Unveiling the Endocrine-Disrupting Potential of Plant-Derived Compounds: An Ecotoxicological Review
Abstract
1. Introduction
2. Environmental Presence and Biological Characteristics of Plant-Derived Secondary Metabolites
2.1. Environmental Presence of Plant-Derived Secondary Metabolites
2.2. Classification and Properties of Plant-Derived Metabolites
| Types | Biosynthesis Pathway | Class | Secondary Metabolites | Reported Endocrine Activity | Ref |
|---|---|---|---|---|---|
| Terpenes | Mevalonate pathway and methylerythritol 4-phosphate pathway | Monoterpenes | Geraniol, citral, nerol, linalool, and linalyl acetate | (Anti-)estrogenic activity and anti-androgenic activity | [25,26] |
| Diterpenes | Steviol, stevioside, and rebaudioside A | Anti-progesterone activity | [27] | ||
| Triterpenes | Ginsenoside-Rh2 | Estrogenic activity | [28] | ||
| Phenols | Acetate-malonate pathway and shikimic acid pathway | Coumarin | Coumarins | Estrogenic activity | [30] |
| Furanocoumarins | Bergapten, xanthotoxol, angelicin, psoralen, and isoimperatorin | Anti-estrogenic activity and progesterone activity | [32,33] | ||
| Lignin | Bisguaiacol A and bissyringol A | Estrogenic activity and developmental toxicity | [34] | ||
| Flavonoids | Luteolin and quercetin | (Anti-)estrogenic activity, anti-androgenic activity, and anti-progesterone activity | [35,36] | ||
| Isoflavonoids | Genistein, equol, and Daidzein | Estrogenic activity and neuroendocrine system | [35,36,37] | ||
| N-containing compounds | Shikimic acid pathway | Alkaloids | α-Erythroidin | Estrogenic activity | [39] |
| Cyanogenic glucosides | Amygdalin | Indirect female reproductive processes | [40] | ||
| S-containing compounds | Sulfur Assimilation | Glucosinolates | Thiocyanate and Isothiocyanates | Negative effects on thyroid function | [42] |
| Phytoalexins | Phaseollin and kievitone | Estrogenic activity and anti-androgenic activity | [43] |
3. Approaches and Models for Environmental Endocrine Disruption Assessment
| System | Model Organism/ Platform | Target Endpoint | Description | Ref |
|---|---|---|---|---|
| In silico | QSAR models | Acute and chronic toxicity endpoints in aquatic species | Prediction of aquatic toxicity based on chemical structure and physicochemical descriptors | [46] |
| Molecular docking and dynamics simulation | Binding affinity and conformational changes over time | Prediction and validation of binding interactions between chemicals and receptors | [47] | |
| AOP (Adverse Outcome Pathway) | Molecular initiating events to population-level outcomes | Mechanistic prediction of endocrine disruption and adverse phenotypes | [48] | |
| HTS data integration | Multi-target bioactivity signatures | Integration of assay data to prioritize and predict endocrine activity | [49,50] | |
| In vitro (Mammalian) | Estrogen and androgen receptor binding affinity | Estrogen receptor (ER) and androgen receptor (AR) binding affinities | Detection of chemical interaction with human ER-α and AR; applicable to estrogenic androgenic hazard screening and prioritization | [51,52] |
| Hormone receptor transactivation and yeast ER/AR screen | ER and AR agonistic or antagonistic activities | Stably transfected cell lines or genetically modified Saccharomyces cerevisiae expressing human Erα or AR linked to a reporter gene (e.g., luciferase) | [53,54,55,56] | |
| H295R Steroidogenesis assay | Steroid hormone synthesis (e.g., estradiol, testosterone) | Human adrenocortical carcinoma cell line to detect chemicals that alter the production of steroid hormones | [57] | |
| Thyroid peroxidase (TPO) inhibition assay | Thyroid peroxidase enzyme inhibition | Detection of chemical interference with TPO activity | [58] | |
| Transthyretin (TTR) binding assay | Disruption of thyroid hormone transport via TTR | Evaluation of interference with thyroid hormone transport via transthyretin | [59] | |
| In vitro (Non-mammalian) | Fish hormone receptor-based transactivation assay | Hormone receptor activation or inhibition | Recombinant reporter gene assay expressing hormone receptors for endocrine activity screening | [60] |
| Primary cell cultures from fish | VTG induction, steroidogenesis, and hormone biosynthesis | Freshly isolated liver or gonadal cells to evaluate endocrine responses | [61,62] | |
| Amphibian thyroid cell assay | Thyroid hormone disruption | Primary cell culture from amphibians to assess thyroid-disrupting effects | [63] | |
| Immortalized fish cell lines | Cytotoxicity, estrogenic response, AhR activation, and oxidative stress | Immortalized cell lines for assessing chemical toxicity and endocrine- related responses | [64,65] | |
| Embryo-based assays | Sex differentiation, thyroid disruption, morphogenesis, and gene expression | Endocrine-disruptive effects with morphological and molecular endpoints | [66,67] | |
| Fish microsome assay | Enzyme activity related to steroidogenesis and xenobiotic metabolism | Microsomes from fish to assess steroid hormone synthesis and metabolism of EDCs | [68] | |
| Primary cell cultures from terrestrial invertebrates | Oxidative stress, DNA damage, and hormone-related gene expression | Primary cell cultures for assessing cytotoxicity, oxidative damage, and endocrine-responsive markers | [69,70] | |
| In vivo | Zebrafish (Danio rerio) | VTG induction, sex ratio, and reproductive behavior | Multiple endocrine endpoints and reproductive endpoints | [71,72,73,74,75,76,77] |
| Rainbow trout (Oncorhynchus mykiss) | Plasma VTG levels, hepatic gene expression, and gonad histopathology | Well-established salmonid model in endocrine and reproductive toxicology | [71,75] | |
| Medaka (Oryzias latipes) | Gonadal development and secondary sexual characteristics | Multiple endocrine endpoints and reproductive endpoints | [71,72,73,74,77,78] | |
