Mechanism of Diphtheria Toxin Catalytic Domain Delivery to the Eukaryotic Cell Cytosol and the Cellular Factors that Directly Participate in the Process
Abstract
:1. Diphtheria Toxin and Diphtheria Toxin-Based Fusion Protein Toxins
2. The Intoxication Process
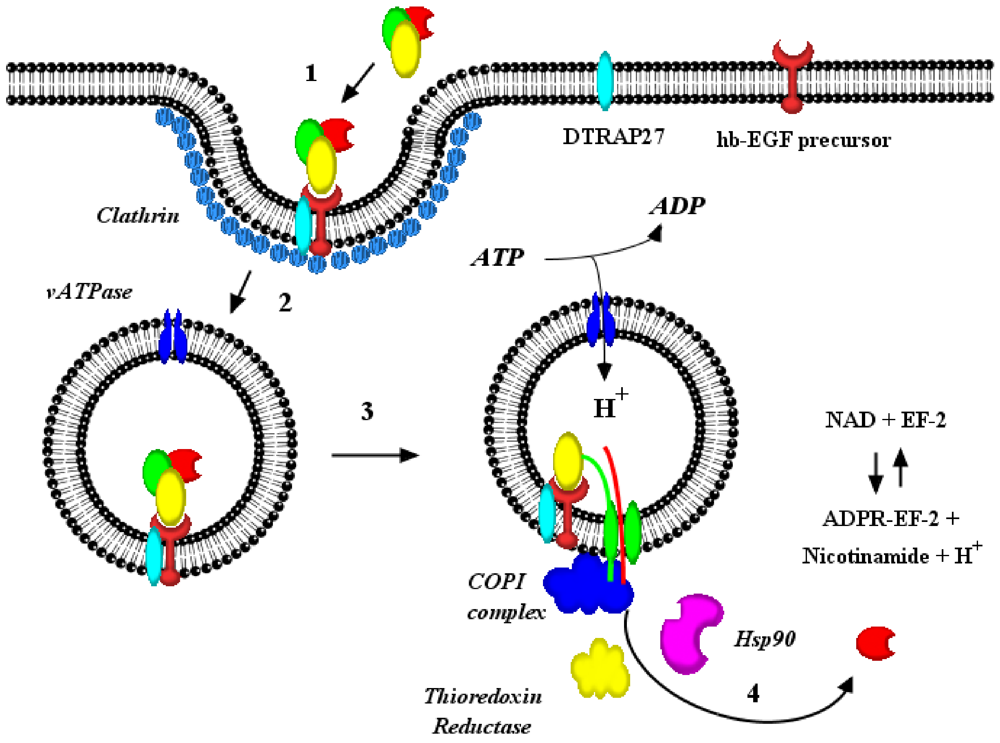
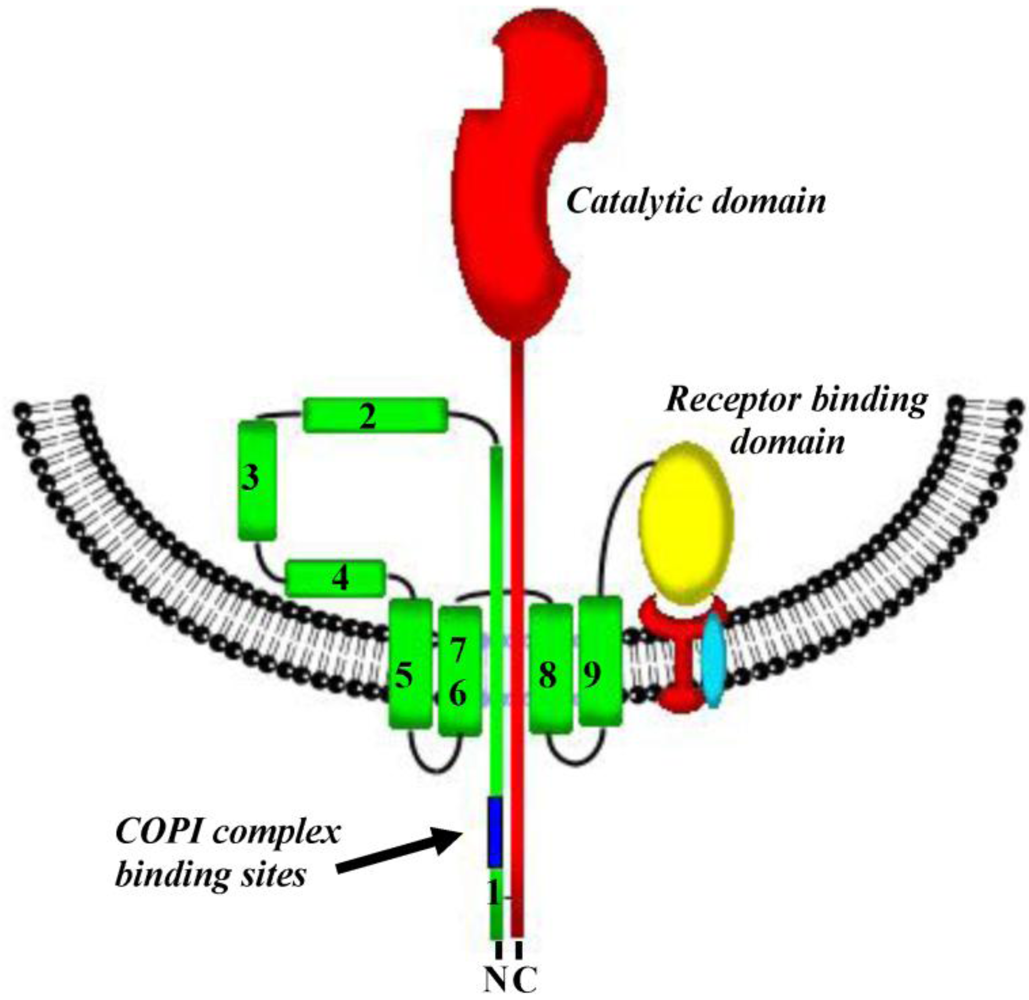
3. Pore Formation, Topography and Catalytic Domain Delivery
4. Autonomous vs. Facilitated Hypotheses of Diphtheria Toxin Catalytic Domain Delivery to the Cytosol
5. Partial Purification and Characterization of Cytosolic Factors Required for Diphtheria Toxin Translocation
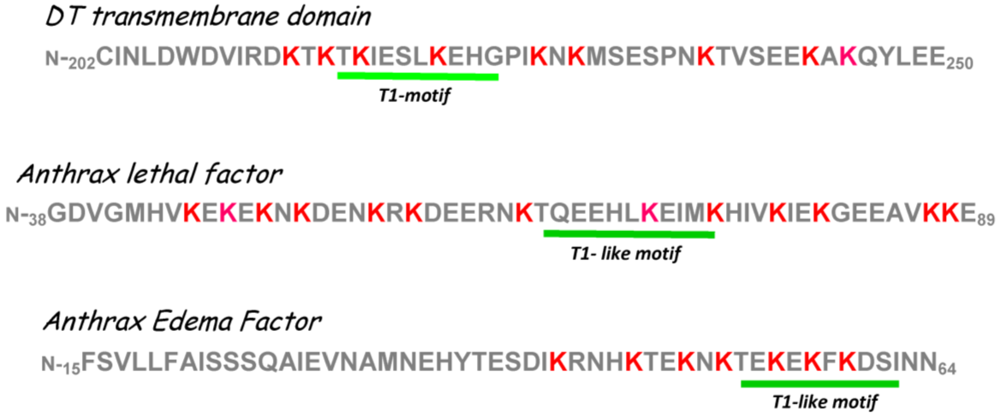
6. Interaction(s) between the Diphtheria Toxin Transmembrane Domain Helix1 and COPI Complex Proteins
Acknowledgements
References
- Pappenheimer, A.M., Jr. Diphtheria toxin. Ann. Rev. Biochem. 1977, 46, 69–94. [Google Scholar]
- Smith, W.P.; Tai, P.C.; Murphy, J.R.; Davis, B.D. Precursor in cotranslational secretion of diphtheria toxin. J. Bacteriol. 1980, 141, 184–189. [Google Scholar]
- Kaczorek, M.; Delpeyroux, F.; Chenciner, N.; Streeck, R.E.; Murphy, J.R.; Boquet, P.; Tiollais, P. Nucleotide sequence and expression of the diphtheria tox228 gene in Escherichia coli. Science 1983, 221, 855–858. [Google Scholar]
- Greenfield, L.; Bjorn, M.J.; Horn, G.; Fong, D.; Buck, G.A.; Collier, R.J.; Kaplan, D.A. Nucleotide sequence of the structural gene for diphtheria toxin carried by corynebacteriophage beta. Proc. Natl. Acad. Sci. USA 1983, 80, 6853–6857. [Google Scholar]
- Collier, R.J.; Kandel, J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J. Biol. Chem. 1971, 246, 1496–1503. [Google Scholar] [PubMed]
- Gill, D.M.; Pappenheimer, A.M., Jr. Structure-activity relationships in diphtheria toxin. J. Biol. Chem. 1971, 246, 1492–1495. [Google Scholar]
- Uchida, T.; Gill, D.M.; Pappenheimer, A.M., Jr. Mutation in the structural gene for diphtheria toxin carried by temperate phage. Nat. New Biol. 1971, 233, 8–11. [Google Scholar]
- Choe, S.; Bennett, M.J.; Fugii, G.; Curmi, P.M.; Kantardjieff, K.A.; Collier, R.J.; Eisenberg, D. The crystal structure of diphtheria toxin. Nature 1992, 357, 216–222. [Google Scholar]
- Bennett, M.J.; Choe, S.; Eisenberg, D. Refined structure of dimeric diphtheria toxin at 2.0 Å resolution. Protein Sci. 1994, 3, 1444–1463. [Google Scholar] [CrossRef] [PubMed]
- Kochi, S.K.; Collier, R.J. DNA fragmentation and cytolysis in U937 cells treated with diphtheria toxin or other inhibitors of protein synthesis. Exp. Cell Res. 1993, 208, 296–302. [Google Scholar]
