Development of a Patient-Centered Outcome Tool for Blepharospasm: A Stepwise Modified Delphi Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
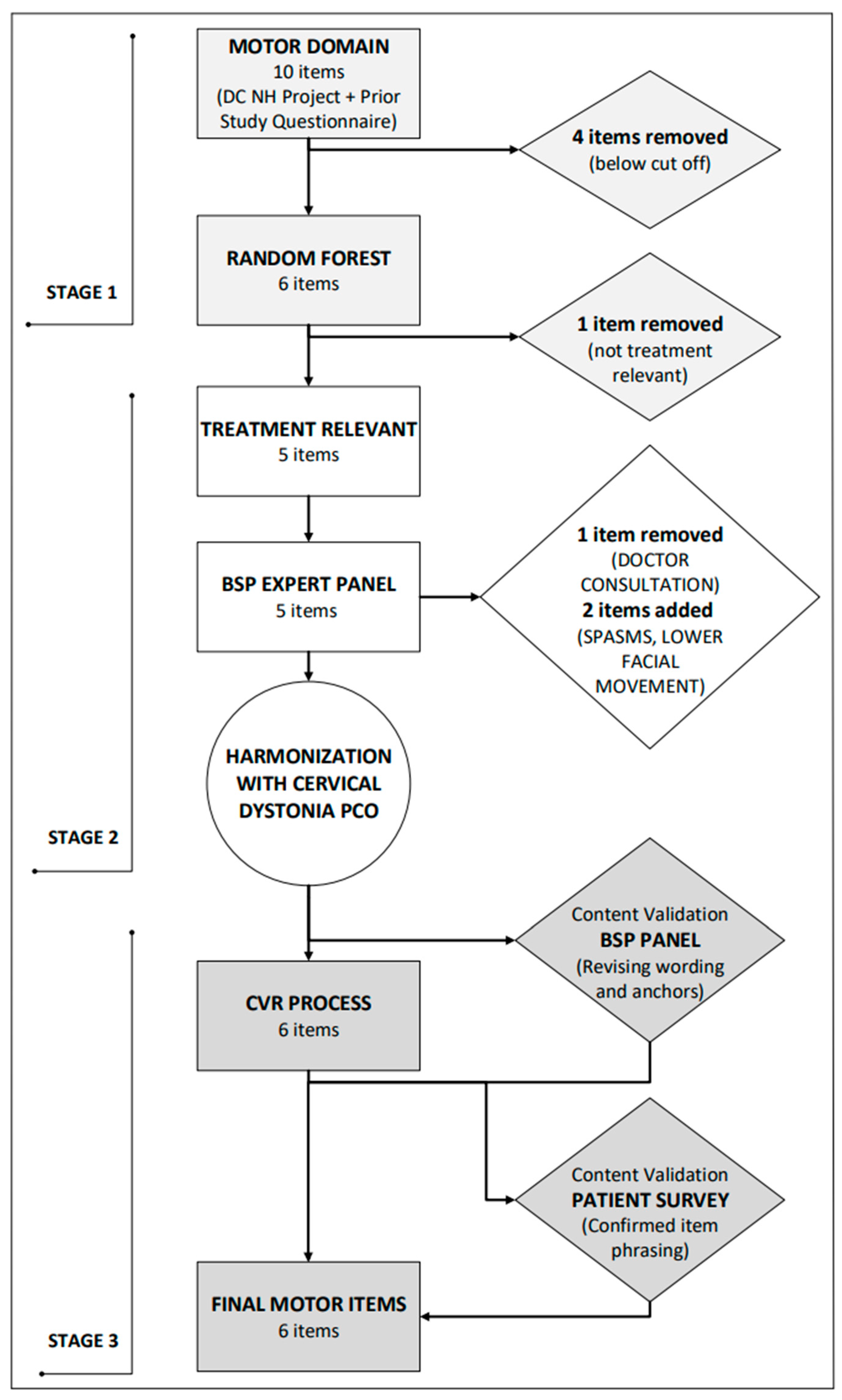
2.1.1. Stage 1: Content Development and Item Generation
2.1.2. Stage 2: Item Improvement and Revision of Items
2.1.3. Stage 3: Content Validity
2.1.4. Stage 4: Testing Performance of PCO
3. Results
4. Discussion
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSP | Blepharospasm |
| BoNT | Botulinum neurotoxin |
| BSDI | Blepharospasm Disability Index |
| CDQ-24 | Craniocervical Dystonia Questionnaire |
| PCO | Patient-centered outcome |
| CVR | Content validity rating |
| SAS | Statistical Analysis Software |
| CGI-C | Clinical Global Impression-Clinician |
| CGI-P | Clinical Global Impression-Patient |
| MCID | Minimum clinically important difference |
References
- Defazio, G.; Jinnah, H.A.; Berardelli, A.; Perlmutter, J.S.; Berkmen, G.K.; Berman, B.D.; Jankovic, J.; Bäumer, T.; Comella, C.; Cotton, A.C.; et al. Diagnostic Criteria for Blepharospasm: A Multicenter International Study. Park. Relat. Disord. 2021, 91, 109–114. [Google Scholar] [CrossRef]
- Scorr, L.M.; Cho, H.J.; Kilic-Berkmen, G.; McKay, J.L.; Hallett, M.; Klein, C.; Baumer, T.; Berman, B.D.; Feuerstein, J.S.; Perlmutter, J.S.; et al. Clinical Features and Evolution of Blepharospasm: A Multicenter International Cohort and Systematic Literature Review. Dystonia 2022, 1, 10359. [Google Scholar] [CrossRef]
- Zhu, L.; Meng, H.; Zhang, W.; Xie, W.; Sun, H.; Hou, S. The Pathogenesis of Blepharospasm. Front. Neurol. 2024, 14, 1336348. [Google Scholar] [CrossRef]
- Berman, B.D.; Groth, C.L.; Sillau, S.H.; Pirio Richardson, S.; Norris, S.A.; Junker, J.; Brüggemann, N.; Agarwal, P.; Barbano, R.L.; Espay, A.J.; et al. Risk of Spread in Adult-Onset Isolated Focal Dystonia: A Prospective International Cohort Study. J. Neurol. Neurosurg. Psychiatry 2020, 91, 314–320. [Google Scholar] [CrossRef]
- Svetel, M.; Pekmezović, T.; Jović, J.; Ivanović, N.; Dragašević, N.; Marić, J.; Kostić, V.S. Spread of Primary Dystonia in Relation to Initially Affected Region. J. Neurol. 2007, 254, 879–883. [Google Scholar] [CrossRef]
- Weiss, E.M.; Hershey, T.; Karimi, M.; Racette, B.; Tabbal, S.D.; Mink, J.W.; Paniello, R.C.; Perlmutter, J.S. Relative Risk of Spread of Symptoms among the Focal Onset Primary Dystonias. Mov. Disord. 2006, 21, 1175–1181. [Google Scholar] [CrossRef]
- Junker, J.; Hall, J.; Berman, B.D.; Vidailhet, M.; Roze, E.; Bäumer, T.; Malaty, I.A.; Shukla, A.W.; Jankovic, J.; Reich, S.G.; et al. Longitudinal Predictors of Health-Related Quality of Life in Isolated Dystonia. J. Neurol. 2024, 271, 852–863. [Google Scholar] [CrossRef]