| Fathead minnow (Pimephales promelas) | Gonad histopathology and reproductive success | Multiple endocrine endpoints and reproductive endpoints | [71,72,74,75,77] | |
| Daphnia (Daphnia magna) | Reproduction rate, molting, and developmental delay | Key aquatic invertebrate for assessing endocrine and reproductive toxicity | [79,80,81] | |
| Xenopus (Xenopus laevis) | Metamorphosis stage, tail resorption, and thyroid gene expression | Amphibian model for thyroid hormone disruption during early development | [82,83,84] | |
| Stickleback (Gasterosteus aculeatus) | Spiggin induction (protein and gene expression) | Detection of androgenic activity via male-specific protein expression in females | [77,85] | |
| Copepod (Amphiascus tenuiremis) | Survival, offspring number, development rate, and sex ratio | Sediment-associated marine invertebrate model for assessing chronic and endocrine toxicity | [86] | |
| Midge (Chironomus riparius) | Emergence timing and gene expression | Sediment-exposed benthic model with endocrine-sensitive endpoints | [87,88] | |
| New Zealand mud snail (Potamopyrgus antipodarum) | Embryo count in brood pouch, survival, and growth | Sediment- and water-associated screening for estrogenic or anti-estrogenic endocrine activity | [89] | |
| Taxonomy browser (Lymnaea stagnalis) | Egg mass production, egg count, survival, growth, and behavior | Assessment of endocrine-related reproductive toxicity and chronic exposure effects | [90] | |
| Springtail (Folsomia candida) | Juvenile production and growth | Soil-dwelling arthropod used for endocrine and developmental effects | [91] | |
| Earthworm (Eisenia fetida) | Cocoon production and survival | Soil organism with reproduction-sensitive endocrine endpoints | [92] | |
| Honeybee (Apis mellifera) | Behavior, learning and reproduction | Pollinator model for assessing sublethal endocrine-disrupting effects | [93,94] |
3.1. In Silico Models
3.2. In Vitro Models
3.3. In Vivo Models
4. Endocrine-Disrupting Effects of Plant-Derived Metabolites
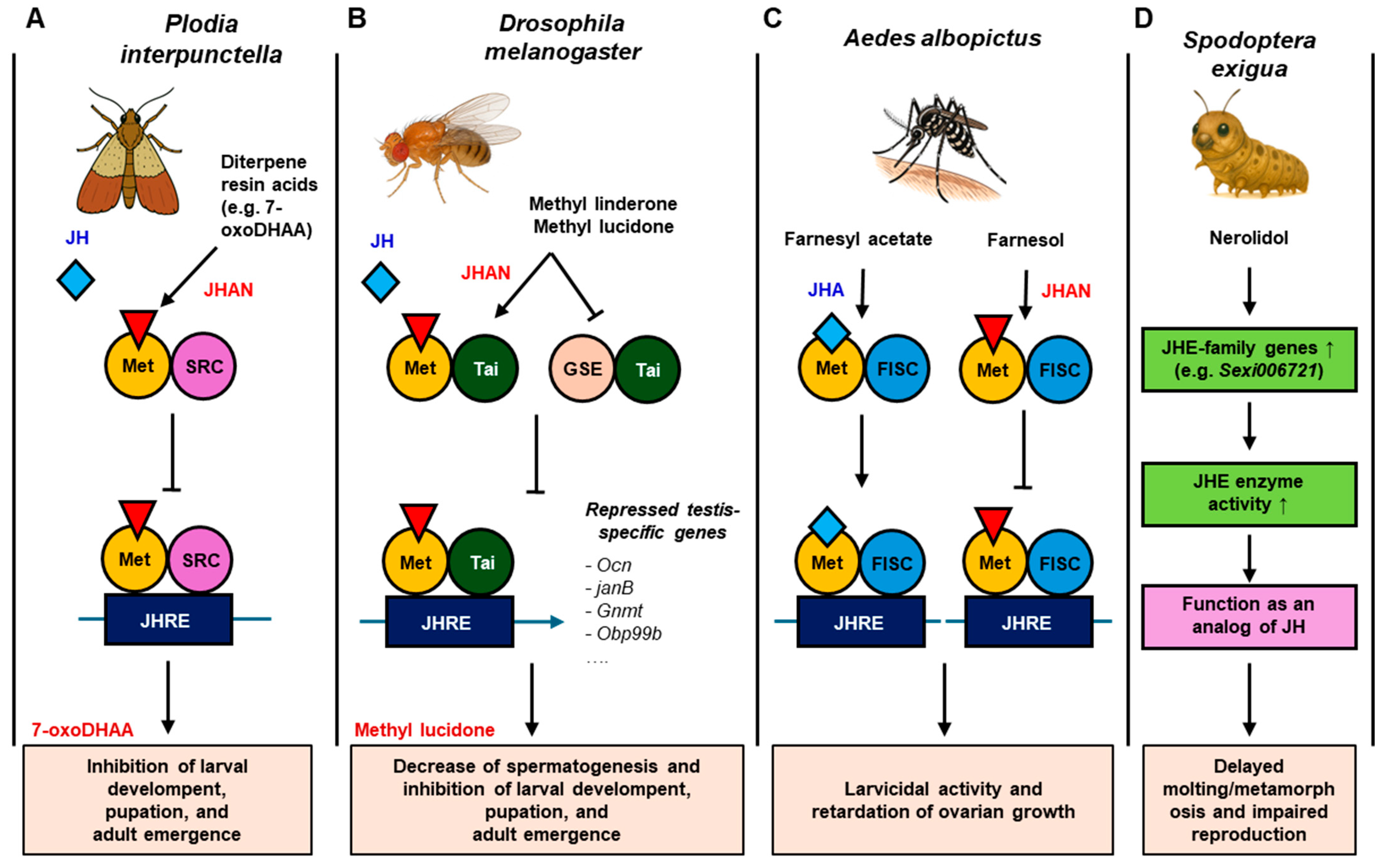
4.1. Terpenes
| Metabolite | System | Model Organism/Platform | Target Endpoint | Effect | Ref |
|---|---|---|---|---|---|
| 7-oxoDHAA | In vitro/ In vivo | Yeast two-hybrid/ Plodia interpunctella | JH-mediated metamorphosis, pupation, and adult emergence | Disruption of JH receptor complex formation; inhibition of larval growth, pupation, and adult emergence | [100] |
| 7α-HDHAA | In vitro/ In vivo | Yeast two-hybrid/ Plodia interpunctella | JH-mediated metamorphosis, pupation, and adult emergence | Inhibition of Met-SRC receptor binding | [100] |
| Abietic acid | In vivo | Rainbow trout (Oncorhynchus mykiss) | Vitellogenin gene expression | Slight induction of estrogenic response via oral exposure through feed mixture (abietic acid 37% and dehydroabietic acid 6%) | [106] |
| In vitro/ In vivo | Yeast two-hybrid/ Plodia interpunctella | JH-mediated metamorphosis, pupation, and adult emergence | Presence of JHAN activity; manifestation of anti-feedant activity without developmental toxicity | [100] | |
| Bakuchiol | In vivo | Medaka (Oryzias melastigma) | Liver-based reporter of estrogenic activity | Elevation of GFP fluorescence in medaka liver as an indication of estrogenic activity | [105] |
| Betulinol | In vivo | Zebrafish (Danio rerio) | Plasma vitellogenin, sex hormones, gonad histology, and reproduction | Reduction in VTG in F0 females; elevation of VTG in F1 males; stimulation of spawning | [107] |
| Dehydroabietic acid | In vitro/ In vivo | Yeast two-hybrid/ Plodia interpunctella | JH-mediated metamorphosis, pupation, and adult emergence | Moderate JH receptor binding interference | [100] |
| In vivo | Rainbow trout (Oncorhynchus mykiss) | Hepatic enzyme activity (EROD, GST), plasma vitellogenin, and E2 response | Modulation of metabolic enzyme activity; attenuation of estradiol-induced vitellogenin synthesis | [108] | |
| Zebrafish (Danio rerio) | Plasma vitellogenin, sex hormones, gonad histology, and reproduction | Reduction in plasma vitellogenin in F0 males; alteration of gonadal development | [107] | ||
| Farnesol | In vivo | Mosquito (Aedes albopictus) | JH signaling disruption, gene expression, and ovarian development | JH antagonist activity; retardation of ovarian growth | [102] |
| Farnesyl acetate | In vivo | Mosquito (Aedes albopictus) | JH signaling disruption, gene expression, and ovarian development | JH agonist activity; retardation of ovarian growth | [102] |
| Gibberellic acid | In vivo | Zebrafish embryos (Danio rerio) | Developmental toxicity (heart, liver, eye, and kidney) and oxidative stress | Inhibition of organogenesis; alteration of gene expression (e.g., Myl7, Vmhc, Fabp10a, Kim1); elevation of ROS | [109] |
| Methyl linderone | In vitro/ In vivo | Yeast two-hybrid/Drosophila melanogaster | JH receptor complex (Met-Taiman, GCE-Taiman) and larval-pupal development | Moderate inhibition of JH receptor dimerization and developmental interference; weaker than methyl lucidone | [101] |
| Yeast two-hybrid/ Plodia interpunctella | JH-mediated metamorphosis, pupation, and adult emergence | Potent JHAN activity | [100] | ||
| Methyl lucidone | In vitro/ In vivo | Yeast two-hybrid/Drosophila melanogaster | JH receptor complex (Met-Taiman, GCE-Taiman) and larval-pupal development | Inhibition of JH-mediated receptor dimerization and gene expression; suppression of larval development | [101] |
| Nerolidol | In vivo | Beet armyworm (Spodoptera exigua) | Juvenile hormone esterase (JHE) gene expression, JH titer, and JHE enzyme activity | Elevation of JH titer and JHE activity; induction of developmental disruption and fecundity reduction | [103] |