- Naglich, J.G.; Metherall, J.E.; Russell, D.W.; Eidels, L. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell 1992, 69, 1051–1061. [Google Scholar]
- Murphy, J.R.; Bishai, W.; Borowski, M.; Miyanohara, A.; Boyd, J.; Nagle, S. Genetic construction, expression, and melanoma-selective cytotoxicity of a diphtheria toxin-related alpha-melanocyte-stimulating hormone fusion protein. Proc. Natl. Acad. Sci. USA 1986, 83, 8258–8262. [Google Scholar]
- vanderSpek, J.C.; Murphy, J.R. Fusion protein toxins based on diphtheria toxin: selective targeting of growth factor receptors of eukaryotic cells. Meth. Enzymol. 2000, 327, 239–249. [Google Scholar]
- Williams, D.; Parker, K.; Bishai, W.; Borowski, M.; Genbauffe, F.; Strom, T.B.; Murphy, J.R. Diphtheria toxin receptor binding domain substitution with interleukin-2: Genetic construction and properties of the diphtheria toxin-related interleukin-2 fusion protein. Protein Eng. 1987, 1, 493–498. [Google Scholar]
- Williams, D.P.; Snider, C.E.; Strom, T.B.; Murphy, J.R. Structure function analysis of IL-2-toxin (DAB486IL-2): Fragment B sequences required for the delivery of fragment A to the cytosol of target cells. J. Biol. Chem. 1990, 265, 11885–11889. [Google Scholar]
- Foss, F.M. DAB(389)IL-2 (ONTAK): A novel fusion toxin therapy for lymphoma. Clin. Lymphoma 2000, 1, 110–116. [Google Scholar]
- Iwamoto, R.; Higashiyama, S.; Mitamura, T.; Taniguchi, N.; Klagsbrun, M.; Mekada, E. Heparin-binding EGF-like growth factor, which acts as the diphtheria toxin receptor, forms a complex with membrane protein DRAP27/CD9, which up-regulates functional receptors and diphtheria toxin sensitivity. EMBO J. 1994, 13, 2322–2330. [Google Scholar]
- Moya, M.; Dautry-Varsat, A.; Goud, B.; Louvard, D.; Boquet, P. Inhibition of coated pit formation in Hep2 cells blocks the cytotoxicity of diphtheria toxin but not that of ricin toxin. J. Cell Biol. 1985, 101, 548–559. [Google Scholar]
- Boquet, P.; Silverman, M.S.; Pappenheimer, A.M., Jr.; Vernon, W.B. Binding of triton X-100 to diphtheria toxin, crossreacting material 45, and their fragments. Proc. Natl. Acad. Sci. USA 1976, 73, 4449–4453. [Google Scholar]
- Donovan, J.J.; Simon, M.I.; Montal, M. Diphtheria toxin forms transmembrane channels in planar lipid bilayers. Proc. Natl. Acad. Sci. USA 1981, 78, 172–176. [Google Scholar]
- Kagan, B.L.; Finkelstein, A.; Colombini, M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc. Natl. Acad. Sci. USA 1981, 78, 4950–4954. [Google Scholar]
