files
References
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Watanabe, H.; Miyamoto, Y.; Otagiri, M.; Maruyama, T. Update on the pharmacokinetics and redox properties of protein-bound uremic toxins. J. Pharm. Sci. 2011, 100, 3682–3695. [Google Scholar] [CrossRef]
- Vanholder, R.; Glorieux, G.; De Smet, R.; Lameire, N. New insights in uremic toxins. Kidney Int. 2003, 63 Suppl. 84, S6–S10. [Google Scholar]
- Vanholder, R.; Baurmeister, U.; Brunet, P.; Cohen, G.; Glorieux, G.; Jankowski, J. A bench to bedside view of uremic toxins. J. Am. Soc. Nephrol. 2008, 19, 863–870. [Google Scholar] [CrossRef]
- Niwa, T. Role of indoxyl sulfate in the progression of chronic kidney disease and cardiovascular disease: Experimental and clinical effects of oral sorbent AST-120. Ther. Apher. Dial. 2011, 15, 120–124. [Google Scholar]
- Goto, S.; Yoshiya, K.; Kita, T.; Fujii, H.; Fukagawa, M. Uremic toxins and oral adsorbents. Ther. Apher. Dial. 2011, 15, 132–134. [Google Scholar]
- Niwa, T.; Miyazaki, T.; Hashimoto, N.; Hayashi, H.; Ise, M.; Uehara, Y.; Maeda, K. Suppressed serum and urine levels of indoxyl sulfate by oral sorbent in experimental uremic rats. Am. J. Nephrol. 1992, 12, 201–206. [Google Scholar]
- Kikuchi, K.; Itoh, Y.; Tateoka, R.; Ezawa, A.; Murakami, K.; Niwa, T. Metabolomic search for uremic toxins as indicators of the effect of an oral sorbent AST-120 by liquid chromatography/tandem mass spectrometry. J. Chromatogr. B 2010, 878, 2997–3002. [Google Scholar] [CrossRef]
- Ueda, S.; Yamagishi, S.; Takeuchi, M.; Kohno, K.; Shibata, R.; Matsumoto, Y.; Kaneyuki, U.; Fujimura, T.; Hayashida, A.; Okuda, S. Oral adsorbent AST-120 decreases serum levels of AGEs in patients with chronic renal failure. Mol. Med. 2006, 12, 180–184. [Google Scholar]
- Toyohara, T.; Suzuki, T.; Morimoto, R.; Akiyama, Y.; Souma, T.; Shiwaku, H.O.; Takeuchi, Y.; Mishima, E.; Abe, M.; Tanemoto, M.; et al. SLCO4C1 transporter eliminates uremic toxins and attenuates hypertension and renal inflammation. J. Am. Soc. Nephrol. 2009, 20, 2546–2555. [Google Scholar] [CrossRef]
- Toyohara, T.; Suzuki, T.; Akiyama, Y.; Yoshihara, D.; Takeuchi, Y.; Mishima, E.; Kikuchi, K.; Suzuki, C.; Tanemoto, M.; Ito, S.; et al. Metabolomic profiling of the autosomal dominant polycystic kidney disease rat model. Clin. Exp. Nephrol. 2011, 15, 676–687. [Google Scholar]
- Toyohara, T.; Akiyama, Y.; Suzuki, T.; Takeuchi, Y.; Mishima, E.; Tanemoto, M.; Momose, A.; Toki, N.; Sato, H.; Nakayama, M.; et al. Metabolomic profiling of uremic solutes in CKD patients. Hypertens. Res. 2010, 33, 944–952. [Google Scholar] [CrossRef]
- Soga, T.; Ohashi, Y.; Ueno, Y.; Naraoka, H.; Tomita, M.; Nishioka, T. Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J. Proteome Res. 2003, 2, 488–494. [Google Scholar] [CrossRef]
- Soga, T.; Heiger, D.N. Amino acid analysis by capillary electrophoresis electrospray ionization mass spectrometry. Anal. Chem. 2000, 72, 1236–1241. [Google Scholar]
- Soga, T.; Igarashi, K.; Ito, C.; Mizobuchi, K.; Zimmermann, H.P.; Tomita, M. Metabolomic profiling of anionic metabolites by capillary electrophoresis mass spectrometry. Anal. Chem. 2009, 81, 6165–6174. [Google Scholar]
- Soga, T.; Ueno, Y.; Naraoka, H.; Ohashi, Y.; Tomita, M.; Nishioka, T. Simultaneous determination of anionic intermediates for Bacillus subtilis metabolic pathways by capillary electrophoresis electrospray ionization mass spectrometry. Anal. Chem. 2002, 74, 2233–2239. [Google Scholar] [CrossRef]