| Sandaracopimaric acid | In vitro/ In vivo | Yeast two-hybrid/ Plodia interpunctella | JH-mediated metamorphosis, pupation, and adult emergence | Receptor-binding disruption | [100] |
| β-sitosterol | In vivo | Rainbow trout (Oncorhynchus mykiss) | Vitellogenin gene expression | Robust upregulation of VTG expression as an indication of estrogenic activity | [106] |
4.2. Flavonoids
| Metabolite | System | Model Organism/Platform | Target Endpoint | Effect | Ref |
|---|---|---|---|---|---|
| 7,4′-dihydroxyflavone | In vitro | Ovarian microsomes (Oncorhynchus mykiss) | Aromatase enzyme inhibition | Potent inhibition, relative potency: 3.7 (vs. flavone = 1.0) | [110] |
| α-naphthoflavone | In vitro | Ovarian microsomes (Oncorhynchus mykiss) | Aromatase enzyme inhibition | Potent inhibition, relative potency: 3.2 (vs. flavone = 1.0) | [110] |
| Apigenin | In vitro | Ovarian microsomes (Oncorhynchus mykiss) | Aromatase enzyme inhibition | Potent inhibition, relative potency: 8.7 (vs. flavone = 1.0) | [110] |
| Biochanin A | In vitro | Ovarian microsomes (Oncorhynchus mykiss) | Aromatase enzyme inhibition | Weak inhibition, relative potency: <0.3 (vs. flavone = 1.0) | [110] |
| Chrysin | In vitro | Hepatic microsomes (Oreochromis niloticus) | Aromatase enzyme inhibition | Potent anti-aromatase effect | [111] |
| Daidzein | In vivo | Male goldfish (Carassius auratus) | Plasma vitellogenin | VTG induction in a fish diet containing high levels of genistein and daidzein | [112] |
| Russian sturgeon (Acipenser gueldenstaedtii) | Expression of sex-related genes (amh, ar, cyp19, dmrt1, erα, erβ, foxl2, sox9, star, vasa, and vtg) | Pronounced downregulation of genes in liver and gonads; endocrine disruption pattern | [113] | ||
| DL-aminoglute- thimide | In vitro | Ovarian microsomes (Oncorhynchus mykiss) | Aromatase enzyme inhibition | Potent inhibition, relative potency: 19 (vs. flavone = 1.0) | [110] |
| Equol | In vitro | Ovarian microsomes (Oncorhynchus mykiss) | Aromatase enzyme inhibition | Potent inhibition, relative potency: 0.9 (vs. flavone = 1.0) | [110] |
| In vivo | Japanese medaka (Oryzias latipes) | Gonadal development, secondary sex characteristics | Impaired spermatogenesis, fibrosis, altered female oocyte development, and sex reversal of external characteristics | [115] | |
| Flavone | In vitro | Ovarian microsomes (Oncorhynchus mykiss) | Aromatase enzyme inhibition | Potent inhibition | [110] |
| Genistein | In vitro | Ovarian microsomes (Oncorhynchus mykiss) | Aromatase enzyme inhibition | Weak inhibition, relative potency: <0.2 (vs. flavone = 1.0) | [110] |
| In vivo | Russian sturgeon (Acipenser gueldenstaedtii) | Expression of sex-related genes (amh, ar, cyp19, dmrt1, erα, erβ, foxl2, sox9, star, vasa, and vtg) | Alteration of gene expression in all tissues, with moderate induction of feminization-related markers | [113] | |
| Male goldfish (Carassius auratus) | Plasma vitellogenin | VTG induction in a fish diet containing high levels of genistein and daidzein | [112] | ||
| In vivo | Japanese medaka (Oryzias latipes) | Gonadal development, secondary sex characteristics | Delayed oocyte maturation, increased oocyte atresia, ovarian fibrosis, and disrupted sex phenotype expression | [115] | |
| Gequol (S-equol) | In vivo | Zebrafish (Danio rerio) | Estrogenic tissue-specific GFP response | Dose-dependent GFP expression in liver (ER-alpha) and heart (ER-beta) | [36] |
| Isobavachin | In vivo | Zebrafish (Danio rerio) | Estrogenic tissue-specific GFP response | Dose-dependent GFP expression in liver (ER-alpha) and heart (ER-beta) | [36] |
| Liquiritigenin | In vivo | Zebrafish (Danio rerio) | Estrogenic tissue-specific GFP response | GFP expression at 1000 nM in heart (ER-beta) | [36] |
| Phenoxodiol | In vivo | Zebrafish (Danio rerio) | Estrogenic tissue-specific GFP response | GFP expression at 1000 nM in liver (ER-alpha) and heart (ER-beta) | [36] |
| Quercetin | In vitro | Hepatic microsomes (Oreochromis niloticus) | Aromatase enzyme inhibition | Weak anti-aromatase effect compared to chrysin | [111] |
| In vitro | Ovarian microsomes (Oncorhynchus mykiss) | Aromatase enzyme inhibition | Potent inhibition, relative potency: 5.3 (vs. flavone = 1.0) | [110] | |
| Tannic acid | In vivo | Plateau pika (Ochotona curzoniae)/Root vole (Microtus oeconomus) | GnRH and plasma androgen and estrogen levels | Elevation of plasma androgen and estrogen without significant change in GnRH. | [114] |
4.3. Other Secondary Metabolites
| Metabolite | System | Model Organism/Platform | Target Endpoint | Effect | Ref |
|---|---|---|---|---|---|
| Coumestrol | In vivo | Russian sturgeon (Acipenser gueldenstaedtii) | Expression of sex-related genes (amh, ar, cyp19, dmrt1, erα, erβ, foxl2, sox9, star, vasa, and vtg) | Mild modulation of gene expression | [113] |
| Scopoletin | In vivo | Zebrafish (Danio rerio) | Metabolic profiles in embryos | Disruption of hormone-relevant metabolic pathways; indirect endocrine disturbance potential | [116] |
| 3,3′-Diindolylmethane (DIM) | In vivo | Rainbow trout (Oncorhynchus mykiss) | Hepatocarcinogenesis and gene expression of vitellogenin and CYP1A | VTG and CYP1A Induction | [117] |
| Caffeine | In vivo | Yellow-tail tetra (Astyanax altiparanae) | Plasma steroid levels (Testosterone, 11-KT), hepatic vitellogenin gene expression, testis and liver histology | Reduction in E2 concentration; reduction in VTG gene expression | [118] |
| Indole-3-carbinol (I3C) | In vivo | Rainbow trout (Oncorhynchus mykiss) | Hepatocarcinogenesis and gene expression of vitellogenin and CYP1A | VTG and CYP1A induction | [117] |
| Nicotine | In vivo | Zebrafish (Danio rerio) | Endocrine biomarker gene expression (vtg1, vtg2, cyp19a1a, and cyp19a1b) | Significant downregulation of all endocrine biomarker genes | [119] |