- Berman, B.D.; Junker, J.; Shelton, E.; Sillau, S.H.; Jinnah, H.A.; Perlmutter, J.S.; Espay, A.J.; Jankovic, J.; Vidailhet, M.; Bonnet, C.; et al. Psychiatric Associations of Adult-Onset Focal Dystonia Phenotypes. J. Neurol. Neurosurg. Psychiatry 2017, 88, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Defazio, G.; Gigante, A.F.; Hallett, M.; Berardelli, A.; Perlmutter, J.S.; Berman, B.D.; Jankovic, J.; Bäumer, T.; Comella, C.; Ercoli, T.; et al. Motor and Psychiatric Features in Idiopathic Blepharospasm: A Data-Driven Cluster Analysis. Park. Relat. Disord. 2022, 104, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Junker, J.; Berman, B.D.; Hall, J.; Wahba, D.W.; Brandt, V.; Perlmutter, J.S.; Jankovic, J.; Malaty, I.A.; Wagle Shukla, A.; Reich, S.G.; et al. Quality of Life in Isolated Dystonia: Non-Motor Manifestations Matter. J. Neurol. Neurosurg. Psychiatry 2021, 92, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Duarte, G.S.; Rodrigues, F.B.; Marques, R.E.; Castelão, M.; Ferreira, J.; Sampaio, C.; Moore, A.P.; Costa, J. Botulinum Toxin Type A Therapy for Blepharospasm. Cochrane Database Syst. Rev. 2020, 2020, CD004900. [Google Scholar] [CrossRef]
- Leplow, B.; Eggebrecht, A.; Pohl, J. Treatment Satisfaction with Botulinum Toxin: A Comparison between Blepharospasm and Cervical Dystonia. Patient Prefer Adherence 2017, 11, 1555–1563. [Google Scholar] [CrossRef]
- Ojo, O.O.; Fernandez, H.H. Is It Time for Flexibility in Botulinum Inter-Injection Intervals? Toxicon 2015, 107, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Fezza, J.; Burns, J.; Woodward, J.; Truong, D.; Hedges, T.; Verma, A. A Cross-Sectional Structured Survey of Patients Receiving Botulinum Toxin Type A Treatment for Blepharospasm. J. Neurol. Sci. 2016, 367, 56–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Comella, C.; Ferreira, J.J.; Pain, E.; Azoulai, M.; Om, S. Patient Perspectives on the Therapeutic Profile of Botulinum Neurotoxin Type A in Cervical Dystonia. J. Neurol. 2021, 268, 903–912. [Google Scholar] [CrossRef]
- Pirio Richardson, S.; Jinnah, H.A. New Approaches to Discovering Drugs That Treat Dystonia. Expert Opin. Drug Discov. 2019, 14, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Comella, C.L.; Jankovic, J.; Hauser, R.A.; Patel, A.T.; Banach, M.D.; Ehler, E.; Vitarella, D.; Rubio, R.G.; Gross, T.M.; on behalf of the ASPEN-1 Study Group; et al. Efficacy and Safety of DaxibotulinumtoxinA for Injection in Cervical Dystonia: ASPEN-1 Phase 3 Randomized Controlled Trial. Neurology 2024, 102, e208091. [Google Scholar] [CrossRef]
- Kilic-Berkmen, G.; Kim, H.; Chen, D.; Yeo, C.I.; Dinasarapu, A.R.; Scorr, L.M.; Yeo, W.; Peterson, D.A.; Williams, H.; Ruby, A.; et al. An Exploratory, Randomized, Double-Blind Clinical Trial of Dipraglurant for Blepharospasm. Mov. Disord. 2024, 39, 738–745. [Google Scholar] [CrossRef]
- Jankovic, J.; Kenney, C.; Grafe, S.; Goertelmeyer, R.; Comes, G. Relationship between Various Clinical Outcome Assessments in Patients with Blepharospasm. Mov. Disord. 2009, 24, 407–413. [Google Scholar] [CrossRef]