- Soga, T.; Baran, R.; Suematsu, M.; Ueno, Y.; Ikeda, S.; Sakurakawa, T.; Kakazu, Y.; Ishikawa, T.; Robert, M.; Nishioka, T.; Tomita, M. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J. Biol. Chem. 2006, 281, 16768–16776. [Google Scholar]
- Vanholder, R.; Van Laecke, S.; Glorieux, G. What is new in uremic toxicity? Pediatr. Nephrol. 2008, 23, 1211–1221. [Google Scholar] [CrossRef]
- Kand’ar, R.; Zakova, P. Allantoin as a marker of oxidative stress in human erythrocytes. Clin. Chem. Lab. Med. 2008, 46, 1270–1274. [Google Scholar]
- Biedron, R.; Ciszek, M.; Tokarczyk, M.; Bobek, M.; Kurnyta, M.; Slominska, E.M.; Smolenski, R.T.; Marcinkiewicz, J. 1-Methylnicotinamide and nicotinamide: Two related anti-inflammatory agents that differentially affect the functions of activated macrophages. Arch. Immunol. Ther. Exp. (Warsz.) 2008, 56, 127–134. [Google Scholar] [CrossRef]
- Chlopicki, S.; Swies, J.; Mogielnicki, A.; Buczko, W.; Bartus, M.; Lomnicka, M.; Adamus, J.; Gebicki, J. 1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway. Br. J. Pharmacol. 2007, 152, 230–239. [Google Scholar] [CrossRef]
- Watala, C.; Kazmierczak, P.; Dobaczewski, M.; Przygodzki, T.; Bartus, M.; Lomnicka, M.; Slominska, E.M.; Durackova, Z.; Chlopicki, S. Anti-diabetic effects of 1-methylnicotinamide (MNA) in streptozocin-induced diabetes in rats. Pharmacol. Rep. 2009, 61, 86–98. [Google Scholar]
- Swendseid, M.E.; Wang, M.; Vyhmeister, I.; Chan, W.; Siassi, F.; Tam, C.F.; Kopple, J.D. Amino acid metabolism in the chronically uremic rat. Clin. Nephrol. 1975, 3, 240–246. [Google Scholar]
- Bain, M.A.; Faull, R.; Fornasini, G.; Milne, R.W.; Evans, A.M. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol. Dial. Transplant. 2006, 21, 1300–1304. [Google Scholar]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Akiyama, Y.; Takeuchi, Y.; Kikuchi, K.; Mishima, E.; Yamamoto, Y.; Suzuki, C.; Toyohara, T.; Suzuki, T.; Hozawa, A.; Ito, S.; et al. A Metabolomic Approach to Clarifying the Effect of AST-120 on 5/6 Nephrectomized Rats by Capillary Electrophoresis with Mass Spectrometry (CE-MS). Toxins 2012, 4, 1309-1322. https://doi.org/10.3390/toxins4111309
Akiyama Y, Takeuchi Y, Kikuchi K, Mishima E, Yamamoto Y, Suzuki C, Toyohara T, Suzuki T, Hozawa A, Ito S, et al. A Metabolomic Approach to Clarifying the Effect of AST-120 on 5/6 Nephrectomized Rats by Capillary Electrophoresis with Mass Spectrometry (CE-MS). Toxins. 2012; 4(11):1309-1322. https://doi.org/10.3390/toxins4111309
Chicago/Turabian StyleAkiyama, Yasutoshi, Yoichi Takeuchi, Koichi Kikuchi, Eikan Mishima, Yasuaki Yamamoto, Chitose Suzuki, Takafumi Toyohara, Takehiro Suzuki, Atsushi Hozawa, Sadayoshi Ito, and et al. 2012. "A Metabolomic Approach to Clarifying the Effect of AST-120 on 5/6 Nephrectomized Rats by Capillary Electrophoresis with Mass Spectrometry (CE-MS)" Toxins 4, no. 11: 1309-1322. https://doi.org/10.3390/toxins4111309
APA StyleAkiyama, Y., Takeuchi, Y., Kikuchi, K., Mishima, E., Yamamoto, Y., Suzuki, C., Toyohara, T., Suzuki, T., Hozawa, A., Ito, S., Soga, T., & Abe, T. (2012). A Metabolomic Approach to Clarifying the Effect of AST-120 on 5/6 Nephrectomized Rats by Capillary Electrophoresis with Mass Spectrometry (CE-MS). Toxins, 4(11), 1309-1322. https://doi.org/10.3390/toxins4111309