| Reserpine | In vivo | Zebrafish (Danio rerio) | CNS neuron differentiation, thyroid development, locomotion, and gene expression | Developmental toxicity; thyroid dysfunction via HPT axis disruption; dysregulation of endocrine related genes | [122] |
4.4. Ecological Implications of Endocrine Disruption Driven by Plant-Derived Secondary Metabolites
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruce, S.O. Secondary Metabolites from Natural Products. In Secondary Metabolites—Trends and Reviews; Vijayakumar, R., Raja, S.S.S., Eds.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The Influence of Environmental Conditions on Secondary Metabolites in Medicinal Plants: A Literature Review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of Botanical Pesticides in Agriculture as an Alternative to Synthetic Pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Smith, C.J.; Perfetti, T.A. A Comparison of the Persistence, Toxicity, and Exposure to High-Volume Natural Plant-Derived and Synthetic Pesticides. Toxicol. Res. Appl. 2020, 4, 2397847320940561. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The Role of Root Exudates in Rhizosphere Interactions with Plants and Other Organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Price, W.E. Occurrence of Phytoestrogens in Municipal Wastewater and Surface Waters. J. Environ. Monit. 2009, 11, 1477–1483. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Yao, L.; Fan, Z.; Han, S.; Sun, N.; Che, H. Apigenin Acts as a Partial Agonist Action at Estrogen Receptors in Vivo. Eur. J. Pharmacol. 2021, 906, 174175. [Google Scholar] [CrossRef] [PubMed]
- van Meeuwen, J.A.; Nijmeijer, S.; Mutarapat, T.; Ruchirawat, S.; de Jong, P.C.; Piersma, A.H.; van den Berg, M. Aromatase Inhibition by Synthetic Lactones and Flavonoids in Human Placental Microsomes and Breast Fibroblasts—A Comparative Study. Toxicol. Appl. Pharmacol. 2008, 228, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Austin, J.; Jinhong, R.; Johnson, M.E.; Lantvit, D.D.; Burdette, J.E. The Flavonoid Apigenin Is a Progesterone Receptor Modulator with in Vivo Activity in the Uterus. Horm. Cancer 2018, 9, 265–277. [Google Scholar] [CrossRef]
- Nordeen, S.K.; Bona, B.J.; Jones, D.N.; Lambert, J.R.; Jackson, T.A. Endocrine Disrupting Activities of the Flavonoid Nutraceuticals Luteolin and Quercetin. Horm. Cancer 2013, 4, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Habza-Kowalska, E.; Kaczor, A.A.; Żuk, J.; Matosiuk, D.; Gawlik-Dziki, U. Thyroid Peroxidase Activity Is Inhibited by Phenolic Compounds-Impact of Interaction. Molecules 2019, 24, 2766. [Google Scholar] [CrossRef]
- Amin Altawash, A.S.; Shahneh, A.Z.; Moravej, H.; Ansari, M. Chrysin-Induced Sperm Parameters and Fatty Acid Profile Changes Improve Reproductive Performance of Roosters. Theriogenology 2017, 104, 72–79. [Google Scholar] [CrossRef]
- Ohno, S.; Shinoda, S.; Toyoshima, S.; Nakazawa, H.; Makino, T.; Nakajin, S. Effects of Flavonoid Phytochemicals on Cortisol Production and on Activities of Steroidogenic Enzymes in Human Adrenocortical H295r Cells. J. Steroid Biochem. Mol. Biol. 2002, 80, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Jana, K.; Yin, X.; Schiffer, R.B.; Chen, J.J.; Pandey, A.K.; Stocco, D.M.; Grammas, P.; Wang, X. Chrysin, a Natural Flavonoid Enhances Steroidogenesis and Steroidogenic Acute Regulatory Protein Gene Expression in Mouse Leydig Cells. J. Endocrinol. 2008, 197, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.L. Flavonoid and Isoflavonoid Distribution in Developing Soybean Seedling Tissues and in Seed and Root Exudates. Plant Physiol. 1991, 95, 594–603. [Google Scholar] [CrossRef]
- Duong, C.N.; Ra, J.S.; Cho, J.; Kim, S.D.; Choi, H.K.; Park, J.H.; Kim, K.W.; Inam, E.; Kim, S.D. Estrogenic Chemicals and Estrogenicity in River Waters of South Korea and Seven Asian Countries. Chemosphere 2010, 78, 286–293. [Google Scholar] [CrossRef]
- Jarošová, B.; Javůrek, J.; Adamovský, O.; Hilscherová, K. Phytoestrogens and Mycoestrogens in Surface Waters—Their Sources, Occurrence, and Potential Contribution to Estrogenic Activity. Environ. Int. 2015, 81, 26–44. [Google Scholar] [CrossRef]
- Lundgren, M.S.; Novak, P.J. Quantification of Phytoestrogens in Industrial Waste Streams. Environ. Toxicol. Chem. 2009, 28, 2318–2323. [Google Scholar] [CrossRef]
- Nanusha, M.Y.; Krauss, M.; Schönsee, C.D.; Günthardt, B.F.; Bucheli, T.D.; Brack, W. Target Screening of Plant Secondary Metabolites in River Waters by Liquid Chromatography Coupled to High-Resolution Mass Spectrometry (Lc–Hrms). Environ. Sci. Eur. 2020, 32, 142. [Google Scholar] [CrossRef]
- Amorati, R.; Baschieri, A.; Cowden, A.; Valgimigli, L. The Antioxidant Activity of Quercetin in Water Solution. Biomimetics 2017, 2, 9. [Google Scholar] [CrossRef]
- Meesschaert, B.; Moons, N.; Steurs, G.; Monballiu, A.; Amery, R.; Jooken, E.; Geuns, J. Degradation of Steviol Glycosides Via Steviol and Monicanone by Soil Microorganisms and Uasb Effluent. J. Environ. Chem. Eng. 2021, 9, 106342. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.M.; Chan, T.F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Howes, M.J.; Houghton, P.J.; Barlow, D.J.; Pocock, V.J.; Milligan, S.R. Assessment of Estrogenic Activity in Some Common Essential Oil Constituents. J. Pharm. Pharmacol. 2002, 54, 1521–1528. [Google Scholar] [CrossRef]