- Defazio, G.; Hallett, M.; Jinnah, H.A.; Stebbins, G.T.; Gigante, A.F.; Ferrazzano, G.; Conte, A.; Fabbrini, G.; Berardelli, A. Development and Validation of a Clinical Scale for Rating the Severity of Blepharospasm. Mov. Disord. 2015, 30, 525–530. [Google Scholar] [CrossRef]
- Defazio, G.; Hallett, M.; Berardelli, A.; Perlmutter, J.S.; Berman, B.D.; Jankovic, J.; Bäumer, T.; Comella, C.; Ercoli, T.; Ferrazzano, G.; et al. Measurement Properties of Clinical Scales Rating the Severity of Blepharospasm: A Multicenter Observational Study. Mov. Disord. Clin. Pract. 2022, 9, 949–955. [Google Scholar] [CrossRef]
- Muller, J. Craniocervical Dystonia Questionnaire (CDQ-24): Development and Validation of a Disease-Specific Quality of Life Instrument. J. Neurol. Neurosurg. Psychiatry 2004, 75, 749–753. [Google Scholar] [CrossRef]
- Pirio Richardson, S.; Berman, B.D.; Hieshetter, J.; Comella, C.; Peterson, D.A.; Kilic-Berkmen, G.; Wright, L.; Pentecost, S.; Reyes, P.; Jankovic, J.; et al. A Digital Patient-Centered Outcome Tool for Cervical Dystonia. Dystonia 2024, 3, 13478. [Google Scholar] [CrossRef]
- Martino, D.; Defazio, G.; Alessio, G.; Abbruzzese, G.; Girlanda, P.; Tinazzi, M.; Fabbrini, G.; Marinelli, L.; Majorana, G.; Buccafusca, M.; et al. Relationship between Eye Symptoms and Blepharospasm: A Multicenter Case–Control Study. Mov. Disord. 2005, 20, 1564–1570. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II 2011; APA PsycNet: Washington, DC, USA, 1996. [Google Scholar] [CrossRef]
- Jenkinson, C.; Coulter, A.; Wright, L. Short Form 36 (SF36) Health Survey Questionnaire: Normative Data for Adults of Working Age. BMJ 1993, 306, 1437–1440. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Strobl, C.; Boulesteix, A.-L.; Kneib, T.; Augustin, T.; Zeileis, A. Conditional Variable Importance for Random Forests. BMC Bioinform. 2008, 9, 307. [Google Scholar] [CrossRef]
- Dixon-Woods, M.; Agarwal, S.; Jones, D.; Young, B.; Sutton, A. Synthesising Qualitative and Quantitative Evidence: A Review of Possible Methods. J. Health Serv. Res. Policy 2005, 10, 45–53. [Google Scholar] [CrossRef]
- O’Keefe-McCarthy, S.; McGillion, M.; Nelson, S.; Clarke, S.; McFetridge-Durdle, J.; Watt-Watson, J. Content Validity of the Toronto Pain Management Inventory-Acute Coronary Syndrome Version. Can. J. Cardiovasc. Nurs. 2014, 24, 11–18. [Google Scholar] [CrossRef]
- Tschuggnall, M.; Grote, V.; Pirchl, M.; Holzner, B.; Rumpold, G.; Fischer, M.J. Machine Learning Approaches to Predict Rehabilitation Success Based on Clinical and Patient-Reported Outcome Measures. Inform. Med. Unlocked 2021, 24, 100598. [Google Scholar] [CrossRef]
- Verma, D.; Jansen, D.; Bach, K.; Poel, M.; Mork, P.J.; d’Hollosy, W.O.N. Exploratory Application of Machine Learning Methods on Patient Reported Data in the Development of Supervised Models for Predicting Outcomes. BMC Med. Inf. Decis. Mak. 2022, 22, 227. [Google Scholar] [CrossRef]