- Ratts, R.; Zeng, H.; Berg, E.A.; Blue, C.; McComb, M.E.; Costello, C.E.; vanderSpek, J.C.; Murphy, J.R. The cytosolic entry of diphtheria toxin catalytic domain requires a host cell cytosolic translocation factor complex. J. Cell Biol. 2003, 160, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Ratts, R.; Trujillo, C.; Bharti, A.; vanderSpek, J.C.; Harrison, R.; Murphy, J.R. A conserved motif in transmembrane helix 1 of diphtheria toxin mediates catalytic domain delivery to the cytosol. Proc. Nat. Acad. Sci. USA 2005, 102, 15635–15640. [Google Scholar]
- Ren, J.; Kachel, K.; Kim, H.; Malenbaum, S.E.; Collier, R.J.; London, E. Interaction of diphtheria toxin T domain with molten globule-like proteins and its implications for translocation. Science 1999, 284, 955–957. [Google Scholar]
- Oh, K.J.; Senzel, L.; Collier, R.J.; Finkelstein, A. Translocation of the catalytic domain of diphtheria toxin across planar phospholipid bilayers by its own T domain. Proc. Nat. Acad. Sci. USA 1999, 96, 8467–8470. [Google Scholar]
- Yamaizumi, M.; Mekada, E.; Uchida, T.; Okada, Y. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell 1978, 15, 245–250. [Google Scholar]
- Deleers, M.; Beugnier, N.; Falgmagne, P.; Cabiaux, V.; Ruysschaert, J.M. Localization in diphtheria toxin fragemnt B of a region that induces pore formation in planar lipid bilayers at low pH. FEBS Lett. 1983, 160, 82–86. [Google Scholar]
- Shiver, J.W.; Donovan, J.J. Interactions of diphtheria toxin with lipid vesicles: Determinants of ion channel formation. Biochim. Biophys. Acta 1987, 903, 48–55. [Google Scholar]
- Almond, B.D.; Eidels, L. The effect of receptor rapid-internalization signals on diphtheria toxin endocytosis and cell sensitivity. Mol. Microbiol. 1995, 18, 623–630. [Google Scholar]
- O’Keefe, D.O.; Cabiaux, V.; Choe, S.; Eisenberg, D.; Collier, R.J. pH-dependent insertion of proteins into membranes: B-chain mutation of diphtheria toxin that inhibits membrane translocation, Glu-349—Lys. Proc. Nat. Acad. Sci. USA 1992, 89, 6202–6206. [Google Scholar]
- Falnes, P.O.; Madshus, I.H.; Sandvig, K.; Olsnes, S. Replacement of negative by positive charges in the presumed membrane-inserted part of diphtheria toxin B fragment. Effect on membrane translocation and on formation of cation channels. J. Biol. Chem. 1992, 267, 12284–12290. [Google Scholar] [PubMed]
- Kim, K.; Groman, N.B. In vitro inhibition of diphtheria toxin action by ammonium salts and amines. J. Bacteriol. 1965, 90, 1552–1556. [Google Scholar]
- Sandvig, K.; Olsnes, S. Entry of the toxic proteins abrin, modeccin, ricin, and diphtheria toxin into cells. II. Effect of pH, metabolic inhibitors, and ionophores and evidence for toxin penetration from endocytotic vesicles. J. Biol. Chem. 1982, 257, 7504–7513. [Google Scholar] [PubMed]