- Hareng, L.; Kolle, S.N.; Gomes, C.; Schneider, S.; Wahl, M. Critical Assessment of the Endocrine Potential of Linalool and Linalyl Acetate: Proactive Testing Strategy Assessing Estrogenic and Androgenic Activity of Lavender Oil Main Components. Arch. Toxicol. 2024, 98, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.; Rehfeld, A.; Frizzell, C.; Livingstone, C.; McGonagle, C.; Skakkebaek, N.E.; Wielogórska, E.; Connolly, L. In Vitro Bioassay Investigations of the Endocrine Disrupting Potential of Steviol Glycosides and Their Metabolite Steviol, Components of the Natural Sweetener Stevia. Mol. Cell Endocrinol. 2016, 427, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, S.N.; Kozhemyako, V.B.; Atopkina, L.N.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I.; Rasskazov, V.A.; Aminin, D.L. Estrogenic Activity of Triterpene Glycosides in Yeast Two-Hybrid Assay. J. Steroid Biochem. Mol. Biol. 2006, 101, 226–231. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Chang, J.; Zhou, J.; Gao, M.; Zhang, H.; Wang, T. Research Advances in the Analysis of Estrogenic Endocrine Disrupting Compounds in Milk and Dairy Products. Foods 2022, 11, 3057. [Google Scholar] [CrossRef]
- Río, J.A.D.; Díaz, L.; García-Bernal, D.; Blanquer, M.; Ortuño, A.; Correal, E.; Moraleda, J.M. Chapter 5—Furanocoumarins: Biomolecules of Therapeutic Interest. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 145–195. [Google Scholar]
- Acharya, R.; Chacko, S.; Bose, P.; Lapenna, A.; Pattanayak, S.P. Structure Based Multitargeted Molecular Docking Analysis of Selected Furanocoumarins against Breast Cancer. Sci. Rep. 2019, 9, 15743. [Google Scholar] [CrossRef]
- Piao, X.L.; Yoo, H.H.; Kim, H.Y.; Kang, T.L.; Hwang, G.S.; Park, J.H. Estrogenic Activity of Furanocoumarins Isolated from Angelica Dahurica. Arch. Pharm. Res. 2006, 29, 741–745. [Google Scholar] [CrossRef]
- Zhang, X.; Mahajan, J.S.; Zhang, J.; Korley, L.T.J.; Epps, T.H., 3rd; Wu, C. Lignin-Derivable Alternatives to Bisphenol a with Potentially Undetectable Estrogenic Activity and Minimal Developmental Toxicity. Food Chem. Toxicol. 2024, 190, 114787. [Google Scholar] [CrossRef]
- Nordeen, S.K.; Kumar, V.; Bona, B.J.; Batson, J.D.; Backos, D.S.; Wempe, M.F. Endocrine-Disrupting Activities of Flavones on Steroid Receptors: Structural Requirements and Synthesis of Novel Flavone with Improved Estrogenic Activity. Biomedicines 2025, 13, 748. [Google Scholar] [CrossRef]
- Bolt, M.J.; Oceguera, J.; Singh, P.K.; Safari, K.; Abbott, D.H.; Neugebauer, K.A.; Mancini, M.G.; Gorelick, D.A.; Stossi, F.; Mancini, M.A. Characterization of Flavonoids with Potent and Subtype-Selective Actions on Estrogen Receptors Alpha and Beta. iScience 2024, 27, 109275. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B. Endocrine Disruption by Dietary Phyto-Oestrogens: Impact on Dimorphic Sexual Systems and Behaviours. Proc. Nutr. Soc. 2017, 76, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Djiogue, S.; Halabalaki, M.; Njamen, D.; Kretzschmar, G.; Lambrinidis, G.; Hoepping, J.; Raffaelli, F.M.; Mikros, E.; Skaltsounis, A.L.; Vollmer, G. Erythroidine Alkaloids: A Novel Class of Phytoestrogens. Planta Med. 2014, 80, 861–869. [Google Scholar] [CrossRef]
- Kolesarova, A.; Baldovska, S.; Roychoudhury, S. The Multiple Actions of Amygdalin on Cellular Processes with an Emphasis on Female Reproduction. Pharmaceuticals 2021, 14, 881. [Google Scholar] [CrossRef]
- Künstler, A.; Gullner, G.; Ádám, A.L.; Kolozsváriné Nagy, J.; Király, L. The Versatile Roles of Sulfur-Containing Biomolecules in Plant Defense—A Road to Disease Resistance. Plants 2020, 9, 1705. [Google Scholar] [CrossRef] [PubMed]
- Galanty, A.; Grudzińska, M.; Paździora, W.; Służały, P.; Paśko, P. Do Brassica Vegetables Affect Thyroid Function?—A Comprehensive Systematic Review. Int. J. Mol. Sci. 2024, 25, 3988. [Google Scholar] [CrossRef] [PubMed]
- Boué, S.M.; Burow, M.E.; Wiese, T.E.; Shih, B.Y.; Elliott, S.; Carter-Wientjes, C.H.; McLachlan, J.A.; Bhatnagar, D. Estrogenic and Antiestrogenic Activities of Phytoalexins from Red Kidney Bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 2011, 59, 112–120. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Burden, N.; Bonnell, M.; Hecker, M.; Hutchinson, T.H.; Jagla, M.; LaLone, C.A.; Lagadic, L.; Lynn, S.G.; Shore, B.; et al. New Approach Methodologies for the Endocrine Activity Toolbox: Environmental Assessment for Fish and Amphibians. Environ. Toxicol. Chem. 2023, 42, 757–777. [Google Scholar] [CrossRef]
- OECD. Revised Guidance Document 150 on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption; OECD Series on Testing and Assessment, No. 150; OECD Publishing: Paris, France, 2018. [Google Scholar]
- Dudek, A.Z.; Arodz, T.; Gálvez, J. Computational Methods in Developing Quantitative Structure-Activity Relationships (Qsar): A Review. Comb. Chem. High Throughput Screen. 2006, 9, 213–228. [Google Scholar] [CrossRef]
- Santos, L.H.S.; Ferreira, R.S.; Caffarena, E.R. Integrating Molecular Docking and Molecular Dynamics Simulations. Methods Mol. Biol. 2019, 2053, 13–34. [Google Scholar] [PubMed]
- Villeneuve, D.L.; Crump, D.; Garcia-Reyero, N.; Hecker, M.; Hutchinson, T.H.; LaLone, C.A.; Landesmann, B.; Lettieri, T.; Munn, S.; Nepelska, M.; et al. Adverse Outcome Pathway (Aop) Development I: Strategies and Principles. Toxicol. Sci. 2014, 142, 312–320. [Google Scholar] [CrossRef]
- Grulke, C.M.; Williams, A.J.; Thillanadarajah, I.; Richard, A.M. Epa’s Dsstox Database: History of Development of a Curated Chemistry Resource Supporting Computational Toxicology Research. Comput. Toxicol. 2019, 12, 100096. [Google Scholar] [CrossRef]
- Dix, D.J.; Houck, K.A.; Martin, M.T.; Richard, A.M.; Setzer, R.W.; Kavlock, R.J. The Toxcast Program for Prioritizing Toxicity Testing of Environmental Chemicals. Toxicol. Sci. 2007, 95, 5–12. [Google Scholar] [CrossRef]
- OECD. Test No. 493: Performance-Based Test Guideline for Human Recombinant Estrogen Receptor (Hrer) In Vitro Assays to Detect Chemicals with er Binding Affinity; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2024. [Google Scholar]
- United States Environmental Protection Agency. Standard Evaluation Procedure: Androgen Receptor Binding Assay (OCSPP 890.1150); US EPA, Office of Chemical Safety and Pollution Prevention: Washington, DC, USA, 2011.