- Cella, D.; Hahn, E.; Jensen, S.; Butt, Z.; Nowinski, C.; Rothrock, N.; Lohr, K. Patient-Reported Outcomes in Performance Measurement; RTI Press: Durham, NC, USA, 2015; ISBN 978-1-934831-14-4. [Google Scholar]
- Jaeschke, R.; Singer, J.; Guyatt, G.H. Measurement of Health Status. Ascertaining the Minimal Clinically Important Difference. Control Clin. Trials 1989, 10, 407–415. [Google Scholar] [CrossRef]
- King, M.T. A Point of Minimal Important Difference (MID): A Critique of Terminology and Methods. Expert Rev. Pharmacoecon. Outcomes Res. 2011, 11, 171–184. [Google Scholar] [CrossRef]
- Copay, A.G.; Subach, B.R.; Glassman, S.D.; Polly, D.W.; Schuler, T.C. Understanding the Minimum Clinically Important Difference: A Review of Concepts and Methods. Spine J. 2007, 7, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Revicki, D.; Hays, R.D.; Cella, D.; Sloan, J. Recommended Methods for Determining Responsiveness and Minimally Important Differences for Patient-Reported Outcomes. J. Clin. Epidemiol. 2008, 61, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Wyrwich, K.W.; Norquist, J.M.; Lenderking, W.R.; Acaster, S. Industry Advisory Committee of International Society for Quality of Life Research (ISOQOL) Methods for Interpreting Change over Time in Patient-Reported Outcome Measures. Qual. Life Res. 2013, 22, 475–483. [Google Scholar] [CrossRef]
- Yost, K.J.; Eton, D.T. Combining Distribution- and Anchor-Based Approaches to Determine Minimally Important Differences: The FACIT Experience. Eval. Health Prof. 2005, 28, 172–191. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, N.; Aidoo, H.; Doroshenko, A.; Antle, D.; Els, C.; Flaschner, D.M.; Gross, D.P.; Guptill, C.; Potter, P.; Tan, M.C.; et al. Botulinum Toxin for the Treatment of Focal Task-Specific Hand Dystonias: Systematic Review and Meta-Analysis. Open Neurol. J. 2019, 13, 32–44. [Google Scholar] [CrossRef]
- Chiaramonte, R.; Vecchio, M. Rehabilitation of Focal Hand Dystonia in Musicians: A Systematic Review of the Studies. Rev. Neurol. 2021, 72, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Yeung, W.; Richards, A.L.; Novakovic, D. Botulinum Neurotoxin Therapy in the Clinical Management of Laryngeal Dystonia. Toxins 2022, 14, 844. [Google Scholar] [CrossRef]


| DOMAIN | ITEMS | Content Validity Ratio |
Patient Concurrence
(Item Relevancy, %) |
Desired
Improvement Threshold (90th Percentile) | |
|---|---|---|---|---|---|
| Relevancy | Clarity | ||||
| MOTOR | How often do you blink too much? | 1.0 | 0.71 | 83 | 40 |
| How much difficulty do you have keeping your eyes fully open? | 1.0 | 1.0 | 94 | 40 | |
| How much discomfort do you have with bright light? | 0.86 | 0.86 | 90 | 35 | |
| How much discomfort do you have due to any gritty, sandy or burning sensations in your eyes? | 0.86 | 1.0 | 74 | 30 | |
| Do spasms close your eyes against your will? | 1.0 | 1.0 | 94 | 37 | |
| How often do you experience uncontrollable movements of your tongue, mouth, or jaw? | 1.0 | 1.0 | 43 | 25 | |