- Bowman, E.J.; Siebers, A.; Altendorf, K. Bafilomycins: A class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. USA 1988, 85, 7972–7976. [Google Scholar]
- Umata, T.; Moriyama, Y.; Futai, M.; Mekada, E. The cytotoxic action of diphtheria toxin and its degradation in intact Vero cells are inhibited by bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase. J. Biol. Chem. 1990, 265, 21940–21945. [Google Scholar]
- Cabiaux, V.; Mindell, J.; Collier, R.J. Membrane translocation and channel-forming activities of diphtheria toxin are blocked by replacing isoleucine 364 with lysine. Infect. Immun. 1993, 61, 2200–2202. [Google Scholar]
- Cabiaux, V.; Quertenmont, P.; Conrath, K.; Brasseur, R.; Capiau, C.; Ruysschaert, J.M. Topology of diphtheria toxin B fragment inserted in lipid vesicles. Mol. Microbiol. 1994, 11, 43–50. [Google Scholar]
- Moskaug, J.O.; Stenmark, H.; Olsnes, S. Insertion of diphtheria toxin B-fragment into the plasma membrane at low pH. Characterization and topology of inserted regions. J. Biol. Chem. 1991, 266, 2652–2659. [Google Scholar] [PubMed]
- Madshus, I.H. The N-terminal alpha-helix of fragment B of diphtheria toxin promotes translocation of fragment A into the cytoplasm of eukaryotic cells. J. Biol. Chem. 1994, 269, 17723–17729. [Google Scholar]
- Madshus, I.H.; Wiedlocha, A.; Sandvig, K. Intermediates in translocation of diphtheria toxin across the plasma membrane. J. Biol. Chem. 1994, 269, 4648–4652. [Google Scholar]
- Trujillo, C.; Taylor-Parker, J.; Harrison, R.; Murphy, J.R. Essential lysine residues within transmembrane helix 1 of diphtheria toxin facilitate COPI binding and catalytic domain entry. Mol. Microbiol. 2010, 76, 1010–1019. [Google Scholar]
- Mindell, J.A.; Silverman, J.A.; Collier, R.J.; Finkelstein, A. Structure function relationships in diphtheria toxin channels: II. A residue responsible for the channel's dependence on trans pH. J. Memb. Biol. 1994, 137, 29–44. [Google Scholar]
- vanderSpek, J.C.; Howland, K.; Friedman, T.; Murphy, J.R. Maintenance of the hydrophobic face of the diphtheria toxin amphipathic transmembrane helix 1 is essential for the efficient delivery of the catalytic domain to the cytosol of target cells. Protein Eng. 1994, 7, 985–989. [Google Scholar]
- Silverman, J.A.; Mindell, J.A.; Collier, R.J.; Finkelstein, A. Structure-function relationships in diphtheria toxin channels: I. Determining a minimal channel-forming domain. J. Membr. Biol. 1994, 137, 17–28. [Google Scholar] [PubMed]