- OECD. Test No. 455: Performance-Based Test Guideline for Stably Transfected Transactivation In Vitro Assays to Detect Estrogen Receptor Agonists; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2021. [Google Scholar]
- OECD. Test No. 458: Stably Transfected Human Androgen Receptor Transactivation Assay for Detection of Androgen Agonist and Antagonist Activity of Chemicals; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2023. [Google Scholar]
- ISO 19040-1:2018; Water Quality—Determination of the Estrogenic Potential of Water and Waste Water—Part 1: Yeast Estrogen Screen (Yes) Assay. ISO: Geneva, Switzerland, 2018.
- ISO 19040-3:2021; Water Quality—Determination of the Estrogenic Potential of Water and Waste Water—Part 3: Yeast Androgen Screen (Yas) Assay. ISO: Geneva, Switzerland, 2021.
- OECD. Test No. 456: H295r Steroidogenesis Assay; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2025. [Google Scholar]
- Paul, K.B.; Hedge, J.M.; Rotroff, D.M.; Hornung, M.W.; Crofton, K.M.; Simmons, S.O. Development of a Thyroperoxidase Inhibition Assay for High-Throughput Screening. Chem. Res. Toxicol. 2014, 27, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Montaño, M.; Cocco, E.; Guignard, C.; Marsh, G.; Hoffmann, L.; Bergman, Å.; Gutleb, A.C.; Murk, A.J. New Approaches to Assess the Transthyretin Binding Capacity of Bioactivated Thyroid Hormone Disruptors. Toxicol. Sci. 2012, 130, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Mitsui-Watanabe, N.; Tatarazako, N.; Onishi, Y.; Katsu, Y.; Miyagawa, S.; Ogino, Y.; Yatsu, R.; Kohno, S.; Takase, M.; et al. Establishment of Transactivation Assay Systems Using Fish, Amphibian, Reptilian and Human Thyroid Hormone Receptors. J. Appl. Toxicol. 2013, 33, 991–1000. [Google Scholar] [CrossRef]
- Ager-Wick, E.; Hodne, K.; Fontaine, R.; von Krogh, K.; Haug, T.M.; Weltzien, F.A. Preparation of a High-Quality Primary Cell Culture from Fish Pituitaries. J. Vis. Exp. 2018, 138, 58159. [Google Scholar]
- He, L.; Zhao, C.; Xiao, Q.; Zhao, J.; Liu, H.; Jiang, J.; Cao, Q. Profiling the Physiological Roles in Fish Primary Cell Culture. Biology 2023, 12, 1454. [Google Scholar] [CrossRef]
- Ortego, L.S.; Olmstead, A.W.; Weltje, L.; Wheeler, J.R.; Bone, A.J.; Coady, K.K.; Banman, C.S.; Burden, N.; Lagadic, L. The Extended Amphibian Metamorphosis Assay: A Thyroid-Specific and Less Animal-Intensive Alternative to the Larval Amphibian Growth and Development Assay. Environ. Toxicol. Chem. 2021, 40, 2135–2144. [Google Scholar] [CrossRef]
- Park, C.G.; Ryu, C.S.; Sung, B.; Manz, A.; Kong, H.; Kim, Y.J. Transcriptomic and Physiological Analysis of Endocrine Disrupting Chemicals Impacts on 3d Zebrafish Liver Cell Culture System. Aquat. Toxicol. 2022, 245, 106105. [Google Scholar] [CrossRef]
- OECD. Test No. 249: Fish Cell Line Acute Toxicity—The Rtgill-W1 Cell Line Assay; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2021; Volume 249. [Google Scholar]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (Fet) Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2025; Volume 236. [Google Scholar]
- Gölz, L.; Blanc-Legendre, M.; Rinderknecht, M.; Behnstedt, L.; Coordes, S.; Reger, L.; Sire, S.; Cousin, X.; Braunbeck, T.; Baumann, L. Development of a Zebrafish Embryo-Based Test System for Thyroid Hormone System Disruption: 3rs in Ecotoxicological Research. Environ. Toxicol. Chem. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Smith, E.M.; Chu, S.; Paterson, G.; Metcalfe, C.D.; Wilson, J.Y. Cross-Species Comparison of Fluoxetine Metabolism with Fish Liver Microsomes. Chemosphere 2010, 79, 26–32. [Google Scholar] [CrossRef]
- Azevedo, P.; Butolo, N.P.; de Alencar, L.D.; Lima, H.M.S.; Sales, V.R.; Malaspina, O.; Nocelli, R.C.F. Optimization of in Vitro Culture of Honeybee Nervous Tissue for Pesticide Risk Assessment. Toxicol. Vitr. 2022, 84, 105437. [Google Scholar] [CrossRef] [PubMed]
- Mincarelli, L.; Vischetti, C.; Craft, J.; Tiano, L. DNA Damage in Different Eisenia Andrei Coelomocytes Sub-Populations after in Vitro Exposure to Hydrogen Peroxide. Springerplus 2016, 5, 302. [Google Scholar] [CrossRef]
- OECD. Test No. 210: Fish, Early-Life Stage Toxicity Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2025. [Google Scholar]
- OECD. Test No. 229: Fish Short Term Reproduction Assay; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2012. [Google Scholar]
- OECD. Test No. 240: Medaka Extended One Generation Reproduction Test (Meogrt); OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2023. [Google Scholar]
- OECD. Test No. 230: 21-Day Fish Assay: A Short-Term Screening for Oestrogenic and Androgenic Activity, and Aromatase Inhibition; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2009. [Google Scholar]
- OECD. Test No. 305: Bioaccumulation in Fish: Aqueous and Dietary Exposure; OECD Guidelines for the Testing of Chemicals, Section 3; OECD Publishing: Paris, France, 2012. [Google Scholar]
- OECD. Test No. 250: Easzy Assay—Detection of Endocrine Active Substances, Acting through Estrogen Receptors, Using Transgenic Tg(Cyp19a1b:Gfp) Zebrafish Embryos; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2023. [Google Scholar]
- OECD. Test No. 234: Fish Sexual Development Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2011. [Google Scholar]
- OECD. Test No. 251: Rapid Androgen Disruption Activity Reporter (Radar) Assay; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2022. [Google Scholar]
- OECD. Test No. 202: Daphnia Sp. Acute Immobilisation Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2004; Volume 202. [Google Scholar]
- OECD. Test No. 211: Daphnia Magna Reproduction Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2012. [Google Scholar]
- OECD. Test No. 253: Short-Term Juvenile Hormone Activity Screening Assay Using Daphnia Magna (Jhasa); OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2024. [Google Scholar]
- OECD. Test No. 248: Xenopus Eleutheroembryonic Thyroid Assay (Xeta); OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2019. [Google Scholar]
- OECD. Test No. 231: Amphibian Metamorphosis Assay; Oecd Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2009. [Google Scholar]
- OECD. Test No. 241: Larval Amphibian Growth and Development Assay (Lagda); OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2015. [Google Scholar]
- OECD. Guidance Document on the Androgenised Female Stickleback Screen; OECD Series on Testing and Assessment, No. 148; OECD Publishing: Paris, France, 2017. [Google Scholar]
- OECD. Guidance Document on Harpacticoid Copepod Development and Reproduction Test with Amphiascus; OECD Series on Testing and Assessment, No. 201; OECD Publishing: Paris, France, 2016. [Google Scholar]
- OECD. Test No. 218: Sediment-Water Chironomid Toxicity Using Spiked Sediment; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2023. [Google Scholar]
- OECD. Test No. 219: Sediment-Water Chironomid Toxicity Using Spiked Water; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2023. [Google Scholar]
- OECD. Test No. 242: Potamopyrgus Antipodarum Reproduction Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2016. [Google Scholar]
- OECD. Test No. 243: Lymnaea Stagnalis Reproduction Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2016. [Google Scholar]
- ISO 11267:2023; Soil Quality—Inhibition of Reproduction of Collembola (Folsomia Candida) by Soil Contaminants. ISO: Geneva, Switzerland, 2023.