| DISABILITY | How much limitation do you have in work performance (either household work or outside employment) due to your eye or mouth problem? | 1.0 | 1.0 | 77 | 28 |
| How much limitation do you have in driving due to your eye or mouth problem? | 1.0 | 1.0 | 83 | 42 | |
| How much limitation do you have in your leisure activities due to your eye or mouth problem? | 0.86 | 0.86 | 86 | 30 | |
| How much limitation do you have in talking and/or eating due to uncontrollable movements of your tongue, mouth, or jaw? | 1.0 | 1.0 | 34 | 23 | |
| PSYCHOSOCIAL | How often do you feel anxious due to your eye or mouth problem? | 1.0 | 1.0 | 86 | 27 |
| How often do you feel down or depressed due to your eye or mouth problem? | 1.0 | 1.0 | 73 | 27 | |
| How often do you feel frustrated due to your eye or mouth problem? | 0.86 | 1.0 | 93 | 30 | |
| How often do you feel embarrassed due to your eye or mouth problem? | 1.0 | 1.0 | 81 | 27 | |
| How much limitation do you have in social situations (e.g., visiting friends and family, attending events outside the home, etc.) due to your eye problem? | 0.86 | 0.71 | 84 | 26 | |
| How much limitation do you have in social situations (e.g., visiting friends and family, attending events outside the home, etc.) due to uncontrollable movements of your tongue, mouth, or jaw? | 0.86 | 0.71 | 41 | 30 | |
| How much is your quality of life affected due to your eye or mouth problem? | 1.0 | 1.0 | 92 | 30 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berman, B.D.; Qeadan, F.; Henderson, A.D.; Harrison, A.R.; Defazio, G.; Hallett, M.; Kilic-Berkmen, G.; Wright, L.; Pentecost, S.; Reyes, P.; et al. Development of a Patient-Centered Outcome Tool for Blepharospasm: A Stepwise Modified Delphi Study. Toxins 2025, 17, 455. https://doi.org/10.3390/toxins17090455
Berman BD, Qeadan F, Henderson AD, Harrison AR, Defazio G, Hallett M, Kilic-Berkmen G, Wright L, Pentecost S, Reyes P, et al. Development of a Patient-Centered Outcome Tool for Blepharospasm: A Stepwise Modified Delphi Study. Toxins. 2025; 17(9):455. https://doi.org/10.3390/toxins17090455
Chicago/Turabian StyleBerman, Brian D., Fares Qeadan, Amanda D. Henderson, Andrew R. Harrison, Giovanni Defazio, Mark Hallett, Gamze Kilic-Berkmen, Laura Wright, Samantha Pentecost, Paul Reyes, and et al. 2025. "Development of a Patient-Centered Outcome Tool for Blepharospasm: A Stepwise Modified Delphi Study" Toxins 17, no. 9: 455. https://doi.org/10.3390/toxins17090455
APA StyleBerman, B. D., Qeadan, F., Henderson, A. D., Harrison, A. R., Defazio, G., Hallett, M., Kilic-Berkmen, G., Wright, L., Pentecost, S., Reyes, P., Tingin, A., Jankovic, J., Boyd, J., Hudgins, C., Hieshetter, J., Perlmutter, J. S., Jinnah, H. A., & Pirio Richardson, S. (2025). Development of a Patient-Centered Outcome Tool for Blepharospasm: A Stepwise Modified Delphi Study. Toxins, 17(9), 455. https://doi.org/10.3390/toxins17090455