- Hu, H.Y.; Huynh, P.D.; vanderSpek, J.C.; Murphy, J.R. The effects of helix breaking mutations in the diphtheria toxin transmembrane domain helix layers of the fusion toxin DAB389IL-2. Protein Eng. 1998, 11, 811–817. [Google Scholar]
- Hayashibara, M.; London, E. Topography of diphtheria toxin A chain inserted into lipid vesicles. Biochemistry 2005, 44, 2183–2196. [Google Scholar]
- Kaneda, Y.; Uchida, T.; Mekada, E.; Nakanishi, M.; Okada, Y. Entry of diphtheria toxin into cells: possible existence of cellular factor(s) for entry of diphtheria toxin into cells was studied in somatic cell hybrids and hybrid toxins. J. Cell Biol. 1984, 98, 466–472. [Google Scholar]
- Lemichez, E.; Bomsel, M.; Devilliers, G.; vanderSpek, J.; Murphy, J.R.; Lukianov, E.V.; Olsnes, S.; Boquet, P. Membrane translocation of diphtheria toxin fragment A exploits early to late endosome trafficking machinery. Mole. Microbiol. 1997, 23, 445–457. [Google Scholar]
- Simpson, J.C.; Smith, D.C.; Roberts, L.M.; Lord, J.M. Expression of mutant dynamin protects cells against diphtheria toxin but not against ricin. Exp. Cell Res. 1998, 239, 293–300. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mole. Biol. 1990, 215, 403–410. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994, 2, 28–36. [Google Scholar]
- Serafini, T.; Stenbeck, G.; Brecht, A.; Lottspiech, F.; Orci, L.; Rothman, J.E.; Wieland, F.T. A coat subunit of Golgi-derived nonclathrin—coated vesicles with homology to the clathrin-coated vesicle protein beta-adaptin. Nature 1991, 349, 215–220. [Google Scholar]
- Waters, M.G.; Serafini, T.; Rothman, J.E. “Coatomer” a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature 1991, 349, 248–251. [Google Scholar]
- Whitney, J.A.; Gomez, M.; Sheff, D.; Kries, T.E.; Mellman, I. Cytoplasmic coat proteins involved in endosome function. Cell 1995, 83, 703–713. [Google Scholar]
- Donaldson, J.G.; Cassel, D.; Kahn, R.A.; Klausner, R.D. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of coatomer protein beta-COP to membranes. Proc. Natl. Acad. Sci. USA 1992, 89, 6408–6412. [Google Scholar]
- Palmer, D.J.; Helms, J.B.; Beckers, C.J.; Orci, L.; Rothman, J.E. Binding of coatomer to Golgi membranes requires ADP-ribosylation factor. J. Biol. Chem. 1993, 268, 12083–12089. [Google Scholar]
- Cosson, P.; Letourneur, F. Coatomer interaction with di-lysine endoplasmic retention motifs. Science 1994, 263, 1629–1631. [Google Scholar]
- Eugster, A.; Frigerio, G.; Dale, M.; Duden, R. The alpha- and beta’-COP WD40 domains mediate cargo-selective interactions with distinct di-lysine motifs. Mol. Biol. Cell 2004, 15, 1011–1023. [Google Scholar]