- OECD. Test No. 222: Earthworm Reproduction Test (Eisenia Fetida/Eisenia Andrei); OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2016; Volume 222. [Google Scholar]
- OECD. Test No. 213: Honeybees, Acute Oral Toxicity Test; Oecd Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 1998. [Google Scholar]
- OECD. Test No. 245: Honey Bee (Apis mellifera L.), Chronic Oral Toxicity Test (10-Day Feeding); OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2017. [Google Scholar]
- Kwon, S.; Bae, H.; Jo, J.; Yoon, S. Comprehensive Ensemble in Qsar Prediction for Drug Discovery. BMC Bioinform. 2019, 20, 521. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. Autodock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.H.P.; Botelho, E.B.; de Souza Gomes, T.J.; Kist, R.; Caceres, R.A.; Zanchi, F.B. Visual Dynamics: A Web Application for Molecular Dynamics Simulation Using Gromacs. BMC Bioinform. 2023, 24, 107. [Google Scholar] [CrossRef] [PubMed]
- Park, C.G.; Singh, N.; Ryu, C.S.; Yoon, J.Y.; Esterhuizen, M.; Kim, Y.J. Species Differences in Response to Binding Interactions of Bisphenol a and Its Analogs with the Modeled Estrogen Receptor 1 and in Vitro Reporter Gene Assay in Human and Zebrafish. Environ. Toxicol. Chem. 2022, 41, 2431–2443. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Kunz, P.Y.; Fent, K. Endocrine Disruption and Chronic Effects of Plant Protection Products in Bees: Can We Better Protect Our Pollinators? Environ. Pollut. 2018, 243, 1588–1601. [Google Scholar] [CrossRef]
- Oh, H.W.; Yun, C.S.; Jeon, J.H.; Kim, J.A.; Park, D.S.; Ryu, H.W.; Oh, S.R.; Song, H.H.; Shin, Y.; Jung, C.S.; et al. Conifer Diterpene Resin Acids Disrupt Juvenile Hormone-Mediated Endocrine Regulation in the Indian Meal Moth Plodia Interpunctella. J. Chem. Ecol. 2017, 43, 703–711. [Google Scholar] [CrossRef]
- Shin, S.W.; Jeon, J.H.; Jeong, S.A.; Kim, J.A.; Park, D.S.; Shin, Y.; Oh, H.W. A Plant Diterpene Counteracts Juvenile Hormone-Mediated Gene Regulation during Drosophila Melanogaster Larval Development. PLoS ONE 2018, 13, e0200706. [Google Scholar] [CrossRef]
- Park, D.H.; Choi, J.Y.; Lee, S.-H.; Kim, J.H.; Park, M.G.; Kim, J.Y.; Wang, M.; Kim, H.J.; Je, Y.H. Mosquito Larvicidal Activities of Farnesol and Farnesyl Acetate Via Regulation of Juvenile Hormone Receptor Complex Formation in Aedes Mosquito. J. Asia-Pac. Entomol. 2020, 23, 689–693. [Google Scholar] [CrossRef]
- Dai, H.; Liu, B.; Yang, L.; Yao, Y.; Liu, M.; Xiao, W.; Li, S.; Ji, R.; Sun, Y. Investigating the Regulatory Mechanism of the Sesquiterpenol Nerolidol from a Plant on Juvenile Hormone-Related Genes in the Insect Spodoptera Exigua. Int. J. Mol. Sci. 2023, 24, 13330. [Google Scholar] [CrossRef]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The Juvenile Hormone Signaling Pathway in Insect Development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Liu, C.C.; Lee, L.S.; Man, C.; Cheng, S.H. Phytoestrogen Bakuchiol Exhibits in Vitro and in Vivo Anti-Breast Cancer Effects by Inducing S Phase Arrest and Apoptosis. Front. Pharmacol. 2016, 7, 128. [Google Scholar] [CrossRef]
- Mellanen, P.; Petänen, T.; Lehtimäki, J.; Mäkelä, S.; Bylund, G.; Holmbom, B.; Mannila, E.; Oikari, A.; Santti, R. Wood-Derived Estrogens: Studiesin Vitrowith Breast Cancer Cell Lines Andin Vivoin Trout. Toxicol. Appl. Pharmacol. 1996, 136, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Christianson-Heiska, I.L.; Haavisto, T.; Paranko, J.; Bergelin, E.; Isomaa, B. Effects of the Wood Extractives Dehydroabietic Acid and Betulinol on Reproductive Physiology of Zebrafish (Danio Rerio)—A Two-Generation Study. Aquat. Toxicol. 2008, 86, 388–396. [Google Scholar] [CrossRef]
- Pandelides, Z.; Guchardi, J.; Holdway, D. Dehydroabietic Acid (DHAA) Alters Metabolic Enzyme Activity and the Effects of 17β-Estradiol in Rainbow Trout (Oncorhynchus mykiss). Ecotoxicol. Environ. Saf. 2014, 101, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gao, Y.; Zhang, S.; Li, Y.; Wang, Z.; Zhang, X.; Li, Z.; Li, J.; Chen, Y.; Wu, D. Gibberellic Acid (GA) Induces Developmental Toxicity in Zebrafish (Danio rerio) Embryos Via Oxidative Stress. Aquat. Toxicol. 2025, 279, 107247. [Google Scholar] [CrossRef]
- Pelissero, C.; Lenczowski, M.J.; Chinzi, D.; Davail-Cuisset, B.; Sumpter, J.P.; Fostier, A. Effects of Flavonoids on Aromatase Activity, an in Vitro Study. J. Steroid Biochem. Mol. Biol. 1996, 57, 215–223. [Google Scholar] [CrossRef]
- Sassa-Deepaeng, T.; Chaisri, W.; Pikulkaew, S.; Okonogi, S. Investigation of Antiaromatase Activity Using Hepatic Microsomes of Nile Tilapia (Oreochromis niloticus). Drug Discov. Ther. 2017, 11, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, H.; Kobayashi, M.; Koshiishi, T.; Moriwaki, T.; Tachibana, K.; Tsuchimoto, M.; Soyano, K.; Iguchi, T.; Mori, C.; Arizono, K. Induction of Plasma Vitellogenin Synthesis by the Commercial Fish Diets in Male Goldfish (Carassius auratus) and Dietary Phytoestrogens. J. Health Sci. 2002, 48, 427–434. [Google Scholar] [CrossRef]
- Fajkowska, M.; Adamek-Urbańska, D.; Ostaszewska, T.; Szczepkowski, M.; Rzepkowska, M. Effect of Genistein, Daidzein and Coumestrol on Sex-Related Genes Expression in Russian Sturgeon (Acipenser gueldenstaedtii). Aquaculture 2021, 530, 735872. [Google Scholar] [CrossRef]
- Dai, X.; Zhaozhi, Z.; Tingling, Z.; Wei, J.; Wanhong, W.; Yang, S.-M. The Effect of Tannic Acid on the Gonad Hormones in Plateau Pikas (Ochotona curzoniae) and Root Voles (Microtus oeconomus). Acta Theriol. Sin. 2011, 31, 278–283. [Google Scholar]
- Kiparissis, Y.; Balch, G.C.; Metcalfe, T.L.; Metcalfe, C.D. Effects of the Isoflavones Genistein and Equol on the Gonadal Development of Japanese Medaka Oryzias Latipes. Environ. Health Perspect. 2003, 111, 1158–1163. [Google Scholar] [CrossRef]
- Yao, W.; Chen, J.; Lin, Z.; Wang, N.; Wang, A.; Wang, B.; Wu, Y.; Xu, Z.; Wang, J. Scopoletin Induced Metabolomic Profile Disturbances in Zebrafish Embryos. Metabolites 2022, 12, 934. [Google Scholar] [CrossRef]
- Gallagher, E.P. Using Salmonid Microarrays to Understand the Dietary Modulation of Carcinogenesis in Rainbow Trout. Toxicol. Sci. 2006, 90, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Godoi, F.G.A.; Muñoz-Peñuela, M.; Gomes, A.D.O.; Tolussi, C.E.; Brambila-Souza, G.; Branco, G.S.; Lo Nostro, F.L.; Moreira, R.G. Endocrine Disruptive Action of Diclofenac and Caffeine on Astyanax Altiparanae Males (Teleostei: Characiformes: Characidae). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 231, 108720. [Google Scholar] [CrossRef]
- Kanungo, J.; Cuevas, E.; Guo, X.; Lopez, A.G.; Ramirez-Lee, M.A.; Trickler, W.; Paule, M.G.; Ali, S.F. Nicotine Alters the Expression of Molecular Markers of Endocrine Disruption in Zebrafish. Neurosci. Lett. 2012, 526, 133–137. [Google Scholar] [CrossRef]
- Montagner, C.C.; Umbuzeiro, G.A.; Pasquini, C.; Jardim, W.F. Caffeine as an Indicator of Estrogenic Activity in Source Water. Environ. Sci. Process. Impacts 2014, 16, 1866–1869. [Google Scholar] [CrossRef]
- Venohr, M.; Beusch, C.; Goldhammer, T.; Nguyen, H.H.; Podschun, S.; Schmalsch, C.; Wolter, C. Spatial Distribution of Nicotine Concentrations in Berlin’s Surface Waters and Their Potential Sources. Environ. Sci. Pollut. Res. 2025, 32, 6784–6803. [Google Scholar] [CrossRef]
- Sun, F.; Xia, L.; Wang, B.; Liu, Y.; Cui, X.; Kang, H.; Stoika, R.; Liu, K.; Jin, M. Reserpine Causes Neuroendocrine Toxicity, Inducing Impairments in Cognition Via Disturbing Hypothalamic–Pituitary–Thyroid Axis in Zebrafish. NeuroSci 2025, 6, 28. [Google Scholar] [CrossRef]
- Matthiessen, P. Is Endocrine Disruption a Significant Ecological Issue? Ecotoxicology 2000, 9, 21–24. [Google Scholar] [CrossRef]
- Farooq, S.; Bhat, N.M.; Dar, S.A.; Malik, M.A. Phytoestrogens in Aquaculture: Friend or Foe to Fish Growth and Reproductive Health? Blue Biotechnol. 2025, 2, 15. [Google Scholar] [CrossRef]
- Badamasi, I.; Odong, R.; Masembe, C. Threats Posed by Xenoestrogenic Chemicals to the Aquatic Ecosystem, Fish Reproduction and Humans: A Review. Afr. J. Aquat. Sci. 2020, 45, 243–258. [Google Scholar] [CrossRef]
- Kidd, K.A.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a Fish Population after Exposure to a Synthetic Estrogen. Proc. Natl. Acad. Sci. USA 2007, 104, 8897–8901. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.J.; Yokota, H.; Oshima, Y.; Tsuruda, Y.; Yamaguchi, T.; Maeda, M.; Imada, N.; Tadokoro, H.; Honjo, T. Effect of 17beta-Estradiol on the Reproduction of Japanese Medaka (Oryzias latipes). Chemosphere 2002, 47, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Hinck, J.E.; Blazer, V.S.; Denslow, N.D.; Myers, M.S.; Gross, T.S.; Tillitt, D.E. Biomarkers of Contaminant Exposure in Northern Pike (Esox lucius) from the Yukon River Basin, Alaska. Arch. Environ. Contam. Toxicol. 2007, 52, 549–562. [Google Scholar] [CrossRef]
- Nash, J.P.; Kime, D.E.; Van der Ven, L.T.; Wester, P.W.; Brion, F.; Maack, G.; Stahlschmidt-Allner, P.; Tyler, C.R. Long-Term Exposure to Environmental Concentrations of the Pharmaceutical Ethynylestradiol Causes Reproductive Failure in Fish. Environ. Health Perspect. 2004, 112, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Blazer, V.S.; Iwanowicz, D.D.; Walsh, H.L.; Sperry, A.J.; Iwanowicz, L.R.; Alvarez, D.A.; Brightbill, R.A.; Smith, G.; Foreman, W.T.; Manning, R. Reproductive Health Indicators of Fishes from Pennsylvania Watersheds: Association with Chemicals of Emerging Concern. Environ. Monit. Assess. 2014, 186, 6471–6491. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Lim, H.B. Unveiling the Endocrine-Disrupting Potential of Plant-Derived Compounds: An Ecotoxicological Review. Toxins 2025, 17, 423. https://doi.org/10.3390/toxins17080423
Park C, Lim HB. Unveiling the Endocrine-Disrupting Potential of Plant-Derived Compounds: An Ecotoxicological Review. Toxins. 2025; 17(8):423. https://doi.org/10.3390/toxins17080423
Chicago/Turabian StylePark, Changgyun, and Heung Bin Lim. 2025. "Unveiling the Endocrine-Disrupting Potential of Plant-Derived Compounds: An Ecotoxicological Review" Toxins 17, no. 8: 423. https://doi.org/10.3390/toxins17080423
APA StylePark, C., & Lim, H. B. (2025). Unveiling the Endocrine-Disrupting Potential of Plant-Derived Compounds: An Ecotoxicological Review. Toxins, 17(8), 423. https://doi.org/10.3390/toxins17080423