- Harter, C.; Weiland, F.T. A single binding site for dilysine retrieval motifs and p23 with the gamma subunit of coatomer. Proc. Natl. Acad. Sci. USA 1998, 95, 11649–11654. [Google Scholar]
- Hudson, R.T.; Draper, R.K. Interaction of coatomer with aminoglycoside antibiotics: evidence that coatomer has at least two dilysine binding sites. Mol. Biol. Cell 1997, 8, 1901–1910. [Google Scholar]
- Barth, H. Exploring the role of host cell chaperones/PPIases during cellular up-take of bacterial ADP-ribosylating toxins as basis for novel pharmacological strategies to protect mammalian cells against these virulence foactors. Naunyn. Schmiedebergs Arch. Pharmacol. 2011, 383, 237–245. [Google Scholar]
- Haug, G.; Leemhuis, J.; Tiemann, D.; Meyeer, D.K.; Aktories, K.; Barth, H. The host cell chaperone Hsp90 is essential for translocation of the binary Clostridium botulinum C2 toxin into the cytosol. J. Biol. Chem. 2003, 278, 32266–32274. [Google Scholar]
- Taylor, M.; Navarro-Garcia, F.; Huerta, J.; Burress, H.; Massey, S.; Ireton, K. Hsp90 is required for transfer of the cholera toxin A1 subunit from the endoplasmic reticulum to the cytosol. J. Biol. Chem. 2010, 285, 31262–31267. [Google Scholar]
- Dmochewitz, L.; Lillich, M.; Kaiser, E.; Jennings, L.D.; Lang, A.E.; Buchner, J.; Fischer, G.; Aktories, K.; Collier, R.J.; Barth, H. Role of CypA and Hsp90 in membrane translocation mediated by anthrax protective antigen. Cell Microbiol. 2011, 13, 359–373. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Murphy, J.R. Mechanism of Diphtheria Toxin Catalytic Domain Delivery to the Eukaryotic Cell Cytosol and the Cellular Factors that Directly Participate in the Process. Toxins 2011, 3, 294-308. https://doi.org/10.3390/toxins3030294
Murphy JR. Mechanism of Diphtheria Toxin Catalytic Domain Delivery to the Eukaryotic Cell Cytosol and the Cellular Factors that Directly Participate in the Process. Toxins. 2011; 3(3):294-308. https://doi.org/10.3390/toxins3030294
Chicago/Turabian StyleMurphy, John R. 2011. "Mechanism of Diphtheria Toxin Catalytic Domain Delivery to the Eukaryotic Cell Cytosol and the Cellular Factors that Directly Participate in the Process" Toxins 3, no. 3: 294-308. https://doi.org/10.3390/toxins3030294
APA StyleMurphy, J. R. (2011). Mechanism of Diphtheria Toxin Catalytic Domain Delivery to the Eukaryotic Cell Cytosol and the Cellular Factors that Directly Participate in the Process. Toxins, 3(3), 294-308. https://doi.org/10.3390/toxins3030294



